Abstract
Background
Stereotactic radiosurgery (SRS) offers excellent local control for brain metastases (BM) with low rates of toxicity. Radiation necrosis (RN) may occur after treatment and is challenging to distinguish from local recurrence (LR). We evaluated enlarging brain lesions following SRS that were subsequently biopsied to differentiate RN versus LR.
Methods
This study reviewed patients receiving SRS for BM between 2008 and 2012 who underwent a biopsy for suspicion of RN versus LR on MRI. Data collection included demographics, radiation parameters, imaging findings, and post-biopsy pathology. Kaplan–Meier methods determined overall survival. Fisher’s exact test assessed for association between lesion biopsy result and variables of interest.
Results
Thirty-four patients with 35 biopsied BM were included. Lesions were biopsied a median of 8.8 months after SRS. Most patients had primary lung cancer (11; 31.4%). Eleven (31.4%) biopsies were positive for LR and 24 (68.6%) showed RN only. Median overall survival was longer for patients with RN (31.0 mo) than for patients with LR (14.5 mo; P = 0.135). Time from SRS to biopsy was significantly different between RN and LR groups; 10 lesions (52.5%) biopsied ≤9 months after SRS showed LR, whereas 1 lesion (6.3%) biopsied >9 months after SRS showed LR (P = 0.004). For 16 (65.7%) lesions, management was changed or directed by the biopsy results.
Conclusions
Stereotactic biopsy for accessible enlarging lesions after SRS appears diagnostically valuable in patients with few lesions and changes clinical management. RN should be suspected in patients with an enlarging lesion more than 9 months post-SRS.
Keywords: brain metastasis, enlarging brain lesion, local recurrence, radiation necrosis, stereotactic biopsy
Importance of the study.
This study reports one of the largest known cohorts of radiographically enlarging lesions after SRS for brain metastases that have been subsequently biopsied. Prior studies have used both imaging criteria and biopsy to inform rates of radionecrosis versus local recurrence. By reporting only biopsy-proven rates of LR versus RN, our study offers clinicians a better understanding of the clinical status of their patients who have failed conventional SRS therapy. We highlight stereotactic biopsy as an important tool in managing patients with worsening intracranial lesions.
Brain metastasis is a frequent and feared complication of solid tumors that is seen in up to one-third of advanced cancer patients.1,2 Stereotactic radiosurgery (SRS) is a primary treatment for intracranial metastases in deep brain structures or near eloquent areas that are not amenable to surgical resection. SRS alone can provide local control rates of over 70% and is often favored over conventional whole brain radiotherapy (WBRT) due to a lower rate of adverse neurocognitive side effects.3–7
Radiation necrosis (RN), or irreversible tissue injury after prior cerebral irradiation, is a rare but major complication of SRS most commonly seen 3–9 months after treatment with SRS.8,9 Treatment for RN is largely supportive, consisting of corticosteroids and close neurological follow-up. In more severe cases, laser interstitial thermal therapy, surgical resection, or use of bevacizumab may be warranted.10–12 A diagnosis of RN is determined by gadolinium-enhanced T1-weighted MRI showing enlarging heterogeneous or ring-enhancing lesions accompanied by surrounding edema at prior SRS site. However, the appearance of RN on MRI is often initially indistinguishable from tumor local recurrence (LR), causing a major diagnostic dilemma. Current diagnostic practice is to use frequent serial imaging, with the expectation that LR manifests as sustained and steady solid-appearing growth and RN remains stable in size or shrinks over time.13 However, there is uncertainty regarding the specificity of this approach, as RN can also present as an enlarging lesion with considerable edema on post-SRS MRI.14 The majority of phase III trials with an SRS-alone treatment arm used imaging criteria to determine LR.3,4,6 Unfortunately, investigative imaging modalities are not standardized or consistently reliable to establish a definitive diagnosis, delaying and complicating management of these patients.15 An accurate diagnosis is clinically relevant in this setting, as a misdiagnosis may have detrimental consequences for patients. When mistaken for LR, RN may be treated with unnecessary salvage therapy and associated risks. When mistaken for RN, diagnosis of LR may be delayed and appropriate treatment pursued too late in the disease course.
Lesions that are accessible via intracranial biopsy or amenable to neurosurgical resection allow for a more definitive understanding of the true rate of RN versus LR. Although the gold standard, tissue diagnosis via biopsy can be challenging to obtain and may involve sampling error. Little has been published regarding histopathological confirmation of post-SRS enlarging lesions; consequently, we set out to evaluate our institutional experience. This study is one of the largest known cohorts of radiographically enlarging lesions after SRS that have been subsequently biopsied.
Materials and Methods
An institutional review board‒approved retrospective review was performed examining 425 patients with brain metastases who received SRS at Duke University Department of Radiation Oncology from March 1, 2008 through December 31, 2012. Of these patients, 34 (8.0%) were found to have enlarging lesions after SRS and were deemed eligible for this study. One patient received 2 separate episodes of SRS to different brain lesions which were later biopsied; therefore, both lesions with their respective SRS treatment parameters were included for a total of 35 lesions in the final analysis.
Primary cancer was diagnosed on tissue biopsy in all patients, and brain metastases were diagnosed via standard MRI brain. Patients were treated with image-guided radiosurgery using a Novalis TX image-guided radiosurgery system. Contouring of gross tumor volume was performed on fine-cut T1-weighted MRIs. Optimal planning target volume (PTV) was created by uniform expansion of the gross tumor volume by 1 mm.16 Dosing parameters for SRS treatment in this study were prescribed per the Radiation Therapy Oncology Group 90-05 algorithm: 20–24 Gy for tumors ≤2 cm, 18 Gy for 2.1–3 cm lesions, and 15 Gy for tumors >3.1 cm maximum dimension.17 No patients were receiving concurrent chemotherapy at time of SRS.
Patient follow-up included a brain MRI and physical exam at 3-month intervals for the first year and 6-month intervals for the second year. Lesions enlarging on MRI following SRS treatment were biopsied when the Response Assessment in Neuro-Oncology criteria for enlargement were exceeded,17 when the patient became symptomatic, or when a decision needed to be made whether or not to re-treat with SRS. Per standard neurosurgical practice, 1 to 3 samples of the enhancing portions of each lesion as indicated on the MRI were taken for an accurate pathological analysis. Any biopsy with evidence of neoplastic cells was categorized as LR. Radiation necrosis was defined as any biopsy without neoplastic cells and evidence of necrosis and reactive gliosis.
For each patient, the diagnostic impression pre-biopsy was obtained from the MRI brain reading at an average of 3 weeks (range, 0–12 wk) before stereotactic biopsy. The impression on imaging was based upon serial changes in lesion size, enhancement, and surrounding edema. The radiology reading was categorized into one of 5 impressions: progression, probable progression, necrosis, probable necrosis, and unclear. These pre-biopsy impressions were subsequently compared with the biopsy result. The clinical management decision post-biopsy was obtained from the first radiation oncology note documented after biopsy. Three categories were generated: change in management, no change in management, and directed management. Change in management indicates that the clinician documented a change in the treatment based upon the biopsy result, whereas no change in management indicates that the biopsy did not result in a change in treatment. “Directed management” designates that biopsies were performed for the sole purpose of guiding treatment and there was no documented evidence of a treatment plan pre-biopsy. Two study personnel independently reviewed the chart documentation in order to minimize interobserver variability.
Statistical Analysis
The primary endpoint was the rate of biopsy-confirmed RN versus LR among all enlarging brain lesions. Survival analyses were performed using the Kaplan–Meier method. Overall survival (OS) was calculated from the date of SRS to the date of death or last follow-up, if alive. Fisher’s exact test was used to assess any association between the lesion biopsy result and specific variables of interest, with a median split used to dichotomize the continuous variables. The variables of interest included age at SRS (≤53/>53 y), maximum radiation dose (≤21/21 Gy), PTV (≤4/>4 cm3), volume of brain receiving 12 Gy or more (V12; ≤16/>16 cm3), months from SRS to biopsy (≤9/>9), primary histology (lung mets/all other mets), resection pre-SRS (yes/no), WBRT pre-SRS (yes/no), and WBRT post-SRS (yes/no). All analyses performed were lesion specific, not patient specific. Consequently, 1 patient with 2 treated and biopsied lesions was included twice in the survival analysis. SAS 9.3 was used for all analyses.
Results
The 34 patients were an average of 52.5 years old (range, 22–71 y) at time of SRS, and 18 (53%) were female. Enlarging lesions on contrast-enhanced MR imaging were biopsied a median of 8.8 months (range, 2.2–39.6) after SRS. Baseline clinicopathologic characteristics and SRS treatment parameters are shown in Table 1. Eleven lesions showed a diagnosis of lung cancer (31.4%), 8 had melanoma (22.9%), 7 had breast cancer (20.0%), and 9 (25.7%) had other primary diagnoses (which included carcinoid, esophageal, head and neck, germinoma, renal cell carcinoma, and rectal cancer). Three lesions (8.5%) were synchronous brain metastases; all others were metachronous. Eleven patients (32.4%) received systemic therapy (chemotherapy, endocrine therapy, or other targeted therapies) within 3 months prior to brain metastasis diagnosis. Most lesions (80.0%) were not surgically resected prior to SRS treatment. Eighteen lesions (51.4%) received WBRT prior to treatment with SRS, while 6 (17.1%) lesions were treated with WBRT post-SRS treatment. Eleven (32.4%) patients had progressing systemic disease at time of SRS treatment, as documented on radiographic imaging within 2 months of SRS treatment date; all others had stable systemic disease. SRS treatment parameters were not normally distributed. The median radiation maximum dose among all lesions biopsied was 20.9 Gy (interquartile range [IQR] = 17.4–23.0). Median PTV was 3.5 mL (IQR = 1.3–9.5), and the median V12 was 15.8 cm3 (IQR = 5.3–27.1).
Table 1.
Clinicopathologic characteristics and SRS treatment parameters
| Characteristic* | N (%)** |
|---|---|
| Primary Diagnosis Type | |
| Breast | 7 (20.0) |
| Lung | 11 (31.4) |
| Melanoma | 8 (22.9) |
| Other+ | 9 (25.7) |
| Chronicity of Brain Metastases | |
| Synchronous with primary | 3 (8.6) |
| Metachronous | 32 (91.4) |
| Concomitant Systemic Therapy at Time of Brain Metastasis Diagnosis # ^ | 11 (32.4) |
| Had Resection Pre-SRS | 7 (20.0) |
| Received WBRT Pre-SRS | 18 (51.4) |
| Received WBRT Post-SRS | 6 (17.1) |
| Age at SRS, y, mean (range) | 52.5 (22–71) |
| Extent of Systemic Disease at Time of SRS #$ | |
| Stable | 23 (67.6) |
| Progressing | 11 (32.4) |
| SRS Dosing Parameters | |
| Max Dose, Gy—median (range) | 20.9 (11.2–27.7) |
| PTV, cm3—median (range) | 3.5 (0.1–39.2) |
| V12, cm3—median (range) | 15.8 (0.0–74.6) |
| Months from SRS to biopsy—median (range) | 8.8 (2.2–39.6) |
| All Lesions | 35 (100.0) |
*All characteristics are lesion specific (denominator of 35) instead of patient specific (denominator of 34), unless otherwise noted.
**N and percentage are reported, except where noted.
+Other includes carcinoid, esophageal, head and neck, germinoma, renal cell carcinoma, and rectal cancers.
#Based on 34 patients instead of 35 lesions.
^Considered concomitant systemic therapy if received within 3 months prior to diagnosis of brain metastases.
$ As documented on radiographic imaging within 2 months of SRS treatment date.
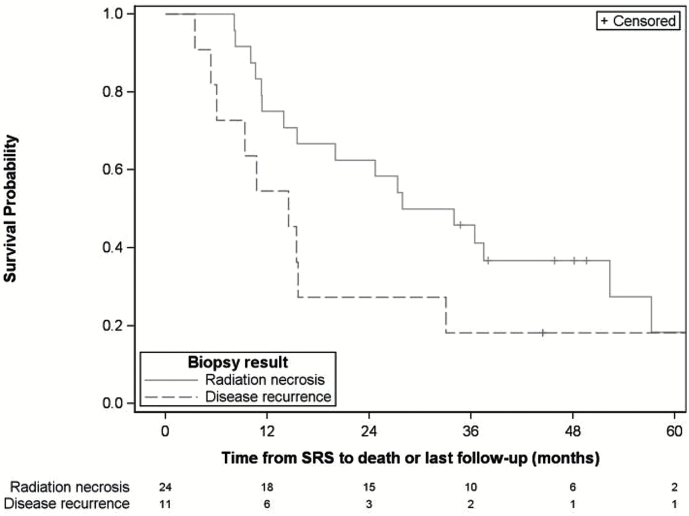
Among the 35 lesions biopsied, 11 (31.4%) were positive for disease recurrence and 24 (68.6%) showed radiation necrosis. The 1 patient with 2 treated and biopsied lesions showed radionecrosis in both biopsied lesions. The median length of follow-up after SRS for all lesions was 49.6 months (95% CI: 38.0–73.5 mo). Median OS was longer for patients with radiation necrosis (31.0 mo, 95% CI: 13.9–52.4 mo) than for those with disease recurrence (14.5 mo, 95% CI: 5.4–33.0 mo), but there was insufficient sample size and power to accurately assess significance (Fig. 1).
Fig. 1.
Kaplan–Meier curve of overall survival from SRS by biopsy status.*
*Note: One patient with 2 lesions treated and biopsied was included twice in this analysis.
Time from SRS to biopsy was significantly associated with the biopsy result and indicated that metastases biopsied 9 months or more post-SRS showed a much lower rate of disease recurrence (Table 2). Ten lesions (52.6%) biopsied ≤9 months after SRS showed local disease recurrence, whereas only one lesion (6.3%) biopsied >9 months after SRS showed LR (P = 0.004). Bivariate analysis showed no association between the biopsy result and primary diagnosis type (P = 0.263), age at SRS (P = 0.364), prior tumor resection (P = 0.652), WBRT pre-SRS (P = 0.146), WBRT post-SRS (P = 0.999), maximum dose (P = 0.471), PTV (P = 0.273), or V12 (P = 0.999).
Table 2.
Histology, treatment, and SRS characteristics for all lesions biopsied by result
| Characteristic | Radiation Necrosis | Disease Recurrence | Exact P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Diagnosis Type | |||||
| All other mets | 18 | 75.0 | 6 | 25.0 | 0.263 |
| Lung mets | 6 | 54.5 | 5 | 45.5 | |
| Age at SRS, y | |||||
| ≤53 | 14 | 73.7 | 5 | 26.3 | 0.364 |
| >53 | 10 | 62.5 | 6 | 37.5 | |
| Resection Pre-SRS | |||||
| No | 20 | 71.4 | 8 | 28.6 | 0.652 |
| Yes | 4 | 57.1 | 3 | 42.9 | |
| WBRT Pre-SRS | |||||
| No | 14 | 82.4 | 3 | 17.6 | 0.146 |
| Yes | 10 | 55.6 | 8 | 44.4 | |
| WBRT Post-SRS | |||||
| No | 20 | 69.0 | 9 | 31.0 | 0.999 |
| Yes | 4 | 66.7 | 2 | 33.3 | |
| Time from SRS to biopsy (mo) | |||||
| ≤9 mo | 9 | 47.4 | 10 | 52.6 | 0.004 |
| >9 mo | 15 | 93.8 | 1 | 6.3 | |
| Max Dose (Gy) | |||||
| ≤21 | 11 | 61.1 | 7 | 38.9 | 0.471 |
| >21 | 13 | 76.5 | 4 | 23.5 | |
| PTV (cm 3) | |||||
| ≤4 | 15 | 78.9 | 4 | 21.1 | 0.273 |
| >4 | 9 | 56.3 | 7 | 43.8 | |
| V12 (cm 3) | |||||
| ≤16 | 13 | 68.4 | 6 | 31.6 | 0.999 |
| >16 | 11 | 68.8 | 5 | 31.3 | |
| All Lesions | 24 | 68.6 | 11 | 31.4 | |
Pre-biopsy diagnostic impressions of the 35 lesions based on MRI appearance are outlined in Table 3, Panel A. The majority of lesions (68.6%) were thought to be “progression” or “probable progression,” while only 11 were thought to represent “probable necrosis” or an “unclear” diagnosis. Of the 11 (31.4%) lesions determined by biopsy to be cancerous, the pre-biopsy clinical impression was progression/probable progression for 7 (63.6%) lesions, probable necrosis for 3 (27.3%) lesions, and unclear for 1 (9.1%) lesion. Of the 24 (68.6%) lesions determined by biopsy to be necrosis, the pre-biopsy clinical impression was progression/probable progression for 17 (70.8%) lesions, probable necrosis for 3 (12.5%) lesions, and unclear for 4 (16.7%) lesions. A change in clinical management based on the biopsy result was seen in 18 (51.4%) lesions (Table 3, Panel B). There was no change in management for 11 (31.4%) lesions. For the 5 lesions (14.3%) where the diagnostic impression was initially “unclear” pre-biopsy, the intracranial biopsy directed management. One patient was lost to follow-up after stereotactic biopsy, so no determination of management change could be made.
Table 3.
Panel A: Pre-biopsy clinical impression and biopsy result. Panel B: Accuracy of the pre-biopsy clinical impression and the impact on clinical management
| Pre-Biopsy Clinical Impression* | Biopsy Result | ||||
|---|---|---|---|---|---|
| Cancer Recurrence/ Progression | Necrosis | Total | |||
| Progression/probable progression | 7 (63.6%) | 17 (70.8%) | 24 | ||
| Probable necrosis | 3 (27.3%) | 3 (12.5%) | 6 | ||
| Unclear | 1 (9.1%) | 4 (16.7%) | 5 | ||
| Total | 11 | 24 | 35 | ||
| Accuracy of the Pre-Biopsy Clinical Impression** | Clinical Management*** | ||||
| Change In Management+ | Directed Management# | No Change in Management^ | No Follow-up | Total | |
| Consistent with biopsy result | 1 (5.6%) | 0 (0.0%) | 9 (81.8%) | 0 (0.0%) | 10 |
| Inconsistent with biopsy result | 17 (94.4%) | 0 (0.0%) | 2 (18.2%) | 1 (100.0%) | 20 |
| Unclear pre-biopsy impression | 0 (0.0%) | 5 (100.0%) | 0 (0.0%) | 0 (0.0%) | 5 |
| Total | 18 | 5 | 11 | 1 | 35 |
*The pre-biopsy clinical impression was obtained from the most recent MRI brain reading prior to biopsy and was based upon serial changes in lesion size, enhancement, and surrounding edema. Five categories were used: progression, probable progression, necrosis, probable necrosis, and unclear.
**Pre-biopsy clinical impressions were compared with biopsy results and determined to be consistent or inconsistent with the biopsy result.
***The clinical management decision post-biopsy was obtained from the first radiation oncology note documented after biopsy.
+Change in management indicates that the clinician documented a change in the treatment plan based upon the biopsy result.
#Directed management designates that biopsies were pursued for the sole purpose of guiding treatment and there was no documented evidence of a treatment plan pre-biopsy.
^No change in management indicates that the biopsy did not result in a change in treatment plan.
Discussion
SRS for treatment of brain metastasis has been repeatedly shown to have impressive local control rates ranging from 67% to 76% at one year follow-up, which increases to approximately 90% when combined with adjuvant WBRT.3,4,6,18 Given a lack of survival benefit of adjuvant WBRT and increased risk for neurocognitive decline,3,6,7,19 SRS alone as a first-line treatment has become standard of care for many patients with a limited number of small or inoperable brain metastases and a good performance status.
With increasing use of high-dose radiation techniques for brain metastasis, RN has become a major concern. Factors correlated with increased risk of RN include prior SRS,13 larger 10- and 12-Gy volumes,13,20 prescription dose,13 and use of single-fraction SRS.21 Miller et al also found that tumor biology (heterogeneity index, human epidermal growth factor receptor 2 amplification, BRAF mutations, and lung adenocarcinoma) is associated with the development of RN after SRS.22 The average overall incidence of RN one year after SRS has been estimated to be between 9% and 14%.13,23 However, this varies with tumor size and dosing volume, and one study reported the incidence to be as high as 39%–82%.20 In a large cohort of 2200 SRS-treated brain metastases, Sneed et al reported 441 (20.0%) enlarging lesions on follow-up imaging suspicious for LR versus adverse radiation effects; of these lesions, the study determined 203 (46.0%) to be LR, 118 (26.8%) to be RN, 30 (6.8%) to be both LR and RN, and 90 (20.4%) to have an indeterminate cause of lesion enlargement.13 Notably, our study of biopsied samples differs, showing a higher proportion of RN (68.6%) and a lower proportion of LR (31.4%) among enlarging lesions treated with SRS. Direct comparison of the Sneed study results to our own is challenging due to our small sample size and possible institutional bias. Moreover, Sneed et al did not report a biopsy rate, so it is unclear how many lesions were definitively diagnosed by biopsy versus being presumptively diagnosed based solely upon imaging. The lower rates of RN in the Sneed study could be attributed to lack of tissue sampling for all patients.
True incidence of RN versus LR is difficult to glean from documented studies for several reasons. First, there is no consensus strategy to diagnose RN via conventional imaging, and differentiating RN from LR has been notoriously challenging on scans. Other than MRI brain with contrast, several non-invasive imaging techniques have been attempted with variable success, including SPECT (single-photon emission CT; sensitivity 85%–90%), MR spectroscopy, and PET + MRI coregistration (sensitivity 65%–85%).24 Second, gold standard histological diagnosis via brain biopsy is rarely pursued16 due to concerns regarding the invasiveness of the procedure, constraints of tumor location, and poor patient performance status. Third, even when definitive diagnosis is recommended via biopsy, patient willingness may be a limiting factor and tissue sampling error may bias result. Our study aimed to avoid the inherent variability in imaging diagnostic techniques for RN by reporting rates of biopsy-proven RN versus LR.
Our study suggests that intracranial biopsy aids the clinical management of patients with enlarging lesions and should be used to guide treatment decisions when clinical impression based on imaging of “pseudoprogression” is unclear. Biopsy often yielded a “true” diagnosis of radionecrosis and changed treatment management (51.4%) even when imaging suggested local recurrence. In 65.7% of our reported cases, clinical management was either changed or directed by the biopsy results. Therefore, the majority of the patients in this study avoided potential misdiagnosis and suboptimal treatment. While needing confirmation in a larger trial, our study suggests that the pre-biopsy impression of “progression/probable progression” in patients evaluated for enlarging lesions >9 months after SRS should be questioned; our data showed a 93.8% incidence rate of RN in this patient population.
The primary limitation of our study was its small sample size and consequent lack of power to detect a statistical difference in volumetric dosing or histopathological factors as reported in prior studies.13,21,22 Several factors may have limited the number of enlarging lesions biopsied at our institution, including substantial risks of brain biopsy, patient unwillingness to undergo the procedure, and unclear clinical algorithms for determination of LR versus RN. In addition to possible tissue sampling error, the lower incidence of cancer at the site of SRS biopsy found in our study could also be a result of other contributing factors. Tertiary referral centers such as ours attract more complex cases that may more frequently represent RN. Moreover, practitioners have different thresholds for actively pursuing a diagnosis versus exploiting the “tincture of time” via short-interval serial imaging. Consequently, the fact that patients were treated at a single institution may predispose to patient selection bias, as well as treatment bias. Finally, it would have been useful to correlate gold standard biopsy with advanced MR imaging findings as explored in other studies.25 To date, however, there remain some issues with reliability and reproducibility of these advanced imaging techniques.15
In conclusion, imaging diagnosis of RN versus LR after SRS for brain metastases can be challenging. We suggest a case-by-case assessment and careful observation of enlarging lesions on MRI after SRS. Lesions that start enlarging greater than 9 months after SRS may more likely be radiation necrosis. Intracranial biopsy of these lesions offers a definitive diagnosis and may result in a substantial change in clinical management.
Funding
Jessica Narloch is funded by TL-1 CTSA Pre-Doctoral Training Grant (5TL1TR001116-03). Fang-Fang Yin, John P. Kirkpatrick, and Grace J. Kim all have research funding from Varian Medical Systems.
Conflict of interest statement. John P. Kirkpatrick owns Clearsight Radiotherapy Products LLP.
References
- 1. Kamar FG, Posner JB. Brain metastases. Semin Neurol. 2010;30(3):217–235. [DOI] [PubMed] [Google Scholar]
- 2. Hedayatizadeh-Omran A, Rafiei A, Alizadeh-Navaei R et al. Role of HER2 in brain metastasis of breast cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16(4):1431–1434. [DOI] [PubMed] [Google Scholar]
- 3. Kocher M, Soffietti R, Abacioglu U et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoyama H, Shirato H, Tago M et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 5. Radiation dose to patients from radiopharmaceuticals. A report of a task group of committee 2 of the International Commission on Radiological Protection. Ann ICRP. 1987;18(1–4):1–377. [PubMed] [Google Scholar]
- 6. Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 7. Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrews DW, Scott CB, Sperduto PW et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 9. Kohutek ZA, Yamada Y, Chan TA et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200. [DOI] [PubMed] [Google Scholar]
- 11. Drezner N, Hardy KK, Wells E et al. Treatment of pediatric cerebral radiation necrosis: a systematic review. J Neurooncol. 2016;130(1):141–148. [DOI] [PubMed] [Google Scholar]
- 12. Fabiano AJ, Qiu J. Post-stereotactic radiosurgery brain metastases: a review. J Neurosurg Sci. 2015;59(2):157–167. [PubMed] [Google Scholar]
- 13. Sneed PK, Mendez J, Vemer-van den Hoek JG et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–386. [DOI] [PubMed] [Google Scholar]
- 14. Stockham AL, Tievsky AL, Koyfman SA et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109(1):149–158. [DOI] [PubMed] [Google Scholar]
- 15. Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirkpatrick JP, Wang Z, Sampson JH et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–108. [DOI] [PubMed] [Google Scholar]
- 17. Lin NU, Lee EQ, Aoyama H et al. ; Response Assessment in Neuro-Oncology (RANO) group. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 18. Ayala-Peacock DN, Peiffer AM, Lucas JT et al. A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro Oncol. 2014;16(9):1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457–464. [DOI] [PubMed] [Google Scholar]
- 20. Korytko T, Radivoyevitch T, Colussi V et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419–424. [DOI] [PubMed] [Google Scholar]
- 21. Minniti G, Salvati M, Muni R et al. Stereotactic radiosurgery plus whole-brain radiotherapy for treatment of multiple metastases from non-small cell lung cancer. Anticancer Res. 2010;30(7):3055–3061. [PubMed] [Google Scholar]
- 22. Miller JA, Bennett EE, Xiao R et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96(5):1060–1069. [DOI] [PubMed] [Google Scholar]
- 23. Shaw E, Scott C, Souhami L et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 24. Hoefnagels FW, Lagerwaard FJ, Sanchez E et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol. 2009;256(6):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. 2017; doi: 10.1158/1078-0432.CCR-16-2265. [DOI] [PubMed] [Google Scholar]