Abstract
Background
Anaplastic oligodendroglioma (AO) and anaplastic oligoastrocytoma (AOA) are chemotherapy-sensitive tumors with prolonged survival after radiochemotherapy. We report a prospective trial using induction temozolomide (TMZ) followed by myeloablative high-dose chemotherapy (HDC) with autologous stem-cell transplant (ASCT) as a potential strategy to defer radiotherapy.
Methods
Patients with AO/AOA received 6 cycles of TMZ (200 mg/m2 × 5/28 day). Responding patients were eligible for HDC (thiotepa 250 mg/m2/day × 3 days, then busulfan 3.2 mg/kg/day × 3 days), followed by ASCT. Genomic characterization was performed using next-generation sequencing.
Results
Forty-one patients were enrolled; 85% had 1p/19q codeleted tumors. After induction, 26 patients were eligible for HDC-ASCT and 21 agreed to proceed. There were no unexpected adverse events or toxic deaths. After median follow-up of 66 months, 2-year progression-free survival (PFS) for transplanted patients was 86%, 5-year PFS 60%, and no patient has died. Among all 1p/19q codeleted patients (N = 33), 5-year PFS was 50% and 5-year overall survival (OS) 93%, with median time to radiotherapy not reached. Next-generation sequencing disclosed typical oligodendroglioma-related mutations, including IDH1, TERT, CIC, and FUBP1 mutations in 1p/19q codeleted patients, and glioblastoma-like signatures in 1p/19q intact patients. Aside from IDH1, potentially oncogenic/actionable mutations were variable, depicting wide molecular heterogeneity within oligodendroglial tumors.
Conclusions
TMZ followed by HDC-ASCT can be safely administered to patients with newly diagnosed 1p/19q codeleted AO. This strategy was associated with promising PFS and OS, suggesting that a chemotherapy-based approach may delay the need for radiotherapy and radiation-related toxicities. Raw data for further genomic and meta-analyses are publicly available at http://cbioportal.org/study?id=odg_msk_2017, accessed 6 January 2017.
Clinicaltrials.gov registry
Keywords: 1p/19q codeletion, anaplastic oligodendroglioma, autologous stem cell transplant, temozolomide
Importance of the study
Multicenter studies of radiation and procarbazine, lomustine, and vincristine chemotherapy have demonstrated median survivals of greater than 14 years for anaplastic oligodendrogliomas. In long-term survivors, the late neurocognitive side effects of radiation therapy are a concerning sequela of treatment. In this multicenter study mostly enrolling patients with 1p/19q codeleted tumors, we investigated a chemotherapy-only approach of TMZ followed by myeloablative chemotherapy with autologous stem cell rescue as an alternative to radiochemotherapy-based treatment. We found acceptable side effects and survival after 5 years, so far comparable to other studies of 1p/19q codeleted patients. Our findings suggest that a chemotherapy-only approach may delay the use of radiation therapy and associated morbidity while maintaining survival. We also provide a comprehensive genomic characterization of oligodendroglial tumors, to be made publicly available along with clinical data and which can be used to guide future studies.
Anaplastic oligodendrogliomas, both pure (AO) and mixed anaplastic oligoastrocytomas (AOA), are uncommon primary brain tumors that are uniquely sensitive to chemotherapy.1–3 While surgery and radiation therapy (RT) may prolong life in many high grade gliomas,4 adjuvant chemotherapy may be especially effective in anaplastic oligodendroglial tumors with loss of heterozygosity of chromosomes 1p and 19q (1p/19q codeleted).5,6 Two large randomized phase III studies of RT with or without adjuvant chemotherapy with procarbazine, lomustine, and vincristine (PCV) demonstrated significant improvement in progression-free survival (PFS) and near doubling of overall survival (OS) to greater than 14 years in 1p/19q codeleted tumors treated with adjuvant RT and PCV.5,6 The question remains whether chemotherapy alone can effectively treat these tumors, thereby delaying RT and its associated neurotoxic effects until relapse or progression. The risk of radiotherapy-induced late-delayed neurotoxicity is particularly relevant in oligodendroglial tumors because of its cumulative nature over time, potentially compromising long-term survivors’ quality of life over decades.
Our group has been interested in exploring high-dose, myeloablative chemotherapy followed by autologous stem cell transplant (ASCT) in newly diagnosed AO.3,7,8 The rationale for this approach is based on (i) the known chemosensitivity of oligodendroglial tumors, (ii) the possibility of overcoming the blood‒brain barrier to achieve higher CNS drug concentrations, (iii) theoretical increased activity on cancer stem cells, and (iv) emerging evidence of an increased antitumor immune response deriving from a reprogramming of T cells following immune reconstitution.9,10 This group previously reported on a phase II trial in AO (N = 69) utilizing dose-intensive PCV, followed by high-dose thiotepa and ASCT in those patients with chemosensitive disease. The median PFS among 39 transplanted patients was 78 months and median OS was not reached, after a median follow-up of 81 months. Subsequently, the group reported on a trial of 20 patients with newly diagnosed AO treated with intensive PCV and a modified myeloablative regimen with high-dose thiotepa and busulfan.7 Preliminary results showed that among the 11 transplanted patients, the median PFS was 78 months and the median OS was not reached. Across both studies, no evidence of delayed neurotoxicity or myelodysplasia has been observed.
As an extension of this work, and based on the emerging evidence of temozolomide (TMZ) activity in this disease,11–13 we conducted a multicenter phase II study in newly diagnosed AO and AOA utilizing TMZ instead of PCV as initial chemotherapy and maintaining the same thiotepa/busulfan myeloablative regimen and ASCT. Our objective was to determine whether such a regimen could result in long-term control, potentially delaying or eliminating the need for RT. Taking advantage of the tissue collection throughout the study, we also performed next-generation gene sequencing for characterization of the molecular landscape of oligodendroglial tumors in patients enrolled in clinical trials.
Materials and Methods
Patients with newly diagnosed AO or AOA were eligible to participate in this prospective multicenter phase II study. The protocol and informed consent were approved by the Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board (IRB) and local IRBs. The protocol was registered at clinicaltrials.gov (NCT00588523). All patients or guardians signed the informed consent. TMZ was provided free of charge by Schering-Plough/Merck.
Inclusion criteria were as follows: diagnosis of AO or AOA according to 2000 World Health Organization (WHO) criteria (mixed tumors should have a minimum of 25% oligodendroglial elements), age ≥18 years, Karnofsky performance status (KPS) ≥60, adequate organ and bone marrow function including a granulocyte count ≥1.5 × 109/L, platelet count of ≥100 × 109/L, aspartate aminotransferase (AST) ≤2× the upper limit of normal (UNL), serum creatinine ≤1.5× UNL, and bilirubin ≤1.5× UNL. Assessment of 1p/19q codeletion was to be performed as part of initial evaluation but was not an inclusion criterion.
Exclusion criteria consisted of systemic or noncontiguous leptomeningeal metastases, prior cranial RT or systemic chemotherapy, other concurrent malignancy with the exception of cervical carcinoma in situ or basal cell carcinoma of the skin, serious illness that would interfere with the prescribed treatment, pregnancy or lactation, and refusal to use effective contraception.
Within 2 weeks of starting treatment, all patients were evaluated with a complete history, physical and neurological examination, contrast enhanced MRI, a biochemistry panel, and complete blood count and underwent screening for hepatitis B and C and HIV.
Induction Chemotherapy
Induction chemotherapy consisted of oral TMZ dosed at 200 mg/m2 days 1–5 in 28-day cycles for 6 cycles. TMZ cycles were delayed for platelets <100 × 109/L or granulocytes <1.5 × 109/L. Doses were modified for toxicities per the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Tumor assessment with MRI was performed after the third and sixth cycles of TMZ. Patients were deemed evaluable for response rate if baseline MRI showed measurable contrast-enhancing lesion ≥10 mm in diameter. Progressive disease (PD) was defined as an increase of 25% or more of the enhancing or residual non-enhancing lesion measured in cross-sectional diameter. Partial response was defined as ≥50% reduction in the size of the lesion; complete response was defined as the disappearance of all enhancing tumor with the patient off corticosteroids and neurologically stable; stable disease (SD) represented all other situations. Disease status in patients without measurable disease at baseline could only be characterized as SD or PD; those patients were deemed non-evaluable for response rate but were evaluable for all other study endpoints.
Patients with PD at any time were removed from study and referred for RT. Patients achieving a response or SD after 6 cycles of TMZ were eligible for high-dose chemotherapy (HDC) with autologous stem cell support.
High-Dose Chemotherapy and Transplant Protocol
Peripheral blood stem cell (PBSC) harvest was performed prior to the start of induction chemotherapy. At the discretion of the treating physician, harvest could be delayed until response to induction treatment was demonstrated. PBSCs were mobilized using granulocyte colony stimulating factor 10–16 μg/kg/day subcutaneously for up to 7 days with cytophoresis beginning on day 4 and repeated daily up to day 7 until a target yield >5 × 106 CD34+ cells/kg was collected (minimum acceptable yield was 2 × 106 CD34+ cells/kg).
High-dose chemotherapy consisted of intravenous thiotepa 250 mg/m2/day for 3 consecutive days on days −8, −7, and −6 prior to transplant, followed by intravenous busulfan 3.2 mg/kg/day on days −5, −4, and −3. All patients were on prophylactic or therapeutic anticonvulsants prior to the administration of busulfan.
PBSCs were reinfused 2 days (48 hours) after completion of busulfan infusion, on day 0. Standard supportive care as defined by each institution was used in all patients. Follow-up clinical, laboratory, and imaging evaluations were performed every 3 months for 2 years, then a minimum of every 6 months for 3 years, then a minimum of once a year, or as needed by symptoms.
Next-Generation Sequencing and 1p/19q Analysis
Determination of 1p/19q codeletion status was performed locally by fluorescence in situ hybridization (FISH). Patients with partial 1p loss were considered non-codeleted. Whenever available, additional tissue was analyzed through massively parallel next-generation sequencing utilizing the MSK-IMPACT assay, as previously described.14 In brief, the MSK-IMPACT is a hybridization capture-based sequencing assay utilizing an Illumina HiSeq 2500 platform, performed in a laboratory compliant with the Clinical Laboratory Improvement Amendments. The assay provides full exon coverage of 410 cancer-related genes, detecting base substitutions, small indels, copy number alterations, and select gene rearrangements.14
Statistical Analysis
The primary objective of this study was to estimate the duration of disease control of newly diagnosed AOs or AOAs treated with dose-intensive chemotherapy and ASCT. The primary endpoint was defined as 2-year PFS among transplanted patients, to be reported along with 95% CIs. An accrual goal of 40 patients total was determined a priori, with stopping rules established upfront. Based on previous experience with high-dose therapy with stem cell rescue,8 it was expected that approximately 50% of the patients would be eligible for high-dose busulfan/thiotepa chemotherapy. The stopping rules were as follows: If 3 deaths attributable to treatment-related toxicity were observed among the first 15 patients, or 4 deaths occurred at any time, the study would be stopped. The probability of stopping the trial early was determined to be 0.07 if the true risk of treatment-related death was 5% and was 0.38 if the true risk of treatment-related death was 20%. Survival times were evaluated by Kaplan–Meier methodology and calculated from the time of registration. PFS was defined as the time to disease progression, death, or last follow-up. For comparison with historical controls, PFS and OS estimates were also calculated for the intent-to-treat population and the 1p/19q codeleted population. Toxicities were recorded utilizing NCI CTCAE version 3.0.
Results
Patient Characteristics
Forty-one patients were enrolled onto this protocol between August 2005 and November 2012. Twenty-seven men and 14 women, with a median age of 44 years (range 30–66) and median KPS of 90 (range 70–100) were treated (Table 1). The histology was AO in 36 tumors (87.8%) and AOA in 5 (12.1%). The 1p/19q status could be established for 39 of the 41 patients; 33 (84.6%) were codeleted and 6 (15.4%) were non-codeleted. Baseline MRI, performed at time of registration, was available for central review in 37 patients; the majority of patients (N = 26; 70.3%) had no measurable disease, where measurable disease was defined as postsurgery residual enhancing disease measuring >10 mm. The remaining 11 patients (29.7%) had lesions evaluable for radiographic response; of these patients, only 6 had 1p/19q codeleted tumors. Due to the small number of evaluable patients, response rate was not characterized, and analysis of efficacy endpoints was restricted to PFS and OS. The median duration of follow-up of survivors is 65.7 months.
Table 1.
Patient characteristics (all patients, N = 41)
| N (% or range) | |
|---|---|
| Sex | |
| Men | 27 (66%) |
| Women | 14 (34%) |
| Median KPS (range) | 90 (70–100) |
| Median age, y (range) | 44 (30–66) |
| Median baseline Mini-Mental State Exam | 30 (23–30) |
| Histology | |
| Anaplastic oligodendroglioma | 36 (88%) |
| Anaplastic oligoastrocytoma | 5 (12%) |
| 1p/19q status | |
| Codeleted | 33 (80%) |
| Non-codeleted | 6 (15%) |
| Unknown | 2 (5%) |
| Baseline disease status | |
| Measurable (>10 mm) | 11 (27%) |
| Nonmeasurable, gross total resection | 26 (63%) |
| Unavailable for review | 4 (10%) |
Induction Therapy
In total, 198 cycles of TMZ were administered to 38 patients, with 29 completing all 6 cycles (70.7%). Three patients withdrew before starting treatment, 2 other patients withdrew during induction chemotherapy, and 1 patient had insurance denial of coverage after 2 cycles and was removed from study. By the end of induction chemotherapy, a total of 9 patients (25.7%) had experienced disease progression. Therefore, 26 patients were deemed eligible for transplant. Table 2 summarizes toxicities observed during TMZ chemotherapy. Treatment was well tolerated, with no unexpected toxicities observed; grades 3 or 4 thrombocytopenia occurred in 5 patients, lymphopenia in 3 patients, neutropenia in 1 patient, and hyponatremia in 1 patient.
Table 2.
Grades 3 and 4 treatment-related toxicities and time to engraftment
| TMZ Toxicities | Grade 3 | Grade 4 |
|---|---|---|
| Thrombocytopenia | 3 | 2 |
| Neutropenia | 1 | |
| Lymphopenia | 2 | 1 |
| Hyponatremia | 1 | 0 |
| ASCT Toxicities | Grade 3 | Grade 4 |
| Febrile neutropenia | 5 | 0 |
| ALT/AST | 3 | 0 |
| Infection | 0 | 1 |
| Diarrhea | 1 | 0 |
| Colitis | 0 | 1 |
| Mucositis | 1 | 0 |
| Rash | 1 | 0 |
| Syncope | 1 | 0 |
| Vasovagal malaise | 1 | 0 |
|
Time to engraftment:
Neutrophils (>500): 9 days (range: 8–12) Platelets (>50,000): 12 days (range: 9–18) | ||
Note: No toxic deaths observed.
High-Dose Chemotherapy and Autologous Stem Cell Transplant
Among the 26 patients eligible for transplant, 21 (51.2% of the initial 41 patients) were treated with myeloablative chemotherapy with thiotepa, busulfan, and stem cell rescue; 5 patients refused. Among the 21 transplanted patients, there were no patients with intact 1p/19q; 20 (95.2%) patients had 1p/19q codeletion, and in 1 patient 1p/19q was unknown. There were no harvesting failures, and the myeloablative regimen was relatively well tolerated, with no toxic deaths. The median time to neutrophil engraftment was 9 days (range 8–12 days) and to platelet engraftment 12 days (range 9–18 days). Nonhematologic toxicities included grade 3 febrile neutropenia (N = 5), grade 3 AST/alanine aminotransferase (ALT) (n = 3), grade 4 infection (N = 1), grade 3 diarrhea (N = 1), grade 4 colitis (N = 1), and grade 3 mucositis, rash, syncope, and vasovagal malaise (N = 1 for each) (Table 2).
Survival and Relapse
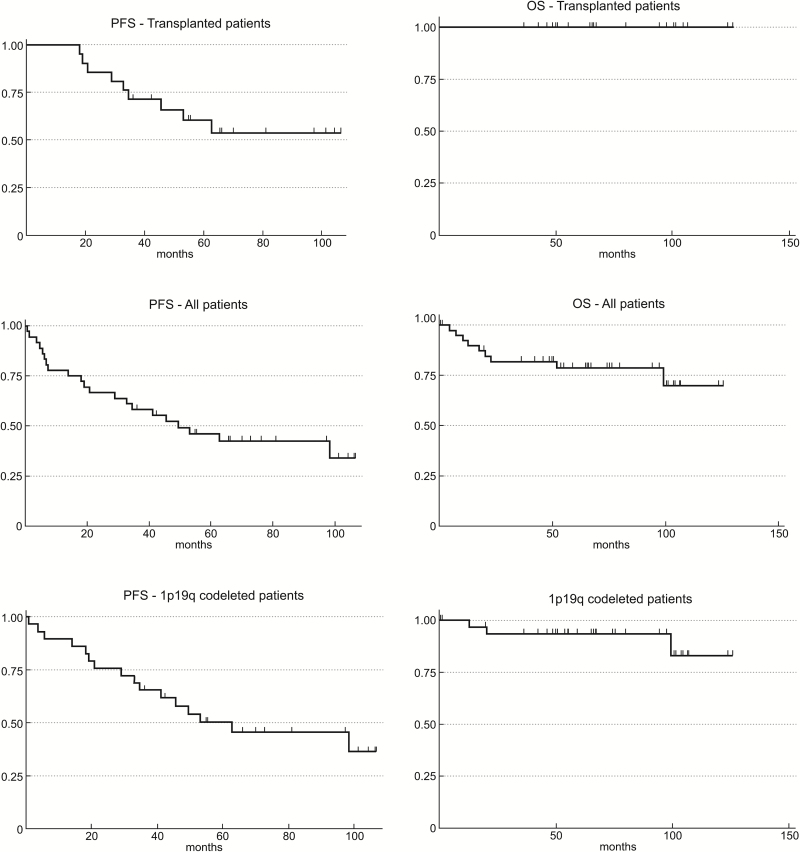
The Kaplan–Meier PFS and OS survival curves are shown in Fig. 1. The median PFS (Fig. 1A) and median OS (Fig. 1B) for the 21 patients who completed HDC-ASCT were not reached, after a median follow-up of survivors of 65.7 months. The 2-year PFS for transplanted patients, the primary endpoint of the study, was 85.7% (95% CI: 62%–95.2%); the 5-year PFS was 60.4% (95% CI: 36%–78%). To date, none of these transplanted patients has died, with a 5-year OS of 100%.
Fig. 1.
Kaplan–Meier survival curves of PFS and OS in transplanted patients (N = 21; A and B), in the intent-to-treat population (all patients, N = 41, C and D), and in all patients with 1p/19q codeletion (N = 33, E and F).
In the intent-to-treat population (N = 41), the median PFS (Fig. 1C) was 49.5 months and the 2-year PFS was 66.7% (95% CI: 48.8%–79.5%). The median OS has not been reached (Fig. 1D), and the 5-year OS was 78.6% (95% CI: 61.6%–88.8%). The median PFS (Fig. 1C) was 49.5 months and the 2-year PFS was 66.7% (95% CI: 48.8%–79.5%). As expected, 1p/19q codeletion was a predictor of PFS (P = 0.03) and OS (P < 0.001) (Appendix 1, online).
Among all patients with 1p/19q codeletion (N = 33), the median PFS (Fig. 1E) was 62.8 months, with 2-year PFS of 75.9% (95% CI: 55.9%–87.7%) and 5-year PFS of 50.3% (95% CI: 30.7%–67%). The median OS in this group (Fig. 1F) has not been reached, and the 5-year OS was 93.4% (95% CI: 76.2%–98.3%). Table 3 demonstrates survival statistics from all patients with 1p/19q codeletion in this trial compared with historical controls from other phase II and III clinical trials in newly diagnosed AO.
Table 3.
Clinical trials for newly diagnosed 1p/19q codeleted anaplastic oligodendroglioma
| First Author | Treatment | RT as Part of Initial Treatment | N | Med Age, y | PFS,mo | OS, mo | 5 y PFS | 5 y OS |
|---|---|---|---|---|---|---|---|---|
| Thomas (present study) |
TMZ 200 mg/m2 days 1–5/28 × 6; thiotepa, busulfan, ASCT | No | 33 | 44 | 63 | NR >66 | 50% | 93% |
| Abrey3 | PCV ×3–4, thiotepa, ASCT | No | 39** | 43 | 78 | NR >80 | 63%* | 78%* |
| Mohile7 *** | PCV ×4, thiotepa, busulfan, ASCT | No | 11 | 46 | 40 | NR >79 | 35% | 55% |
| Ahluwalia30 | TMZ 150 mg/m2 days 1–7,15–21/28 × 8 | No | 20 | 43 | 62 | NR >50 | 55%* | 90%* |
| Van den Bent6 | RT 59.4 Gy | Yes | 37 | 50 | 50 | 112 | 50% | 73% |
| RT, then PCV ×6 | Yes | 43 | 49 | 157 | NR | 71% | 76% | |
| Cairncross5 | RT 59.4 Gy | Yes | 67 | 44 | 35 | 88 | 27% | 75% |
| PCV ×4, then RT | Yes | 59 | 43 | 101 | 176 | 57% | 70% | |
| Jaeckle27 **** | RT 59.4 Gy ± concomitant/adjuvant TMZ | Yes | 24 | — | NR >36 | — | — | — |
| TMZ 200 mg/m2 ×12 cycles | No | 12 | — | 30 | — | — | — | |
| Wick26 | RT | Yes | 35 | 44 | 104 | NR | — | — |
| TMZ | No | 16 | 42 | 54 | 97 | — | — | |
| PCV | No | 17 | 42 | 113 | NR | — | — |
*5-year PFS and OS estimated from Kaplan‒Meier curves; **1p/19q codeletion status unknown for most patients. These survival numbers are for all patients in the study. ***Updated results (personal data). ****Preliminary results, presented in the form of abstract.
At the time of progression, patients were referred for RT. The median time to RT in patients with 1p/19q codeleted tumors has not been reached. At 2 years, 82% of patients (95% CI: 0.62%–0.92%) with 1p/19q codeleted tumors were alive without RT and at 5 years 52% were alive without RT (95% CI: 0.3%–0.74%).
To investigate whether the choice of TMZ for the induction chemotherapy in this trial had a detrimental effect compared with PCV, we performed an exploratory, unplanned analysis comparing results with the previously reported clinical trial of a similar group of patients with newly diagnosed AO or AOA (N = 20), which utilized PCV followed by the same HDC and ASCT protocol, and identical inclusion criteria.7 Updated results from that cohort of patients are shown in Table 3 and Appendix 2 (online). In comparison to the TMZ cohort, patients enrolled in the PCV induction cohort achieved a shorter median OS among all patients (median 49.8 mo, hazard ratio [HR]: 3.38, P = 0.005). This difference was also statistically significant among patients with 1p/19q codeletion (N = 11, median OS not reached, HR 5.68, P = 0.018). The median PFS of the PCV cohort was 14.4 months (HR = 1.79, P = 0.086) among all patients and 39.7 months (HR = 1.54, P = 0.34) among 1p/19q codeleted patients.
Next-Generation Sequencing
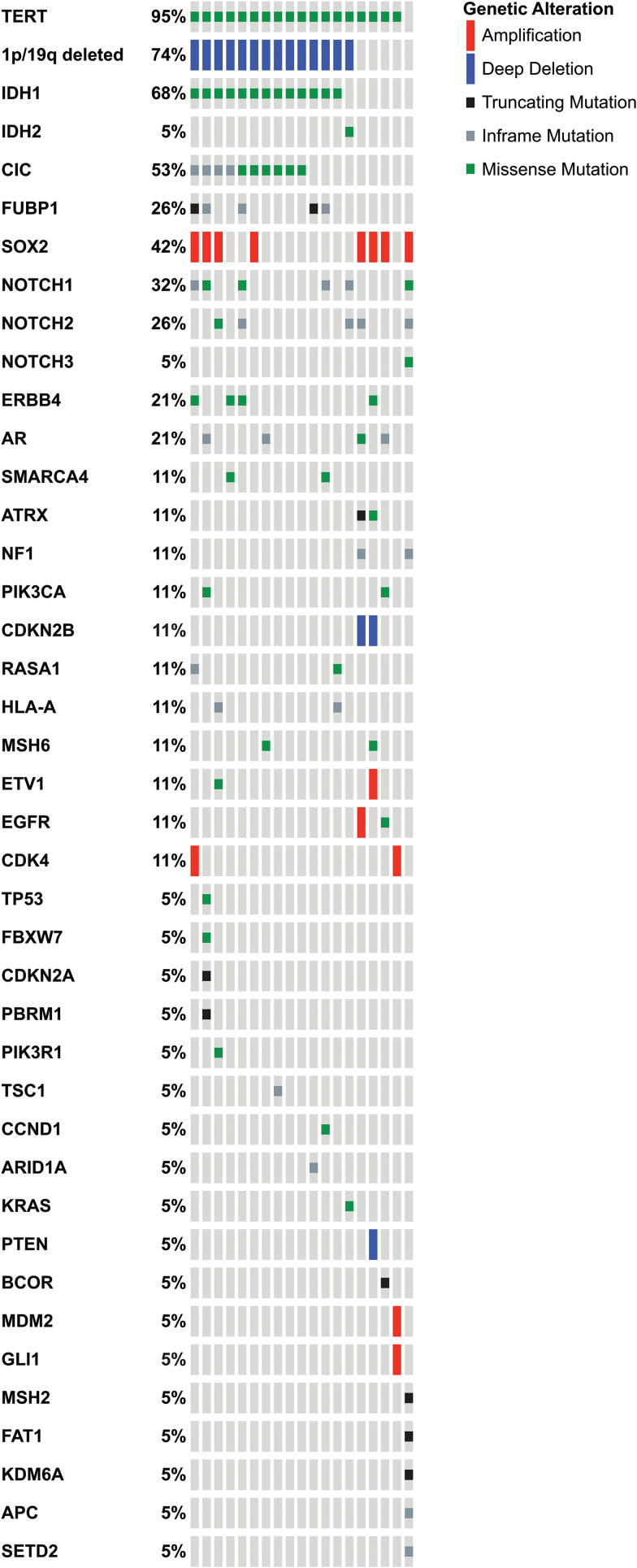
A total of 19 patients had available tissue with adequate DNA quality and quantity for gene sequencing analysis, including 14 patients with confirmed 1p/19q codeletion and 5 with 1p/19q intact. As shown in Fig. 2, and in line with previously reported mutational landscapes in oligodendroglial tumors, TERT promoter mutation was the most frequent abnormality, present in 18/19 patients, followed by IDH1 (13/19), CIC (10/19), Notch1 (6/19), Notch2 (5/19), and FUBP1 (5/19). None of these abnormalities were predictive of PFS or OS. The molecular landscape of patients with 1p/19q codeletion was substantially different from the non-codeleted tumors, which often displayed signatures of de novo glioblastoma-like tumors, including wild-type IDH1 and 2, EGFR amplification and mutation, NF1 mutation, and MDM2 amplification. One patient thought to have 1p/19q codeletion on FISH had a glioblastoma-like signature with PTEN, CDKN2B, CDKN2AP16INK4A, and CDKN2AP14ARF with no evidence of 1p/19q loss or IDH1 or 2 mutation on gene sequencing, suggesting a false-positive 1p/19q codeletion on FISH. That patient progressed on the first MRI and died after 13 months.
Fig. 2.
Distribution of mutations and copy number alterations as determined by next-generation sequencing utilizing a panel of 410 cancer-related genes (1p/19q loss shown determined by FISH).
To provide guidance for future clinical trials, and to help in the differentiation between oncogenic and passenger mutations, we matched results of our gene sequencing with the OncoKB database (available at http://oncokb.org, accessed 6 January 2017). The OncoKB database is an inventory of known somatic mutations and corresponding potential treatments throughout the cancer spectrum, curated by the MSKCC faculty. The OncoKB classifies potentially actionable mutations in levels of evidence, based on existing literature comprising preclinical and clinical evidence. As shown in Table 4, a wide spectrum of potentially oncogenic mutations is represented, although aside from IDH1, the frequency of each mutation is low, depicting a wide molecular heterogeneity within oligodendroglial tumors.
Table 4.
Results of next-generation sequencing showing potentially actionable and other oncogenic mutations in the enrolled population of oligodendrogliomas (N = 19)
| Level of Evidence | Description | Gene | Mutations | N | % |
|---|---|---|---|---|---|
| 1 | FDA-recognized biomarker predictive or response to an FDA-approved drug in this indication | NA | NA | ||
| 2A | Standard of care biomarker predictive of response to an FDA-approved drug in this indication | NA | NA | ||
| 2B | FDA-recognized biomarker predictive of response to an FDA-approved drug in another indication, but not standard of care for this indication | CDK4 | amplification | 2 | 10.53 |
| TSC1 | total | 1 | 5.26 | ||
| V46Wfs | 1 | 5.26 | |||
| 3A | Clinical evidence supports biomarker as being predictive of response to a drug in this indication but neither biomarker nor drug is standard of care | NA | NA | ||
| 3B | Clinical evidence supports biomarker as being predictive of response to a drug in another indication but neither biomarker nor drug is standard of care | IDH1 | total | 13 | 68.42 |
| R132H | 13 | 68.42 | |||
| IDH2 | total | 1 | 5.26 | ||
| R172K | 1 | 5.26 | |||
| KRAS | total | 1 | 5.26 | ||
| Q61H | 1 | 5.26 | |||
| PIK3CA | total | 2 | 10.53 | ||
| E365K | 1 | 5.26 | |||
| H1047R | 1 | 5.26 | |||
| Hotspot | Hotspot mutations with unknown therapeutic implications | EGFR | total | 1 | 5.26 |
| A289V | 1 | 5.26 | |||
| FBXW7 | total | 1 | 5.26 | ||
| R465H | 1 | 5.26 | |||
| PIK3R1 | total | 1 | 5.26 | ||
| G376R | 1 | 5.26 | |||
| SMARCA4 | total | 1 | 5.26 | ||
| G1232S | 1 | 5.26 | |||
| Other potentially oncogenic mutations | Other potentially oncogenic mutations of unknown therapeutic implications | APC | total | 1 | 5.26 |
| V2194Ffs*5 | 1 | 5.26 | |||
| ARID1A | total | 1 | 5.26 | ||
| Y551Lfs*72 | 1 | 5.26 | |||
| ATRX | total | 1 | 5.26 | ||
| C1531 | 1 | 5.26 | |||
| BCOR | total | 1 | 5.26 | ||
| R810 | 1 | 5.26 | |||
| CCND1 | total | 1 | 5.26 | ||
| K114del | 1 | 5.26 | |||
| CDKN2A | total | 1 | 5.26 | ||
| W110 | 1 | 5.26 | |||
| CDKN2B | deletion | 2 | 10.53 | ||
| EGFR | amplification | 1 | 5.26 | ||
| ETV1 | amplification | 1 | 5.26 | ||
| FAT1 | total | 1 | 5.26 | ||
| R937 | 1 | 5.26 | |||
| GLI1 | amplification | 1 | 5.26 | ||
| KDM6A | total | 1 | 5.26 | ||
| Q1037 | 1 | 5.26 | |||
| MDM2 | amplification | 1 | 5.26 | ||
| MSH2 | total | 1 | 5.26 | ||
| R680 | 1 | 5.26 | |||
| NF1 | total | 4 | 21.05 | ||
| F1247Ifs*18 | 1 | 5.26 | |||
| F1455Lfs*9 | 1 | 5.26 | |||
| F2386 | 1 | 5.26 | |||
| K2595Sfs*5 | 1 | 5.26 | |||
| NOTCH1 | total | 5 | 26.32 | ||
| A1701S | 1 | 5.26 | |||
| F357del | 1 | 5.26 | |||
| P1443Afs*36 | 1 | 5.26 | |||
| T633Dfs*12 | 1 | 5.26 | |||
| V324Qfs*6 | 1 | 5.26 | |||
| PBRM1 | total | 1 | 5.26 | ||
| S275 | 1 | 5.26 | |||
| PTEN | deletion | 1 | 5.26 | ||
| RASA1 | total | 1 | 5.26 | ||
| E208Vfs*16 | 1 | 5.26 | |||
| SETD2 | total | 1 | 5.26 | ||
| E282Rfs*9 | 1 | 5.26 | |||
| TP53 | total | 1 | 5.26 | ||
| E258K | 1 | 5.26 |
Discussion
Intensive chemotherapy with TMZ followed by HDC-ASCT achieved favorable disease control and survival with minimal toxicity in a select population of patients with 1p/19q codeleted tumors. Among all transplanted patients, the 2-year PFS was 86% and 5-year PFS was 60%, with no deaths after a median follow-up of 66 months. Importantly, treatment was reasonably well tolerated, with no toxic deaths and no harvest failures.
AO is a uniquely chemosensitive glioma, but an optimal treatment remains to be determined.2,15–18 Two large multicenter clinical trials have demonstrated a survival benefit of tandem PCV chemotherapy and RT compared with RT alone.5,6 The survival benefit of this dual-modality treatment is evident in patients with 1p/19q codeleted tumors, a hallmark of pure AOs. This molecular feature has now been incorporated into the 2016 WHO classification of CNS tumors, as part of an integrated phenotypic/genotypic diagnosis.19 In the present study, 33 of 39 patients had confirmed 1p/19q codeletion and 96% of the transplant-eligible patients had codeleted tumors, which is in keeping with reports that 1p/19q codeletion is important for chemosensitivity. Our results also suggest that chemotherapy alone is not an appropriate strategy for oligodendroglial tumors without 1p/19q codeletion, and support current trends of requiring 1p/19q assessment for eligibility in clinical trials. Our study demonstrates a favorable survival compared with other multidrug chemotherapy regimens for AO. The question remains if transplant confers a specific survival advantage or if non-myeloablative regimens are equally efficacious.
Taking advantage of tissue collection throughout the study, we performed next-generation sequencing in available tissue from enrolled patients. The observed genomic landscape is in line with The Cancer Genome Atlas and other reports,20–24 depicting a clear distinction in molecular signatures between 1p/19q codeleted and 1p/19q intact tumors. The gene signatures observed in 1p/19q intact tumors were suggestive of “de novo” glioblastoma-like signatures,21 with wild-type IDH1 and 2, and mutations in EGFR, NF1, and MDM2. No patient displayed an IDH1 mutant astrocytoma signature, suggesting that the risk in the interpretation of pathology findings in oligodendroglial tumors is to miss a diagnosis of glioblastoma, rather than other IDH1 mutant tumors. Interestingly, one of the patients with 1p/19q codeletion on FISH who died early at 13 months was found to have a glioblastoma-like signature on gene sequencing, with IDH1 and 2 wild type and PTEN loss, indicating false-positive FISH results. This suggests that gene sequencing may constitute a more reliable tool to identify 1p/19q codeleted tumors25 than FISH, and should be considered as a screening tool in future trials, especially for those cases where upfront RT is to be deferred.
As part of the genomic characterization in this trial, we also sought to determine the frequency of potentially actionable mutations for which there are existing targeted agents. In addition to IDH1 and 2 mutations, potentially actionable mutations were found in a wide variety of genes (Table 4), including CDK4 amplification and TSC1 and PIK3CA mutation, although no one leading abnormality was observed in terms of frequency. This highlights a feasibility issue in the development of future treatments for oligodendroglial tumors, with each actionable mutation only found in very rare patients. Enrolling such patients in so-called basket trials, which allow for inclusion of different cancer types sharing the same mutation, may be a more realistic path for investigating new targeted agents in this disease.
This study was modeled after our previous study, in which patients were given 3–4 cycles of PCV chemotherapy followed by ASCT.7 A major controversy in the treatment of oligodendroglial tumors is whether single-agent TMZ can be used instead of PCV, which has been historically the best studied chemotherapy regimen in this disease but is fairly toxic. Although not an objective of our study, we conducted an exploratory analysis and compared results of this study with our previous cohort of patients treated with PCV. The limitations of such comparison are considerable, including small number of patients, differing length of follow-up, and lack of randomization. Regardless, we found no evidence of diminished efficacy, but rather an opposite trend, with patients treated in the TMZ cohort faring better. It must be noted, however, that our cohort received a multidrug myeloablative regimen and these results may not be generalizable to TMZ single agent without transplant. The long-term survival data from the NOA-04 study of chemotherapy versus RT for newly diagnosed anaplastic glioma found longest survival in patients initially treated with PCV chemotherapy alone (N = 17), followed by RT (N = 35), and then shortest survival in patients treated with TMZ (N = 16). While limited by the small number of patients, this raises the question of whether delivering a line of treatment containing a multidrug regimen may be important in this disease.26
Patients with 1p/19q codeletion may survive decades with disease. With long-term survival, late-delayed side effects of treatment become a relevant concern, particularly RT-related neurotoxicity. The sequelae of cranial irradiation often appear months to years after treatment and most commonly manifest as cognitive impairment, and in some cases may include neuroendocrine disorders, cerebral necrosis, and full-blown dementia. In our study, when all 1p/19q patients are considered, the median PFS of 63 months seems comparable to RT alone but inferior to RT combined with PCV (Table 3). This translated into an RT-free survival of 82% at 2 years and 52% at 5 years. With an OS that to date is comparable, if not superior, to RT/PCV studies, our study suggests that salvage RT may be an effective treatment for these patients, and an upfront chemotherapy-alone approach a viable alternative to defer radiation. Other studies of chemotherapy-alone approaches also seem to suggest comparable OS (Table 3). However, preliminary results of a randomized trial of 12 patients treated with TMZ alone versus 24 patients treated with RT with or without TMZ favored the RT arms, leading to discontinuation of the TMZ-alone arm in a newly redesigned study (CODEL, NCT00887146).27 Considering the small number of patients and the fact that results in these 12 patients seem an outlier compared with other prospective studies of chemotherapy-alone approaches (Table 3), such decision may have been questionable.
Our study has some limitations. (1) Although we report on a follow-up that now spans more than 5 years, continuous assessment of survival is still needed to fully characterize later endpoints. In addition to survival, prospective collection of imaging data beyond progression to characterize response and patterns of progression following salvage RT would have been of interest, but this was not part of the original study design. (2) While neurotoxicity has not been reported in progression-free patients, formal neuropsychological evaluation was not performed. (3) The trial did not include evaluation of T-cell repertoire and other correlative studies to elucidate a potential increase in antitumor immunologic response, a question particularly relevant given the disproportionally favorable OS results compared with PFS. (4) Although comparable to or larger than other studies of 1p/19q codeleted tumors (Table 3), the number of patients remains relatively small, and inherent to transplant studies, some patients refused treatment. Because available trials are all small, larger meta-analyses would be helpful to test and generate hypotheses, especially when coupled with genomic data. To this end, raw results of this trial, including both genomic characterization and clinical data, will be made publicly available upon publication of this manuscript at our institutional database portal Cbioportal,28,29 available at http://cbioportal.org/study?id=odg_msk_2017, accessed 6 January 2017.
In summary, we found that TMZ followed by HDC and ASCT can be safely administered to patients with newly diagnosed 1p/19q codeleted AOs, with promising PFS and OS survival. Whether myeloablative chemotherapy with ASCT confers any advantage over other multidrug chemotherapy regimens remains to be seen. Nevertheless, results suggest that further exploration of chemotherapy-only approaches in oligodendrogliomas remains an important avenue of investigation.
Funding
This is an investigator-initiated prospective multicenter phase II trial sponsored by Memorial Sloan Kettering Cancer Center, supported in part by NIH grant P30 CA 008748. Schering-Plough/Merck provided temozolomide and additional partial financial support.
Conflict of interest statement. The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Macdonald DR, Gaspar LE, Cairncross JG. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Ann Neurol. 1990;27(5):573–574. [DOI] [PubMed] [Google Scholar]
- 2. Cairncross JG, Macdonald DR. Chemotherapy for oligodendroglioma. Progress report. Arch Neurol. 1991;48(2):225–227. [DOI] [PubMed] [Google Scholar]
- 3. Abrey LE, Childs BH, Paleologos N et al. . High-dose chemotherapy with stem cell rescue as initial therapy for anaplastic oligodendroglioma: long-term follow-up. Neuro Oncol. 2006;8(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker MD, Alexander E Jr, Hunt WE et al. . Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross G, Wang M, Shaw E et al. . Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Bent MJ, Brandes AA, Taphoorn MJ et al. . Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 7. Mohile NA, Forsyth P, Stewart D et al. . A phase II study of intensified chemotherapy alone as initial treatment for newly diagnosed anaplastic oligodendroglioma: an interim analysis. J Neurooncol. 2008;89(2):187–193. [DOI] [PubMed] [Google Scholar]
- 8. Abrey LE, Childs BH, Paleologos N et al. . High-dose chemotherapy with stem cell rescue as initial therapy for anaplastic oligodendroglioma. J Neurooncol. 2003;65(2):127–134. [DOI] [PubMed] [Google Scholar]
- 9. Dummer W, Niethammer AG, Baccala R et al. . T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sampson JH, Aldape KD, Archer GE et al. . Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gan HK, Rosenthal MA, Dowling A et al. . A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol. 2010;12(5):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taliansky-Aronov A, Bokstein F, Lavon I, Siegal T. Temozolomide treatment for newly diagnosed anaplastic oligodendrogliomas: a clinical efficacy trial. J Neurooncol. 2006;79(2):153–157. [DOI] [PubMed] [Google Scholar]
- 13. Mikkelsen T, Doyle T, Anderson J et al. . Temozolomide single-agent chemotherapy for newly diagnosed anaplastic oligodendroglioma. J Neurooncol. 2009;92(1):57–63. [DOI] [PubMed] [Google Scholar]
- 14. Cheng DT, Mitchell TN, Zehir A et al. . Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairncross G, Macdonald D, Ludwin S et al. . Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12(10):2013–2021. [DOI] [PubMed] [Google Scholar]
- 16. Chamberlain MC, Kormanik PA. Salvage chemotherapy with paclitaxel for recurrent oligodendrogliomas. J Clin Oncol. 1997;15(12):3427–3432. [DOI] [PubMed] [Google Scholar]
- 17. Friedman HS, Lovell S, Rasheed K, Friedman AH. Treatment of adults with progressive oligodendroglioma with carboplatin (CBDCA): preliminary results. Writing Committee for The Brain Tumor Center at Duke. Med Pediatr Oncol. 1998;31(1):16–18. [DOI] [PubMed] [Google Scholar]
- 18. Wick W, Hartmann C, Engel C et al. . NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 19. Louis DN, Perry A, Reifenberger G et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 20. Brat DJ, Verhaak RG, Aldape KD et al. ; The Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brennan CW, Verhaak RG, McKenna A et al. ; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckel-Passow JE, Lachance DH, Molinaro AM et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dubbink HJ, Atmodimedjo PN, Kros JM et al. . Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016;18(3):388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubbink HJ, Atmodimedjo PN, van Marion R et al. . Diagnostic detection of allelic losses and imbalances by next-generation sequencing: 1p/19q co-deletion analysis of gliomas. J Mol Diagn. 2016;18(5):775–786. [DOI] [PubMed] [Google Scholar]
- 26. Wick W, Roth P, Hartmann C et al. ; Neurooncology Working Group (NOA) of the German Cancer Society. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaeckle K, Vogelbaum M, Ballman K et al. . CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): Phase III Randomized Study of RT vs. RT+TMZ vs. TMZ for Newly Diagnosed 1p/19q-Codeleted Anaplastic Oligodendroglial Tumors. Analysis of Patients Treated on the Original Protocol Design (PL02.005). Neurology. 2016;88(16):Suppl PL02.005. [Google Scholar]
- 28. Cerami E, Gao J, Dogrusoz U et al. . The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao J, Aksoy BA, Dogrusoz U et al. . Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahluwalia MS, Xie H, Dahiya S et al. . Efficacy and patient-reported outcomes with dose-intense temozolomide in patients with newly diagnosed pure and mixed anaplastic oligodendroglioma: a phase II multicenter study. J Neurooncol. 2015;122(1):111–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.