Abstract
AIM
To compare the optic disc blood flow of non-arteritic ischemic optic neuropathy (NAION) eyes with normal eyes.
METHODS
The optic disc blood flow densities of diagnosed non-acute phase NAION eyes (21 eyes, 14 individuals) and normal eyes (19 eyes, 12 individuals) were detected via Optovue optical coherence tomography angiography (OCTA). The optic disc blood flow was measured via Image J software. Correlations between optic disc perfusion and visual function variables were assessed by linear regression analysis.
RESULTS
The average percentage of the optic disc non-perfusion areas in the non-acute phase NAION patients (17.84%±6.18%) was increased, when compared to the normal control eyes (8.61%±1.65%), and the difference was statistically significant (P<0.01). Moreover, there was a proportional correlation between the visual field mean defect (MD) and the optic disc non-perfusion area percentage, and the relationship was statistically significant (t=3.65, P<0.01, R2=0.4118). In addition, the critical correlation between the best corrected visual acuity (BCVA) and the optic disc non-perfusion area percentage was statistically significant (t=4.32, P<0.01, R2=0.4957).
CONCLUSION
The optic disc non-perfusion area percentages detected via OCTA in NAION eyes were significantly increased when compared with the normal eyes. Both the BCVA and MD were correlated with the optic disc flow detected, revealing that OCTA may be valuable in the diagnosis and estimation of NAION.
Keywords: non-arteritic anterior ischemic optic neuropathy, optical coherence tomography angiography, visual field, visual acuity
INTRODUCTION
Anterior ischemic optic neuropathy (AION) is the result of disease in the small vessels supplying the anterior portion of the optic nerve[1]–[2]. AION can be divided into arteritic ischemic optic neuropathy and non-arteritic ischemic optic neuropathy (NAION). NAION makes up approximately 85% of all cases of AION, and is a common cause of sudden vision loss due to optic nerve dysfunction in adults over 55 years of age. Generally, it causes sudden, painless, unilateral vision loss, leading to swelling of the optic nerve head[3]. To date, there is no consistently effective treatment, either to improve vision in an eye affected by NAION or to prevent visual loss from NAION in the fellow eye[1].
Optical coherence tomography (OCT) can measure optic disc oedema, ganglion cell layer (GCL) thinning and retinal nerve fibre layer (RNFL) loss in different NAION eye stages. The macula and GCL are thinner in NAION eyes, and show stronger correlation with the visual field[4]–[7]. However, the specific correlation between the optic disc blood flow perfusion and the visual function remains unknown. Although optic disc perfusion changes can be observed in some NAION eyes via fundus fluorescence angiography (FFA), its application value is limited because it is invasive and the result is not digital. In recent years, a newly developed optical coherence tomography angiography (OCTA) technology, which is non-invasive, reproducible and sensitive, has demonstrated an ability to quantify the disc blood flow both rapidly and accurately[8]–[9]. This has prompted us to examine the role of the optic disc blood flow perfusion in the pathogenesis of NAION. In this study, we retrospectively reviewed the clinical data of non-acute phase NAION patients, focusing on the visual field and visual acuity, to explore the features of OCTA and its correlation with visual function.
SUBJECTS AND METHODS
Participants
Fourteen patients diagnosed with non-acute phase NAION in the Outpatient Clinic of our hospital from May 2015 to November 2016 were included in this research. The diagnostic criteria of NAION consisted of the following as previous described[10]–[11]: 1) a history of sudden visual loss and absence of other ocular and neurologic diseases that might influence or explain the patient's visual symptoms; 2) optic disc-related visual field defects in the eye; 3) limited or diffuse papillary oedema and constant linear peripheral haemorrhage; 4) a relative afferent papillary defect (RAPD) (+) and/or aberrant visual evoked potential (VEP); 5) ruled out other optic nerve diseases. All of the patients involved underwent medical optometry, an intraocular pressure measurement, slit-lamp microscopic and fundus examinations, fundus photography, and visual field and VEP examinations. FFA was performed when a patient demonstrated a good general physical condition. This research followed the tenets of the Declaration of Helsinki (as revised in Brazil in 2013) and was approved by the First Affiliated Hospital of Soochow University. Written consent was obtained from all of the patients before participating in this study.
Standards of Inclusion and Exclusion
The inclusion criteria were non-acute phase NAION patients, 18-80 years old, with 3 or more months since the onset of disease. The exclusion criteria were the following: 1) non-NAION or acute phase NAION patients; 2) non-acute phase NAION patients with other eye diseases; 3) poor cooperation with fixation or the OCTA examination; 4) abnormal morphological optic discs leading to inaccurate OCTA data; 5) a refractive error greater than 5.0 diopters (D) of spherical equivalent or 3.0 D of astigmatism. The control group consisted of healthy individuals without systemic or eye diseases, with normal optic discs.
Observation Index
The best corrected visual acuity (BCVA) was tested at each visit using the standard Snellen decimal-acuity chart. The visual fields were tested using Octopus 101 perimeter (Haag-Streit, Koeuiz, Switzerland) and the visual field mean defect (MD) was obtained.
OCTA was performed using the RTVue XR Avanti with Angio Vue (Optovue, Fremont, California, USA). The optic disc flow index was set as the optic disc non-perfusion area percentage detected via OCTA. Retinal scanning using a wide-field en-face swept-source OCTA contributed to the optic disc-centred graphical reports (4.50×4.50 mm2). The graphs were analysed using Image J software. We defined the optic disc areas as A (except any major branch retinal blood vessels), and the optic disc non-perfusion areas as B by setting different threshold values. Subsequently, we defined the optic disc non-perfusion area percentages as C, and calculated C using the following formula: C=B/A×100%. We adopted the same threshold value, and scoped the optic disc margin according to the fundus photograph and previous reports (termination of the retinal pigment epithelium/Bruch's membrane complex)[12]–[14], as far as possible, to reduce human error.
Statistical Analysis
The results were expressed as the mean±standard deviation (SD) using SPSS 22.0 software (SPSS, Los Angeles, CA, USA). A linear regression analysis was performed between the optic disc non-perfusion area percentages and MD, as well as the BCVA. One-way ANOVA was used to compare the measurements between the eyes with NAION and the normal controls. A value of P<0.05 was considered to be statistically significant.
RESULTS
Participant Characteristics
For this study, 14 patients (8 females, 6 males) with non-acute phase NAION were included. The analysis showed that the age of the subjects varied between 38 and 76 years old, with an average age of 59.76±8.77 years old. The onset time varied from 3 to 120mo, with an average time of 12.90±24.97mo. The BCVA varied from 0.04 to 1.2, with an average acuity of 0.66±0.33.
For the control group, we collected information on 19 normal eyes of 12 healthy participants without any eye nor systemic diseases (8 females, 4 males). The mean age of the control group was 31.58±10.66 years old, and none of their BCVAs were lower than 1.0.
Optic Disc Blood Flow Index via Optical Coherence Tomography Angiography
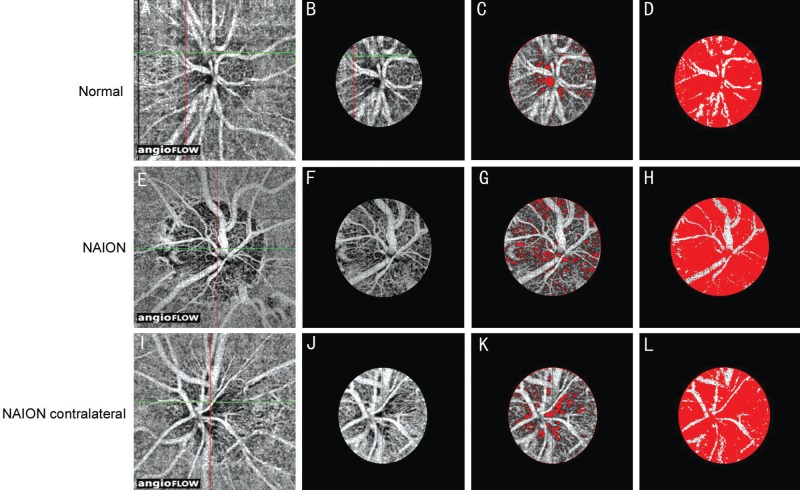
The vascular scanning model of the Optovue OCTA revealed precise perfusion in four disparate layers, including the nerve head layer, vitreous layer, radial peripapillary capillaries and choroid. In this study, the optic nerve head layer was chosen to detect the optic disc blood flow (Figure 1A, 1E and 1I).
Figure 1. Optic disc blood flow index via optical coherence tomography angiography.
A, E, I: Optic disc-centred 4.50×4.50 mm2 OCT angiograms; B, F, J: Selected optic disc OCT angiograms; C, G, K: Optic disc non-perfusion areas; D, H, L: Optic disc areas (except major branch retinal blood vessels). A-D: In the eyes of a normal subject; E-H: NAION eye; I-L: Contralateral eye of a NAION patient. The non-perfusion areas are marked in red (C, D, G, H, K, L).
First, we selected the optic disc margin threshold (Figure 1B, 1F and 1J), showing the optic disc non-perfusion areas (Figure 1C, 1G and 1K), and the optic disc region, with the exception of the major branch retinal blood vessels (Figure 1D, 1H and 1L). Figure 1 shows the top, middle and bottom panels as a normal eye, NAION eye and contralateral NAION eye, respectively.
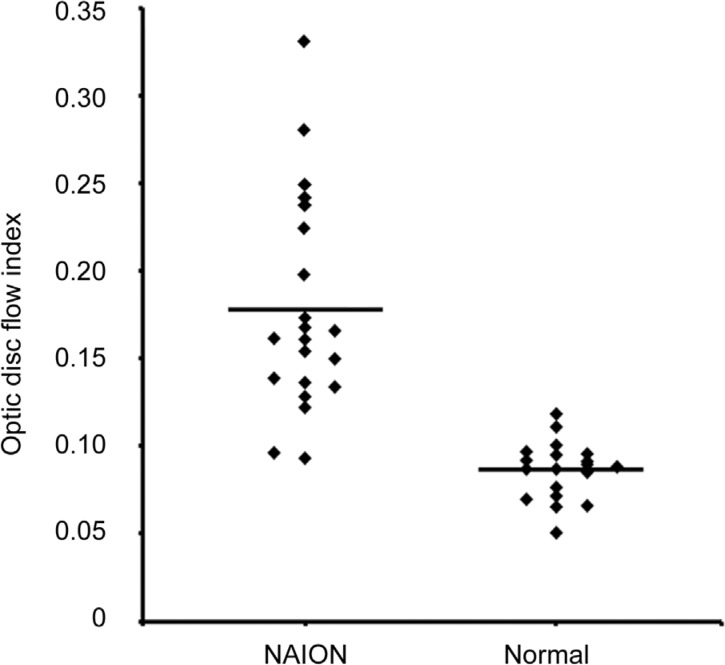
Regardless of bilaterality, whether or not the central vision was normal, the average optic disc non-perfusion area percentage in the non-acute phase NAION eyes (Figure 2) was 17.84%±6.18%. It was 8.61%±1.65% in the normal eyes of the control group. The statistically significant difference between the two groups (P<0.01) suggests an obvious increase in the optic disc non-perfusion areas of the non-acute phase NAION eyes when compared to the control group. Furthermore, to avoid type I error[15]–[16], ANOVA (randomly choose one eye in the 7 patients with bilateral NAION and 7 health control data with bilateral eyes) was conducted, which further confirmed the difference.
Figure 2. Optic disc non-perfusion measurements.
The optic disc non-perfusion area percentage was significantly higher in the NAION eyes than in the normal eyes (P<0.01).
Visual Field Alterations and Optic Disc Non-perfusion Area Percentages in Non-acute Phase Eyes
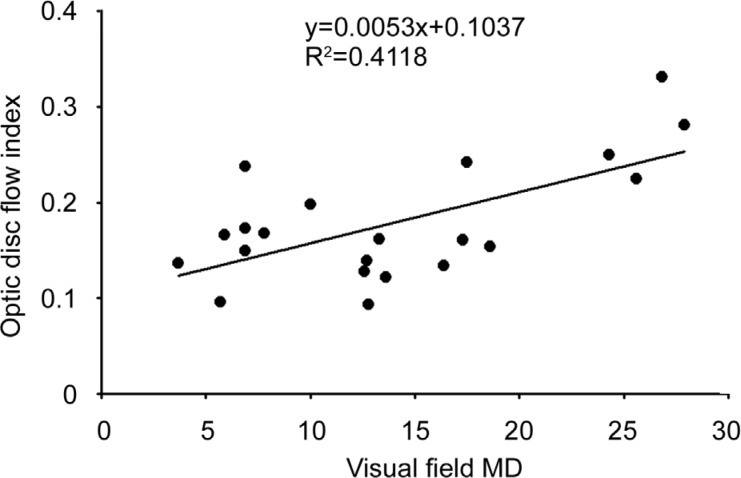
Following the above observation, we sought to explore the relationship between the blood flow in the optic disc and the visual damage. We found that the larger the optic disc non-perfusion area percentage, the greater the visual field MD of the NAION eye. A further correlation analysis revealed a proportional relationship (t=3.65, P<0.01, R2=0.4118) between these two factors (Figure 3).
Figure 3. Correlation between the optic disc non-perfusion area and the visual field in the NAION eyes.
The visual field MD and optic disc non-perfusion area percentages in the NAION eyes are shown.
Visual Acuity and Optic Disc Non-perfusion Area Percentages in Non-acute Phase Eyes
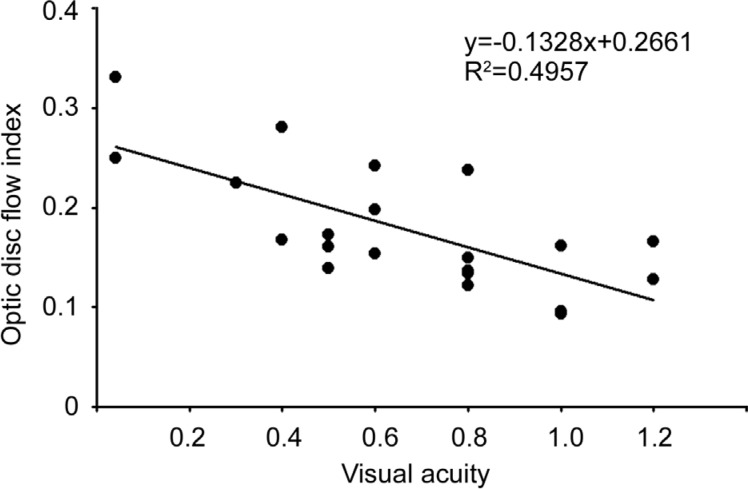
Having shown the critical role of the optic disc blood flow perfusion in visual field alterations, we suspected that the optic disc blood flow perfusion may be also be involved in the visual acuity. Therefore, we analysed the relationship between the optic disc non-perfusion area percentages and the BCVAs of our non-acute phase NAION eyes. As expected, the results indicated an obvious significant correlation (t=4.32, P<0.01, R2=0.4957) between these two factors (Figure 4).
Figure 4. Correlation between the optic disc non-perfusion area and visual acuity in the NAION eyes.
The BCVAs and optic disc non-perfusion area percentages in the NAION eyes are shown.
DISCUSSION
We have shown OCTA can non-invasively detect and estimate the optic disc blood flow of non-acute phase NAION eyes. We revealed that both the visual acuity and visual field are correlated with the optic disc blood flow perfusion, as detected via OCTA. Since the visual field is representative of peripheral vision acuity, these two critical relationships suggest that the vision function of non-acute phase NAION hinges on, at least partly, the degree of optic disc blood flow perfusion. Therefore, more attention should be focused on the role of optic disc perfusion alterations in the pathogenesis of non-acute phase NAION, while considering that NAION can cause irreversible visual damage.
Clinically, NAION is diagnosed by examining the fundus, visual field, FFA and VEP; however, the disadvantages of these methods in monitoring the progression and estimating the prognosis of NAION remain. Previous reports have indicated that the OCT can measure optic disc oedema, GCL thinning and RNFL loss. The RNFL and GCL thickness show stronger correlations with the visual field in NAION eyes[4]–[7]. Using scanning laser polarimetry, a few authors have reported that in acute phase NAION eyes with optic disc swelling, scanning laser polarimetry may be better than OCT for predicting the visual field loss[17]–[18]. OCTA is a novel technology developed to scan blood vessels, and it has been applied to determine choroidal neovascularization in patients with age-related macular degeneration[19]–[20]. It is widely acknowledged that the advantages of OCTA consist of its non-invasiveness, datamation and repeatability. Moreover, with its tracking technology to overcome eye movement, OCTA can offer high resolution retinal and choroidal graphs[9]. Accumulating evidence suggests that the optic disc perfusion detected via OCTA can be correlated to the GCL and RNFL thickness and visual field loss in glaucoma patients[21]–[22]. Essentially, the main vascular pathology of NAION is focused on ischemia of the anterior lamina cribrosa around the optic disc and metarterioles of the posterior ciliary vessels, leading to a local infarct of the optic disc, favouring the progression of optic dysneuria. Including these vessels makes OCTA ideal for predicting the optic disc blood flow and supply in NAION eyes.
This research has provided evidence that the optic disc blood flow of non-acute phase NAION was significantly decreased, when compared to a normal control group, which suggests the poor blood flow recovery of the optic disc, coinciding with the poor visual function prognosis of NAION. In addition, OCTA revealed a dramatic decline in the optic disc blood flow in a portion of NAION eyes that did not show any alterations in the visual acuity or VEP. Considering that the subjectivity of a visual field examination is inevitably inferior to the objectivity of OCTA, we suggest that OCTA could be utilized as a potential diagnosis method for NAION.
The main limitation of this research was that it was a single-centre, small-sample size, retrospective study; these results should be confirmed by multi-centre, large-sample, prospective studies. In addition, the potential involvement of age in the optic disc blood flow requires further exploration. There have been conflicting reports of the effects of age on the optic nerve head blood flow, possibly due to the different number of subjects and detection techniques used in those studies[23]–[24]. Although the age range of our research subjects varied widely, we acquired a preliminary understanding of the scope of normal optic disc non-perfusion area percentages. Moreover, in view of the fact that the optic discs of patients with acute phase NAION exhibit constant oedema, we excluded these subjects to maintain the accuracy of our detection. In addition, abnormal morphologies of the optic disc were excluded accordingly, due to their influence on OCTA.
In conclusion, here we have shown that both the visual acuity and visual field are significantly correlated with the optic disc non-perfusion area percentages of non-acute phase NAION eyes. This indicates, at least in part, the prominent role of OCTA in estimating the progression of NAION. In addition, our results suggest the possibility of future use of OCTA alone, or in combination with automated perimetry, for the early diagnosis of NAION.
Acknowledgments
Foundations: Supported in part by Jiangsu Province's Outstanding Medical Academic Leader Program (No.CXTDA2017039); the Soochow Scholar Project of Soochow University.
Conflicts of Interest: Ling JW, None; Yin X, None; Lu QY, None; Chen YY, None; Lu PR, None.
REFERENCES
- 1.Bernstein SL, Johnson MA, Miller NR. Nonarteritic anterior ischemic optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res. 2011;30(3):167–187. doi: 10.1016/j.preteyeres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428–2436. doi: 10.1056/NEJMra1413352. [DOI] [PubMed] [Google Scholar]
- 3.Mathews MK, Guo Y, Langenberg P, Bernstein SL. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION) Br J Ophthalmol. 2015;99(1):133–137. doi: 10.1136/bjophthalmol-2014-305969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebolleda G, de Dompablo E, Muñoz-Negrete FJ. Ganglion cell layer analysis unmasks axonal loss in anterior optic neuritis. J Neuroophthalmol. 2015;35(2):165–167. doi: 10.1097/WNO.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 5.Kernstock C, Beisse F, Wiethoff S, Mast A, Krapp E, Grund R, Dietzsch J, Lagrèze W, Fischer D, Schiefer U. Assessment of functional and morphometric endpoints in patients with non-arteritic anterior ischemic optic neuropathy (NAION) Graefes Arch Clin Exp Ophthalmol. 2014;252(3):515–521. doi: 10.1007/s00417-014-2572-z. [DOI] [PubMed] [Google Scholar]
- 6.Gonul S, Koktekir BE, Bakbak B, Gedik S. Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013;97(8):1045–1050. doi: 10.1136/bjophthalmol-2013-303438. [DOI] [PubMed] [Google Scholar]
- 7.Papchenko T, Grainger BT, Savino PJ, Gamble GD, Danesh-Meyer HV. Macular thickness predictive of visual field sensitivity in ischemic optic neuropathy. Acta Ophthalmol. 2012;90(6):e463–469. doi: 10.1111/j.1755-3768.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- 8.Jia Y, Morrison JC, Tokayer J, Tan Q, Lombardi L, Baumann B, Lu CD, Choi W, Fujimoto JG, Huang D. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3(12):3127–3137. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG, Huang D. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–1332. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: their pattern and prevalence at initial examination. Arch Ophthalmol. 2005;123(11):1554–1562. doi: 10.1001/archopht.123.11.1554. [DOI] [PubMed] [Google Scholar]
- 11.Neuro-ophthalmology Group, Ophthalmology Branch of Chinese Medical Association Expert consensus for the diagnosis and management of non-arteritic ischemic optic neuropathy. Chin J Ophthalmol. 2015;51(5):323–326. [Google Scholar]
- 12.Strouthidis NG, Yang H, Reynaud JF, Grimm JL, Gardiner SK, Fortune B, Burgoyne CF. Comparison of clinical and spectral domain optical coherence tomography optic disc margin anatomy. Invest Ophthalmol Vis Sci. 2009;50(10):4709–4718. doi: 10.1167/iovs.09-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strouthidis NG, Yang H, Fortune B, Downs JC, Burgoyne CF. Detection of optic nerve head neural canal opening within histomorphometric and spectral domain optical coherence tomography data sets. Invest Ophthalmol Vis Sci. 2009;50(1):214–223. doi: 10.1167/iovs.08-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z, Abràmoff MD, Kwon YH, Lee K, Garvin MK. Automated segmentation of neural canal opening and optic cup in 3D spectral optical coherence tomography volumes of the optic nerve head. Invest Ophthalmol Vis Sci. 2010;51(11):5708–5717. doi: 10.1167/iovs.09-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33(1):7–14. doi: 10.1111/opo.12009. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82(8):971–973. doi: 10.1136/bjo.82.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupersmith MJ, Anderson S, Durbin M, Kardon R. Scanning laser polarimetry, but not optical coherence tomography predicts permanent visual field loss in acute nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2013;54(8):5514–5519. doi: 10.1167/iovs.13-12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata M, Miyamoto K, Oishi A, Kimura Y, Nakagawa S, Horii T, Yoshimura N. Measurement of retinal nerve fiber layer thickness in eyes with optic disc swelling by using scanning laser polarimetry and optical coherence tomography. Clin Ophthalmol. 2014;8:105–111. doi: 10.2147/OPTH.S46769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung L, Lima VC, Garcia P, Landa G, Rosen RB. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology. 2009;116(6):1158–1167. doi: 10.1016/j.ophtha.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, Shi G, Sun X. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1557–1564. doi: 10.1007/s00417-015-3095-y. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, Davis E, Morrison JC, Huang D. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045–1052. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groh MJ, Michelson G, Langhans MJ, Harazny J. Influence of age on retinal and optic nerve head blood circulation. Ophthalmology. 1996;103(3):529–534. doi: 10.1016/s0161-6420(96)30662-3. [DOI] [PubMed] [Google Scholar]
- 24.Boehm AG, Koeller AU, Pillunat LE. The effect of age on optic nerve head blood flow. Invest Ophthalmol Vis Sci. 2005;46(4):1291–1295. doi: 10.1167/iovs.04-0987. [DOI] [PubMed] [Google Scholar]