Abstract
AIM
To compare the effectiveness and safety between modified cross-linking (MC) and standard cross-linking (SC) in mild or moderate progressive keratoconus.
METHODS
Eligible studies were retrieved from four electronic databases, including CENTRAL, Clinical Trials gov, PupMed and OVID MEDLINE. We set post-surgical maximum K value (Kmax) as the primary outcome. In addition, uncorrected and corrected distant visual acuity (UDVA and UDVA), spherical equivalent (SE), endothelial cell density (ECD), central cornea thickness (CCT) and depth of demarcation line (DDL) were Meta-analyzed as secondary outcomes. Mean differences for these outcomes were pooled through either a random-effect model or fixed-effect model according to data heterogeneity.
RESULTS
Twenty-four comparative studies either on accelerated cross-linking (AC) compared with SC or on trans-epithelial cross-linking (TC) compared with SC were included and pooled for analysis. The results indicated that MC was significantly inferior to SC at delaying Kmax deterioration [AC vs SC 0.49 (95% CI: 0.04-0.94, I2=75%, P=0.03); TC vs SC 1.15 (95% CI: 0.54-1.75, I2=50%, P=0.0002)]. SE decreased significantly for SC when compared to AC [0.62 (95% CI: 0.38-0.86, I2=22%, P<0.00001)]. DDL of SC was more significantly deeper than that of TC [-133.49 (95% CI: -145.94 to -121.04, I2=33%, P<0.00001)]. Other outcomes demonstrated comparable results between MC and SC.
CONCLUSION
SC is more favorable at halting the progression of keratoconus, but visual acuity improvement showed comparable results between MCs and SC.
Keywords: progressive keratoconus, cross-linking, standard cross-linking, accelerated cross-linking, trans-epithelial cross-linking, Meta-analysis
INTRODUCTION
Keratoconus is the most common corneal degeneration disease, characterized by cornea conical protrusion, progressive local stroma thinning, increased cornea curvature and irregular astigmatism[1]. The incidence rate of keratoconus is as high as 54.5 per 100 000, which means one person would suffer the disorder within a general population of 2000[2]. Spectacles and contact lens, especially rigid gas permeable lens (RGP), are routine ways to treat mild or moderate keratoconus[3]. However, ocular infection, cornea pannus and other complications from improper wearing and poor hygiene habits are not rare[4]. In addition, some studies have suggested that RGP could not halt progressive keratoconus effectively in the long run[5]–[6]. Thus, it is essential to exploit new ways to stop the progression of keratoconus more effectively and more safely.
In 2003, Wollensak et al[7] first reported their practice of using cross-linking (CXL) to halt progressive keratoconus effectively, and the protocol they used was established as standard cross-linking (SC)-cornea epithelium stripping, riboflavin instillment and 370 nm ultraviolet A (UVA) radiation with an intensity of 3 mW/cm2 for 30min. Although more and more clinical trials have attested to the effectiveness and safety of SC[8]–[11], complications caused by epithelium stripping and long exposure to ultraviolet radiation, such as unbearable postoperative ocular pain, sub-epithelial haze, sterile infiltration and infectious keratitis, could not be avoided completely[12]. Given that, several modifications have been made to SC to avoid these complications[13], including keeping the corneal epithelium in situ (trans-epithelial CXL, TC)[14] and using radiation of higher intensity and shorter duration (accelerated CXL, AC)[15].
Although these modified cross-linkings (MCs) are superior to SC at reducing associated complications, it is still controversial whether the ability of MCs to stop progression of keratoconus is equivalent to that of SC. Al Fayez et al[16] reported that Kmax decreased 2.4 D in the SC group while it increased 1.1 D in the TC group postoperatively, which showed more effectiveness for SC in halting progressive keratoconus (P<0.0001). However, Magli et al[17] found equivalent effects between TC and SC since there was no significant difference in terms of Kmax or mean K (P>0.05). This controversial situation is also observed in some studies regarding comparison between AC and SC. Ng et al[18] reported that significant reductions for Kmax and mean K were found in the SC group when compared to AC group (P=0.001 and 0.015, respectively). In contrast, Hashemi et al[19] found that the mean decrease in neither Kmax (P=0.865) nor mean K (P=0.974) was significantly different between the AC group and SC group. For this reason, it is difficult for clinical practitioners to judge which CXL protocol is more excellent at halting progressive keratoconus and which CXL protocol should be carried out in their own clinical settings, especially when MCs have obvious benefits for certain keratoconic patients. To answer this question, it is essential to conduct a systematic review and Meta-analysis on the basis of current comparative clinical trials to compare the effectiveness and visual improvement comprehensively between SC and MCs in the treatment of progressive keratoconus.
MATERIALS AND METHODS
Search Strategy
We utilized four main electronic databases to retrieve clinical trials on comparison between SC and MCs, including CENTRAL, Clinical Trials gov, PUBMED, and OVID MEDLINE. As the earliest CXL clinical practice was reported in 2003, our searching data ranged from Jan 2003 to Aug 2016, and the language was restricted to English only. To expand the search, alternative text words used for standard CXL, accelerated CXL and trans-epithelial CXL were “conventional, epithelium-off, epithelium-without CXL”, “high-tense, high-fluence CXL” and “epithelium-on, epithelium-with CXL” respectively. Meanwhile, Boolean logic operators, wildcard and position characters were employed to combine the text words to obtain more precise outcomes. In addition, we also scanned the reference lists of included citations to identify any additional reports. However, we did not search any journals or conference proceedings manually, so there was no “gray literature” in this review.
Studies, Participants and Interventions
Considering that MCs are relatively new techniques and only a few comparative studies could be harvested, studies with respect to comparison between MCs and SC, either retrospective case series (RCS) or prospective case series (PCS) or randomized controlled trials (RCT), were all included.
Patients with mild or moderate progressive keratoconus, regardless of gender, age or ethnic group, regardless of how long the disease had progressed, and regardless of when the surgery was carried out, were all included. Progressive keratoconus was defined as continuous increases in K value and astigmatism or a decrease in cornea stroma thickness, regardless of specific definition criteria of each study. TC was defined as corneal epithelium in situ with or without methods used to change epithelial barrier permeability; AC was defined as intensity greater than 3 mW/cm2 and exposure duration less than 30min no matter the specific parameters used in each study.
Outcomes
Since the primary aim of treating keratoconus with CXLs is to halt the progression of the disorder, we chose Kmax at terminal follow-up point as our primary outcome because it is the most sensitive and significant parameter for demonstrating progression of keratoconus. Secondary outcomes included not only visual functional parameters but also histological and morphological indices, including uncorrected distant visual acuity (UDVA), corrected distant visual acuity (CDVA), spherical equivalent (SE), depth of demarcation line (DDL), central cornea thickness (CCT), endothelium cell density (ECD) and adverse events.
Selection of Studies
Two reviewers selected the literatures independently by the same method. Primary selection was conducted through browsing titles and abstracts so that obviously unrelated studies could be excluded; then, the full copies of the remaining studies were obtained to determine whether inclusion or not. At last, the two reviewers compared their reviewing results and solved disagreements with discussion.
Assessment of Risk of Bias in Included Studies
The two independent reviewers assessed bias of the included studies by referring to a validated checklist consisted of 14 questions (http://links.lww.com/ICO/A265)[20]. This checklist is suitable for evaluating both RCT and non-RCT, as the 14 questions cover every element of a clinical study. According to the checklist, we defined “long enough follow-up” as 12mo, defined “all important outcomes considered” as primary and main secondary outcomes included in the study, and defined “representative sample” as patients with mild or moderate progressive keratoconus. Three ranks marked “yes”, “no” and “unclear” were used to score each question of the checklist and as tudy with 8-9 “yes” answers could be deemed as high qualification[21]. Results from the two independent reviewers were compared, and discrepancies were resolved through discussion or consultation with a third reviewer.
Data Extraction and Management
Study characteristics, such as study design, participant demographics, definition of progressive keratoconus, details of intervention (e.g. riboflavin ingredientsand frequency of riboflavin instillment, wave length of UVA, intensity and radiation duration), clinical outcomes and adverse events, were extracted by the two independent reviewers. Disagreements between the two reviewers were resolved through discussion until consensus was reached.
Measures of Treatment Effect and Statistical Analysis
Review Manager 5.3 (www.ims.cochrane.org/revman) was used for data entry and Meta-analysis. Since Kmax, visual acuity (VA; logMAR), SE, CCT, DDL and ECD were all continuous data, mean difference (MD) and its 95% confidence interval (CI) were utilized as the measure of treatment effect. To reduce heterogeneity generated from variations of baseline and increase comparability among the included studies, the difference value (terminal value minus baseline) and its standard deviation (SD) of each outcome was calculated for comparison. Moreover, we addressed statistical heterogeneity systematically through three different methods: assessing forest plot overlap, calculating Chi-square and I2. If heterogeneity proved significantly by Chi-square or I2 (either P<0.1 or I2>50%), a randomized effects pattern was used for pooling the data; otherwise fixed effects was used. A P value of 0.05 was used as the threshold for statistical significance.
RESULTS
Study Selection
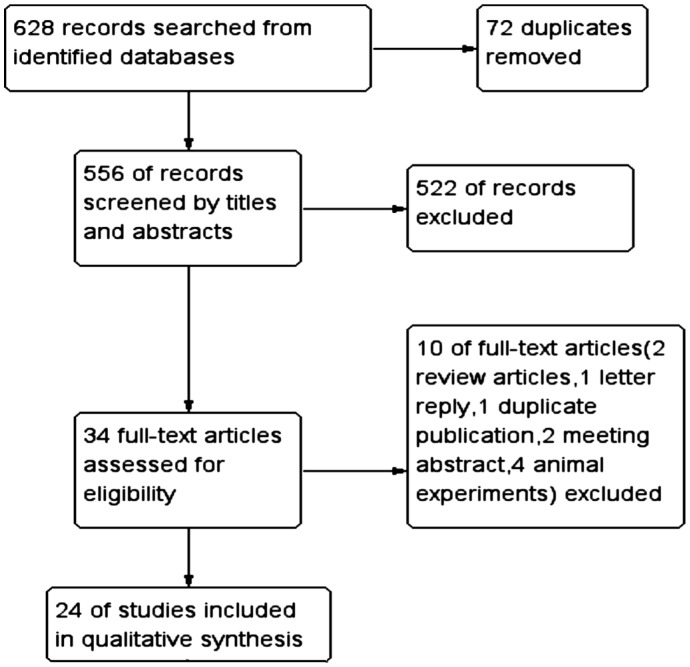
Figure 1 shows the procedure for selecting citations. A total of 628 records were retrieved by searching the electronic databases and by indexing references of related literature. There were 72 duplications and 522 obviously unrelated records, which were recognized by titles and abstracts easily. Then, we excluded 10 citations by browsing full texts, and 24 eligible studies were included finally.
Figure 1. Flow diagram for selecting citations.
Characteristics of Included Studies
Characteristics of all the 24 eligible studies are shown in Tables 1 and 2. Among these studies, only one study (4%)[22] was with respect to comparison of the three CXLs,13 studies (54%)[18],[23]–[34] were comparing between AC and SC, 10 studies (42%)[16]–[17],[35]–[42] were on TC versus SC, and as for study design, 11 studies (46%)[16],[26]–[28],[32]–[33],[35],[39]–[42] were RCT, 8 studies (33%)[22]–[24],[29]–[30],[34],[37]–[38] were PCS and 5 studies (21%)[17]–[18],[25],[31],[36] were RCS. The sample size varied widely among the studies, the largest sample size enrolled 153 patients (153 eyes)[27], the smallest one just enrolled 13 patients (13 eyes)[35], and the sample sizes of most were 30-70 eyes.
Table 1. Characteristics of included studies (Accelerated CXL vs Standard CXL).
| Author | Region | Study design | Group | Eye/patient (n) | Age (a) | Gender (M/F) | Baseline balance (Y/N) | Follow-up (mo) | Definition for progressive keratcous | Intensity (mW/cm2) | Duration (min) | Wave legth (nm) | Riboflavie | Apparatus |
| Bouheraouaet al[22] | France | PCS | SC TC AC |
15/15 15/15 15/15 |
25.4±5.6 32.4±6.6 26.7±6.2 |
12/3 11/4 9/6 |
N | 6 | Kmax≥0.75 D/3mo, AST≥0.75 D/12mo, CCT↓≥30 µm/ 6mo | 3.0 10 30 |
30 9 5 |
370 NA NA |
Ri 0.1% in 20% dex | X-Vega (Sooft, SPA) |
| Chow et al[23] | China | PCS | SC AC |
19/32 19/32 |
27.8±10.9 26.3±3.7 |
13M 12M |
N | 12 | Kmax≥1 D/1y, AST≥1 D/1y | 3.0 18 |
30 5 |
365 365 |
Ri 0.1% in 20% dex | UV-X CCL-Vario |
| Cinar et al[24] | Turkey | PCS | SC AC |
13/13 13/13 |
17.0±2.7 18.8±4.5 |
6/7 2/11 |
Y | 6 | Kmax≥1 D/1y, CDVA≥1/2y | 3.0 9 |
30 10 |
NA NA |
Ri 0.1% in 20% dex | Vega CBM X CCL-Vario |
| Cummings et al[25] | Ireland | RCS | SC AC |
66/53 36/34 |
30.0±8.00 27.9±7.6 |
39/14 28/6 |
NA NA |
12 | Kmax≥1 D/2y, ASTIG≥1 D/2y, SE≥0.5 D/3y | 3 9 |
30 10 |
365 365 |
Ri 0.1% in 0-20% dex | UV-X 1000 lamp |
| Hashemianet al[26] | Iran | RCT | SC AC |
31/31 31/31 |
25.13±4.21 |
18/13 | NA NA |
18 | Kmax≥1 D/y, AST≥1 D/y, VA↓≥2 line/y | 3 18 |
30 5 |
370 370 |
Ri 0.1% in 20% dex | UV-X system |
| Hashemianet al[27] | Iran | RCT | SC AC |
76/76 77/77 |
22.3±4 22.6±4 |
38/38 32/45 |
Y | 15 | Kmean≥1.5 D/6mo, CCT↓≥5% | 3 30 |
30 3 |
365 365 |
Ri 0.1% in 20% dex | CCL-Vario |
| Kanellopoulos[28] | Greece | RCT | SC AC |
21/21 21/21 |
NA NA |
NA NA |
NA NA |
48 | Kmax≥1 D/y | 3 7 |
30 15 |
370 370 |
Ri 0.1% | NA NA |
| Kymionis et al[29] | Greece | PCS | SC AC |
16/29 16/29 |
27.56±6.20 25.06±6.34 |
22/7 | Y | 1 | Kmax≥0.75 D/6mo, SE≥0.75 D/6mo | 3 18 |
30 7 |
365 365 |
Ri 0.1% in 20% dex | CCL-365 |
| Kymionis et al[30] | Greece | PCS | SC AC |
26/43 26/43 |
26.15±6.32 26.23±6.9 |
34/9 | Y | 1 | NA NA |
3 9 |
30 14 |
NA NA |
Ri 0.1% in 20% dex | UV-A illuminator |
| Ng et al[18] | China | RCS | SC AC |
14/12 12/12 |
36.1±10.7 32.6±6.6 |
13/1 9/3 |
Y | 13.9±6.3 | Kmax≥1 D/6mo, AST≥1 D/6mo | 3 9 |
30 10 |
NA NA |
Ri 0.1% in 20% dex | UV-X 1000 UV-X 2000 |
| Ng et al[31] | China | RCS | SC AC |
18/17 15/14 |
32.8±9.3 33.0±6.1 |
16/2 12/3 |
Y | 1 | Kmax≥1 D/12mo, ASTIG ≥1 D/12mo, SE≥0.5 D/12mo | 3 9 |
30 10 |
NA NA |
Ri 0.1% in 20% dex | UV-X 1000 UV-X 2000 |
| Sherif[32] | Eygpt | RCT | SC AC |
11/18 14/18 |
23.64±4.03 21.58±5.78 |
6/2 5/5 |
Y | 12 | Kmax≥1 D/6mo, AST≥1 D/6mo, SE≥0.5 D/6mo | 3 30 |
30 4 |
370 370 |
Ri 0.1% | UV-X KXL® system |
| Shetty et al[33] | The Netherlands | RCT | SC AC AC AC |
36/36 36/36 33/33 33/33 |
22.8±5.0 23.1±4.7 19.9±5.8 24.2±7.1 |
NA NA NA NA |
N | 15.32±3.39 | Kmax≥1-1.5 D/6mo, AST ≥1-1.5 D/6mo, thinnest CT↓ ≥5%/6mo | 3 9 18 30 |
30 10 5 3 |
365 365 365 365 |
Ri 0.1% in 20% dex | Avedro KXL system |
| Tomita et al[34] | Japan | PCS | SC AC |
18/18 30/21 |
30.83±5.2 31.17±5.5 |
NA NA |
NA NA |
12 | NA NA |
3 30 |
30 3 |
365 365 |
Ri 0.1% in 20% dex | CCL-365 Vario KXL |
SC: Standard cross-linking; AC: Accelerated cross-linking; TC: Trans-epithelial cross-linking; Kmax: Maximum K value; UDVA: Uncorrected distant visual acuity; CDVA: Corrected distant visual acuity; AST: Astigmatism; SE: Spherical equivalent; CCT: Central cornea thickness; RCT: Randomized clinical trial; RCS: Retrospective case series; PCS: Prospective case series; Ri: Riboflavin; Dex: Dextran; BZK: Benzalkonium chloride; THAM: Tromethamine.
Table 2. Characteristics of included studies (Trans-epithelial CXL vs Standard CXL).
| Author | Region | Study design | Group | Eye/patient (n) | Age (a) | Gender (M/F) | Baseline balance (Y/N) | Follow-up (mo) | Definition for progressive keratcous | Intensity (mW/cm2) | Duration (min) | Wave legth (nm) | Riboflavie | Apparatus |
| Acar et al[35] | Turkey | RCT | SC TC |
7/7 6/6 |
22.71±10.14 24.50±68.11 |
3/4 4/2 |
Y | 6 | Kmax≥1 D/y, SE≥0.5 D/2y, AST≥1.0 D/2y | 3 3 |
30 30 |
NA NA |
Ri 0.1% in 20% dex Ri 0.1%+dex 20%+THAM+EDTA |
Peschke Meditrade, GmbH, |
| Al Fayez et al[16] | Saudi Arabia | RCT | SC TC |
36/36 34/34 |
24.1±5.3 24.8±4.2 |
15/21 16/18 |
Y | 36 | Kmax≥1 D/y, AST≥1 D/y | 3 3 |
30 30 |
NA NA |
Ri 0.1% in 20% dex Ri + with dex |
UV-X unit |
| Cerman et al[36] | Turkey | RCS | SC TC |
30/20 30/23 |
23.7±3.9 22.8±4.7 |
NA NA |
Y | 18 | Kmax≥1 D/6mo, AST≥1 D/6mo | 3 3 |
30 30 |
365 365 |
Ri 0.1% in 20% dex Ri 0.1%+dex 20%+THAM+EDTA |
Ricrolin TE |
| Eraslan et al[37] | Turkey | PCS | SC AC |
18/12 18/15 |
15.5±1.7 15.4±1.7 |
6/6 7/8 |
Y | 24 | Kmax≥1 D/6mo, AST≥1 D/6mo, VA↓≥1 line | 3 3 |
30 30 |
365 365 |
Ri 0.1% in 20% dex 0.25% ri 1.2%+THAM+0.01% BZK |
Vega |
| Kocak et al[38] | Turkey | PCS | SC TC |
19/19 17/17 |
27.16±2.4 27.35±5.95 |
9/10 8/9 |
Y | 12 | Kmax≥1 D/6mo, AST≥1 D/6mo | 3 3 |
30 30 |
366-374 366-374 |
Ri 0.1% in 20% dex Ri 0.1%+15% dex+EDTA+THAM |
CBM-X-Linker |
| Magli et al[17] | Italy | RCS | SC TC |
23/19 16/11 |
14.75±2.1 15±4.2 |
14/5 8/3 |
Y | 12 | Kmax≥1 D/6mo, AST≥1 D/6mo | 3 3 |
30 30 |
365 NA |
Ri 0.1% in 20% dex Ri 0.1%+15% dex+EDTA+THAM |
Vega CBM X linker Vega |
| Nawaz et al[39] | India | RCT | SC TC |
20/20 20/20 |
23.95±4.08 22.35±3.95 |
17/3 15/5 |
Y | 6 | Kmax≥1 D/y | 3 3 |
30 30 |
765 765 |
NA | CL-UVR machine |
| Rossi et al[40] | Italy | RCT | SC TC |
10/10 10/10 |
30.4±7.3 28±3.8 |
5/5 6/6 |
Y | 12 | UDVA, CDVA↓≥1 line/6mo, Kmax≥1 D/6mo, AST≥1 D/6mo | 3 3 |
30 30 |
370 NA |
Ri 0.1% in 20% dex Ri 0.1%+15% dex+EDTA+THAM |
UV-X System NA |
| Soeters et al[41] | The Netherlands | RCT | SC TC |
26/26 35/26 |
24 24 |
19/7 28/7 |
N | 12 | Kmax,Kmean and/or topographic cylinder≥0.5 D/6-12mo | 3 3 |
30 30 |
365 365 |
Ri 0.1% in 20% dex Ri 0.1%+15% dex+EDTA+THAM |
UV-X |
| Stojanovic et al[42] | Norway | RCT | SC TC |
10/20 10/20 |
29.5 | 17/3 | NA NA |
12 | AST or myopia≥1 D/12mo, Sim K≥1.5 D/12mo | 3 3 |
30 30 |
365 365 |
Ri 0.5% without dextran | UV-X lamp |
SC: Standard cross-linking; AC: Accelerated cross-linking; TC: Trans-epithelial cross-linking; Kmax: Maximum K value; UDVA: Uncorrected distant visual acuity; CDVA: Corrected distant visual acuity; AST: Astigmatism; SE: Spherical equivalent; CCT: Central cornea thickness; RCT: Randomized clinical trial; RCS: Retrospective case series; PCS: Prospective case series; Ri: Riboflavin; Dex: Dextran; BZK: Benzalkonium chloride; THAM: Tromethamine.
All the studies enrolled progressive keratoconus patients as their participants. Three studies (13%)[17],[24],[37] took juveniles (less than 18 years old) as their objects, the others (87%) were all adult patients. All eligible studies included both genders, and 18 studies[16]–[18],[22]–[24],[27],[29]–[32],[35]–[41] mentioned the demographic balance within inter-groups. Moreover, the most common participant race was Caucasian (10 studies, 42%)[17],[22],[25],[28]–[30],[33],[40]–[42], the others were Mongolian (4 studies, 17%)[18],[23],[31],[43], Middle Eastern Ethnicity (4 studies, 17%)[16],[26],[27],[32], Turks (5 studies, 21%)[24],[35]–[38] and Indian (1 study, 4%)[39].
All SC in the studies[16]–[17],[22]–[42] used UVA radiation for 30min with 3 mW/cm2. However, the combinations of duration and intensity used for AC were different in some studies, e.g. 30 mW/cm2 with 3min, 30 mW/cm2 with 4min, 18 mW/cm2 with 5min, 9 mW/cm2 with 10min, etc[18],[23]–[27],[31]–[33]. Except for one TC protocol[22] that used 10 mW/cm2 with 9min, all the other TC followed SC protocol. However, the riboflavin used in TC were different from that in SC and AC, containing some loosened or permeable ingredients, such as EDTA and tromethamine[17],[35]–[38],[40]–[41]. As an essential process for SC and AC, most studies scraped the corneal epithelium mechanically, but others used excimer laser or chemical means to remove epithelium such as ethanol or topical anesthetics[28],[31] and the diameter of epithelium removal varied in the range of 6.5-10 mm[27]–[28],[38]. Although the apparatus and wave length of UVA used in these studies were different, the 365 nm or 370 nm wavelength was the most commonly used except for one study that used 765 nm UVA[39].
Risk of Bias in Included Studies
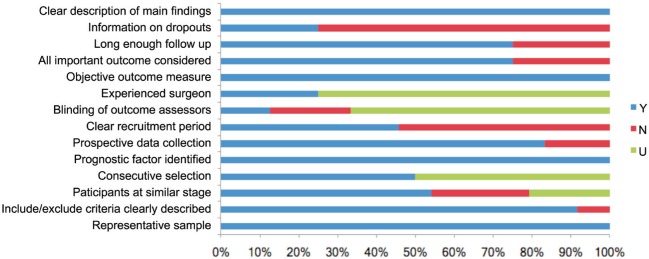
Qualities of included studies assessed according to the checklist consisted of 14 questions are shown in Table 3 and Figure 2. The included studies could be considered as qualified, for all of them scored more than 8 “yes” which conformed to our evaluation standard. For each question marked from 1 to 14, “yes” took account for 100% in the questions 1, 5, 10, 14, “no” accounted for more than 50% in the questions 7 and 13, and “unclear” was higher in the questions 4, 8 and 9.
Table 3. Evaluation of included studies according to the checklist.
| First author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Acar[35] | Y | Y | Y | U | Y | Y | N | Y | Y | Y | N | N | Y | Y |
| Al Fayez[16] | Y | Y | Y | U | Y | Y | N | Y | U | Y | Y | Y | Y | Y |
| Bouheraoua[22] | Y | Y | N | Y | Y | Y | Y | Y | U | Y | Y | N | N | Y |
| Cerman[36] | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | N | Y |
| Chow[23] | Y | Y | N | Y | Y | Y | Y | N | U | Y | Y | Y | Y | Y |
| Cinar[24] | Y | Y | Y | U | Y | Y | N | U | U | Y | Y | N | N | Y |
| Cummings[25] | Y | Y | U | Y | Y | N | Y | U | U | Y | Y | Y | N | Y |
| Eraslan[37] | Y | Y | Y | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
| Hashemian 2015[26] | Y | Y | U | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Hashemian2014[27] | Y | Y | Y | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
| Kanellopoulos[28] | Y | N | U | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
| Kocak[38] | Y | Y | Y | Y | Y | Y | Y | N | U | Y | Y | Y | N | Y |
| Kymionis2016[29] | Y | Y | Y | U | Y | Y | N | U | U | Y | N | N | N | Y |
| Kymionis2014[30] | Y | Y | Y | U | Y | Y | N | N | Y | Y | N | N | N | Y |
| Magli[17] | Y | Y | Y | Y | Y | N | Y | N | U | Y | Y | Y | N | Y |
| Nawaz[39] | Y | N | Y | Y | Y | Y | N | U | U | Y | Y | N | N | Y |
| Ng 2016[18] | Y | Y | Y | Y | Y | N | Y | U | Y | Y | N | Y | N | Y |
| Ng 2015[31] | Y | Y | Y | Y | Y | N | Y | U | Y | Y | N | Y | N | Y |
| Rossi[40] | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y |
| Sherif[32] | Y | Y | Y | U | Y | Y | N | U | U | Y | N | Y | N | Y |
| Shetty[33] | Y | Y | N | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
| Soeters[41] | Y | Y | N | Y | Y | Y | Y | U | U | Y | Y | Y | Y | Y |
| Stojanovic[42] | Y | Y | U | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
| Tomita[34] | Y | Y | U | U | Y | Y | N | U | U | Y | Y | Y | N | Y |
Y: Yes; N: No; U: Unclear.
Figure 2. Evaluation of included studies.
Y: Yes; N: No; U: Unclear.
Effects of Interventions
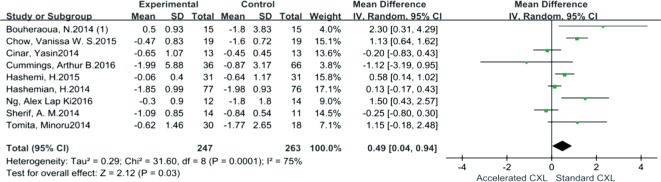
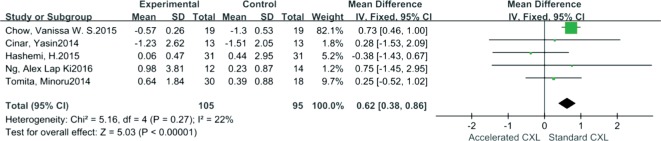
Comparative outcomes between AC and SC are shown in Table 4. As shown in Figure 3, Kmax reduction was significantly greater in SC than in AC; the pooled mean difference of Kmax was 0.49 (95% CI: 0.04-0.94, I2=75%, P=0.03). In addition, SE decreased significantly for SC when compared to AC. The mean difference which was 0.62 (95% CI: 0.38-0.86, I2=22%, P<0.00001) (Figure 4). However, comparative outcomes of UDVA, CDVA, DDL, CCT and ECD indicated no significant differences between the two CXLs shown by the pooled data.
Table 4. Comparative outcomes by pooled data.
| Item | Sample size (Na, Ns, Nt) | Mean difference (95%CI) | Heterogeneity (I2) | P | Effect-model (Ra/Fi) |
| Accelerated CXL vs Standard CXL | |||||
| Kmax | Na=247, Ns=263 | 0.49 (0.04, 0.94) | 75% | 0.03a | Ra |
| UDVA | Na=140, Ns=139 | 0.01 (-0.06, 0.09) | 65% | 0.74 | Ra |
| CDVA | Na=167, Ns=168 | -0.02 (-0.12, 0.07) | 93% | 0.63 | Ra |
| SE | Na=105, Ns=95 | 0.62 (0.38, 0.86) | 22% | <0.00001a | Fi |
| DDL | Na=98, Ns=91 | -38.84 (-116.32, 38.64) | 96% | 0.33 | Ra |
| CCT | Na=91, Ns=90 | 0.54 (-2.52, 3.06) | 6% | 0.73 | Fi |
| ECD | Na=196, Ns=183 | 4.70 (-9.36, 18.7) | 1% | 0.51 | Fi |
| Trans-epithelial CXL vs Standard CXL | |||||
| Kmax | Nt=161, Ns=161 | 1.15 (0.54, 1.75) | 50% | 0.0002a | Ra |
| UDVA | Nt=126, Ns=126 | 0.01 (-0.01, 0.03) | 34% | 0.57 | Fi |
| CDVA | Nt=161, Ns=161 | -0.01 (-0.05, 0.02) | 46% | 0.47 | Ra |
| SE | Nt=130, Ns=12 | -0.53 (-1.19, 0.13) | 67% | 0.11 | Ra |
| DDL | Nt=56, Ns=51 | -133.49 (-145.94, -121.04) | 33% | <0.00001a | Fi |
| CCT | Nt=119, Ns=120 | 0.36 (-5.14, 5.87) | 48% | 0.90 | Ra |
| ECD | Nt=67, Ns=75 | -5.19 (-36.15, 25.76) | 0 | 0.74 | Fi |
UDVA: Uncorrected distant visual acuity; CDVA: Corrected distant visual acuity; SE: Spherical equivalent; DDL: Depth of demarcation line; CCT: Central cornea thickness; ECC: Endothelium cell density; Na: Number of eyes for accelerated CXL; Ns: Number of eyes for standard CXL; Nt: Number of eyes for trans-epithelial CXL; Ra: Random; Fi: Fix. aP<0.05.
Figure 3. Forest plot for comparison of Kmax changes between AC and SC.
Figure 4. Forest plot for comparison of SE changes between AC and SC.
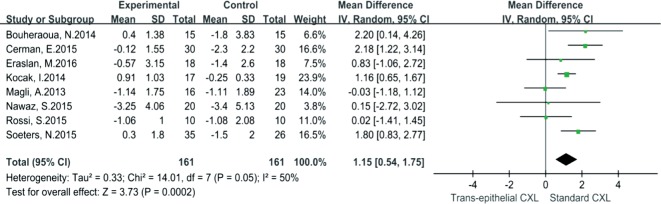
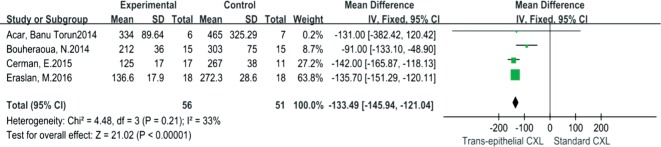
When comparing TC to SC, significant difference of Kmax between the groups was observed by pooled mean difference, which was 1.15 (95% CI: 0.54-1.75, I2=50%, P=0.0002) (Figure 5). Similarly, the DDL of SC was more significantly deeper than that of TC, the mean difference of DDL was -133.49 (95% CI: -145.94 to -121.04, I2=33%, P<0.00001) (Figure 6). However, UDVA, CDVA, CCT, ECD and SE demonstrated no significant differences between TC and SC.
Figure 5. Forest plot for comparison of Kmax changes between TC and SC.
Figure 6. Forest plot for comparison of DDL between TC and SC.
The main side effects reported in these studies were delayed epithelium healing and anterior stromal scarring or opacity. In all 24 included studies, only two studies reported postoperative side effects. In one study, Sherif et al[32] mentioned that one patient had infant anterior stromal opacity for one year after SC treatment. In the other study, Shetty et al[33] noticed two patients in the SC group and four patients in the AC group had delayed epithelial healing and two patients had anterior scarring after AC.
DISCUSSION
Both SC and MCs have been proved to halt progressive keratoconus effectively by more and more studies[7]–[11],[43]–[46], but whether MCs are equivalent to SC in effectiveness has remained unclear. From our Meta-analysis, pooled data showed significant inferiority for MCs relative to SC at halting Kmax deterioration in progressive keratoconus. In addition, SE and DDL showed significant differences when comparing SC with AC and TC, respectively. However, UDVA, CDVA, CCT and ECD demonstrated no significant differences in comparison of MCs and SC. These findings illustrated that SC is superior to MCs athalting progression of keratoconus, but improvements for visual acuity and safety showed equivalence between MCs and SC.
The rationale for CXL is mainly about photochemical effects generated from reactions between ultraviolet radiation and riboflavin (vitamin B2) in the cornea stroma. This procedure can lead to more covalent bond formation within cornea lamellar fibers through which the thinner part of the keratoconic cornea can be consolidated and cornea curvature could also be decreased[47]–[48]. Moreover, the cross-linked corneal collagen fibers can delay the progression of keratoconus via resisting the intraocular pressure (IOP) effectively[49].
The corneal epithelium is the critical obstacle to riboflavin permeation into the corneal stroma, and it affects CXL's effects significantly because a complete and intact epithelial layer is a tough lipophilic barrier to hydro-soluble riboflavin[11],[47]. Franch et al[50] testified that through an enhancer used in the riboflavin solution, the concentration of riboflavin in epi-on cornea was much lower than in epi-off cornea in vivo. This can explain to a large extent why TC was significantly inferior to SC at halting progression of Kmax value, which was also confirmed by superficial DDL in TC caused by shallower infiltration of riboflavin and lower absorption. Similarly, Wollensak and Iomdina[51] suggested that the therapeutic effect of TC was only about one fifth that of SC. Although the corneal epithelium is also an obstacle for UVA radiation, it is not significant enough to influence CXL's effects. Bottos et al[52] assumed that the main obstacle caused by the cornea epithelium in TC is prevention of riboflavin penetration rather than limitation of UVA transmittance. Other authors estimated that approximately 30% of UVA radiation and approximately 80% of riboflavin could be absorbed by intact cornea epithelium[53]–[54].
In contrast to TC, the corneal epithelium is usually removed by mechanical scraping or excimer laser cutting in order to allow more riboflavin to permeate into the cornea stroma in AC and SC[11],[47]. As a standard step for both AC and SC, the riboflavin penetration depth is greater than in TC after the epithelium is removed. In addition, the position that the reactions occur in the corneal stroma should be identical between AC and SC theoretically, because the similar procedures and riboflavin are used. Since DDL indicates the depth of riboflavin permeation and the reacted position in the cornea, this assumption is consistent with our pooled result that DDL was not significantly different between AC and SC.
Ultraviolet radiation intensity and duration are other significant factors that influence CXL's effects. Most AC protocols used in the included studies employed different combinations that had an energy dose (5.4 J/cm2) equal to SC, such as 30 mW/cm2 for 3min and 18 mW/cm2 for 5min[26]–[27]. According to Bunsen-Roscoe's law of reciprocity that effects of CXL mainly depend on the energy absorbed by tissue[55]–[56], the effect of AC should be equivalent to SC through the similar radiation dose used in these studies. However, we found that it was significantly superior for SC to AC at halting progression of Kmax values by the pooled data. Paralleling to our result, Wernli et al[57] found that higher intensities, e.g. from 50 mW/cm2 up to 90 mW/cm2, could not reach the same stiffness effects as lower intensities did even though they complied with Bunsen-Roscoe's law. The reasons accounting for this, inferred by some authors, are limitation of intrastromal oxygen diffusion and more oxygen consumption from higher intensity UVA radiation, which could reduce the biomechanical effects of AC[58].
It is somewhat contradictory to explain that UDVA and CDVA from our pooled results for TC and AC could be comparable to SC even though they were inferior to SC at halting the progression of keratoconus. We assume that the effects generated from CXL could not exert enough impact to improve visual acuity and refractive condition dramatically, no matter what CXL protocol is used. In other words, the effects of CXL mainly reflect the biomechanical impact on stiffening the thinning cornea rather than reforming cornea shape. Even though the pooled SE showed more decrease in SC when compared to AC, we assume that the result was caused by one included study[23] that was given too much weight in the analysis.
In most cases, CCT decreases after CXL have been observed regardless of what CXL protocol was used[8]–[11],[43]–[46]. Greenstein et al[59] explained this phenomenon by compactness of cornea fibers after CXL caused by thermal and photochemical effects, but other authors attribute this to measurement errors from different apparatus[60]. We assume that thermal and photochemical effects are relatively minor for both MCs and SC, so the CCT decrease from the three CXLs did not show any trend of significance in our pooled data. The safety of MCs and SC have been proved by pooled ECD data that indicated reactions between UVA and riboflavin had no influence on endothelial layer[18],[22],[31],[35]–[37].
To the best of our knowledge, there is no published Meta-analysis comparing MCs and SC until now, but this Meta-analysis still has some unavoidable limitations. One objective limitation was that the definition criteria for progressive keratoconus, demographic baseline and follow-up period varied within the included studies. Moreover, a small sample size of participants was enrolled in most studies and all of them were single centered, consecutive case serials without randomization. Lastly, different UVA instruments, riboflavin ingredients, surgical procedures and postoperative medications were used in these studies. In the future, accompanied by more participants enrolled into multi-center randomized clinical trials and by standardization for apparatus and riboflavin ingredients, more reliable outcomes should be obtained and more confirmed conclusions could be made.
In conclusion, SC was more favorable at halting the progression of keratoconus, but visual acuity improvement showed comparable results between MCs and SC. MCs are more suitable for pediatrics regarding epithelium-on and short duration, and TC could be carried out for patients with cornea thickness less than 400 µm due to its shallower DDL.
Acknowledgments
Authors contributions: Study design (Yang Liu, Zhi-Qiang Pan); literature retrieval (Yang Liu, Yi Liu, Ying-Nan Zhang, Jing Zhang); data extraction (Yang Liu, Yi Liu, Jing Zhang, Ai-Peng Li); statistical analysis (Yang Liu, Ying-Nan Zhang, Ai-Peng Li); manuscript writing (Yang Liu); review ( Zhi-Qiang Pan, Qing-Feng Liang, Ying Jie).
Conflicts of Interest: Liu Y, None; Liu Y, None; Zhang YN, None; Li AP, None; Zhang J, None; Liang QF, None; Jie Y, None; Pan ZQ, None.
REFERENCES
- 1.Randleman JB, Crosby MB. Cornea Handbook. In: Trattler WB, Majmudar PA, Luchs JI, Swartz TS, editors. Corneal ectatic disorders. US: Slack Inc; 2010. pp. 109–122. [Google Scholar]
- 2.Wilson SE, Klyce SD. Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology. 1994;101(1):147–152. doi: 10.1016/s0161-6420(94)31372-8. [DOI] [PubMed] [Google Scholar]
- 3.Hartstein J. Keratoconus that developed in patients wearing corneal contact lenses. Report of four cases. Arch Ophthalmol. 1968;80(3):345–346. doi: 10.1001/archopht.1968.00980050347009. [DOI] [PubMed] [Google Scholar]
- 4.Forister JF, Forister EF, Yeung KK, Ye P, Chung MY, Tsui A, Weissman BA. Pevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009;35(4):176–180. doi: 10.1097/ICL.0b013e3181a7bda1. [DOI] [PubMed] [Google Scholar]
- 5.Korb DR, Finnemore VM, Herman JP. Apical changes and scarring in keratoconus as related to contact lens fitting techniques. J Am Optom Assoc. 1982;53(3):199–205. [PubMed] [Google Scholar]
- 6.McMonnies CW. Keratoconus fittings: apical clearance or apical support? Eye Contact Lens. 2004;30(3):147–155. doi: 10.1097/01.icl.0000138717.57592.36. [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 8.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33(12):2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Caporossi A, Mazzotta C, Baiocchi S. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: The Siena Eye Cross Study. Am J Ophthalmol. 2010;149(4):585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Vinciguerra P, Albe E, Trazza S. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009;127(10):1258–1265. doi: 10.1001/archophthalmol.2009.205. [DOI] [PubMed] [Google Scholar]
- 11.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26(4):385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 12.Chan CC, Sharma M, Wachler BS. Effect of inferior-segment Intacs with and without C3-R on keratoconus. J Cataract Refract Surg. 2007;33(1):75–80. doi: 10.1016/j.jcrs.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal cross-linking. J Cataract Refract Surg. 2009;35(8):1358–1362. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Hayes S, O'Brart DP, Lamdin LS, Doutch J, Samaras K, Marshall J, Meek KM. Effect of complete epithelial debridement before riboflavin-ultraviolet-A corneal collagen crosslinking therapy. J Cataract Refract Surg. 2008;34(4):657–661. doi: 10.1016/j.jcrs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Rocha KM, Ramos-Esteban JC, Qian Y, Herekar S, Krueger RR. Comparative study of riboflavin-UVA cross-linking and “flash-linking” using surface wave elastometry. J Refract Surg. 2008;24(7):S748–S751. doi: 10.3928/1081597X-20080901-20. [DOI] [PubMed] [Google Scholar]
- 16.Al Fayez MF, Alfayez S, Alfayez Y. Transepithelial versus epithelium-off corneal collagen cross-linking for progressive keratoconus: a prospective randomized controlled trial. Cornea. 2015;34(Suppl 10):S53–S56. doi: 10.1097/ICO.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 17.Magli A, Forte R, Tortori A, Capasso L, Marsico G, Piozzi E. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea. 2013;32(5):597–601. doi: 10.1097/ICO.0b013e31826cf32d. [DOI] [PubMed] [Google Scholar]
- 18.Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44(1):8–14. doi: 10.1111/ceo.12571. [DOI] [PubMed] [Google Scholar]
- 19.Hashemi H, Fotouhi A, Miraftab M, Bahrmandy H, Seyedian MA, Amanzadeh K, Heidarian S, Nikbin H, Asgari S. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J Cataract Refract Surg. 2015;41(3):533–540. doi: 10.1016/j.jcrs.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146(2):251–259. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34(5):592–600. doi: 10.1097/ICO.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 22.Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C, Loriaut P, Basli E, Borderie V, Laroche L. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 2014;55(11):7601–7609. doi: 10.1167/iovs.14-15662. [DOI] [PubMed] [Google Scholar]
- 23.Chow VW, Chan TC, Yu M, Wong VW, Jhanji V. One-year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci Rep. 2015;5:14425. doi: 10.1038/srep14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cınar Y, Cingü AK, Türkcü FM, Çınar T, Yüksel H, Özkurt ZG, Çaça I. Comparison of accelerated and conventional corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 2014;33(3):218–222. doi: 10.3109/15569527.2013.834497. [DOI] [PubMed] [Google Scholar]
- 25.Cummings AB, McQuaid R, Naughton S, Brennan E, Mrochen M. Optimizing corneal cross-linking in the treatment of keratoconus: a comparison of outcomes after standard- and high-intensity protocols. Cornea. 2016;35(6):814–822. doi: 10.1097/ICO.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi H, Miraftab M, Seyedian MA, Hafezi F, Bahrmandy H, Heidarian S, Amanzadeh K, Nikbin H, Fotouhi A, Asgari S. Long-term results of an accelerated corneal cross-linking protocol (18 mW/cm2) for the treatment of progressive keratoconus. Am J Ophthalmol. 2015;160(6):1164–1170.e1. doi: 10.1016/j.ajo.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Hashemian H, Jabbarvand M, Khodaparast M, Ameli K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: a randomized controlled trial. J Refract Surg. 2014;30(12):837–842. doi: 10.3928/1081597X-20141117-02. [DOI] [PubMed] [Google Scholar]
- 28.Kanellopoulos AJ. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kymionis GD, Tsoulnaras KI, Liakopoulos DA, Skatharoudi CA, Grentzelos MA, Tsakalis NG. Corneal stromal demarcation line depth following standard and a modified high intensity corneal cross-linking protocol. J Refract Surg. 2016;32(4):218–222. doi: 10.3928/1081597X-20160216-01. [DOI] [PubMed] [Google Scholar]
- 30.Kymionis GD, Tsoulnaras KI, Grentzelos MA, Liakopoulos DA, Tsakalis NG, Blazaki SV, Paraskevopoulos TA, Tsilimbaris MK. Evaluation of corneal stromal demarcation line depth following standard and a modified-accelerated collagen cross-linking protocol. Am J Ophthalmol. 2014;158(4):671–675.e1. doi: 10.1016/j.ajo.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Ng AL, Chan TC, Lai JS, Cheng AC. Comparison of the central and peripheral corneal stromal demarcation line depth in conventional versus accelerated collagen cross-linking. Cornea. 2015;34(11):1432–1436. doi: 10.1097/ICO.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 32.Sherif AM. Accelerated versus conventional corneal collagen cross-linking in the treatment of mild keratoconus: a comparative study. Clin ophthalmol. 2014;8:1435–1440. doi: 10.2147/OPTH.S59840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty R, Pahuja NK, Nuijts RM, Ajani A, Jayadev C, Sharma C, Nagaraja H. Current protocols of corneal collagen cross-linking: visual, refractive, and tomographic outcomes. Am J Ophthalmol. 2015;160(2):243–249. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40(6):1013–1020. doi: 10.1016/j.jcrs.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Acar BT, Utine CA, Ozturk V, Acar S, Ciftci F. Can the effect of transepithelial corneal collagen cross-linking be improved by increasing the duration of topical riboflavin application? An in vivo confocal microscopy study. Eye Contact Lens. 2014;40(4):207–212. doi: 10.1097/ICL.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 36.Cerman E, Toker E, Ozarslan Ozcan D. Transepithelial versus epithelium-off crosslinking in adults with progressive keratoconus. J Cataract Refract Surg. 2015;41(7):1416–1425. doi: 10.1016/j.jcrs.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 37.Eraslan M, Toker E, Cerman E, Ozarslan D. Efficacy of epithelium-off and epithelium-on corneal collagen cross-linking in pediatric keratoconus. Eye Contact Lens. 2017;43(3):155–161. doi: 10.1097/ICL.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 38.Kocak I, Aydin A, Kaya F, Koc H. Comparison of transepithelial corneal collagen crosslinking with epithelium-off crosslinking in progressive keratoconus. J Fr Ophtalmol. 2014;37(5):371–376. doi: 10.1016/j.jfo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Nawaz S, Gupta S, Gogia V, Sasikala NK, Panda A. Trans-epithelial versus conventional corneal collagen crosslinking: A randomized trial in keratoconus. Oman J Ophthalmol. 2015;8(1):9–13. doi: 10.4103/0974-620X.149855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi S, Orrico A, Santamaria C, Romano V, De Rosa L, Simonelli F, De Rosa G. Standard versus trans-epithelial collagen cross-linking in keratoconus patients suitable for standard collagen cross-linking. Clin Ophthalmol. 2015;9:503–509. doi: 10.2147/OPTH.S73991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821–828. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Stojanovic A, Zhou W, Utheim TP. Corneal collagen cross-linking with and without epithelial removal: a contralateral study with 0.5% hypotonic riboflavin solution. Biomed Res Int. 2014;2014:619398. doi: 10.1155/2014/619398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ameen SS, Mehboob MA, Ali K. Efficacy and safety of transepithelial collagen cross linking for progressive keratoconus. Pak J Med Sci. 2016;32(5):1111–1115. doi: 10.12669/pjms.325.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koc M, Uzel MM, Tekin K, Kosekahya P, Ozulken K, Yilmazbas P. Effect of preoperative factors on visual acuity, corneal flattening, and corneal haze after accelerated corneal crosslinking. J Cataract Refract Surg. 2016;42(10):1483–1489. doi: 10.1016/j.jcrs.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Koç M, Uzel MM, Koban Y, Tekin K, Taşlpnar AG, Ylmazbaş P. Accelerated corneal cross-linking with a hypoosmolar riboflavin solution in keratoconic thin corneas: short-term results. Cornea. 2016;35(3):350–354. doi: 10.1097/ICO.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Jian W, Sun L, Li M, Han T, Son J, Zhou X. One-year follow-up of changes in corneal densitometry after accelerated (45 mW/cm2) transepithelial corneal collagen cross-linking for keratoconus: a retrospective study. Cornea. 2016;35(11):1434–1440. doi: 10.1097/ICO.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66(1):97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 48.Milne PJ, Zika RG. Crosslinking of collagen gels: photochemanical measurements. Proc SPIE Int Soc Opt Eng. 1992;1644:115–124. [Google Scholar]
- 49.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 50.Franch A, Birattari F, Dal Mas G, Lužnik Z, Parekh M, Ferrari S, Ponzin D. Evaluation of intrastromal riboflavin concentration in human corneas after three corneal cross-linking imbibition procedures: a pilot study. J Ophthalmol. 2015;2015:794256. doi: 10.1155/2015/794256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35(3):540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 52.Bottós KM, Schor P, Dreyfuss JL, Nader HB, Chamon W. Effect of corneal epithelium on ultraviolet-A and riboflavin absorption. Arq Bras Oftalmol. 2011;74(5):348–351. doi: 10.1590/s0004-27492011000500008. [DOI] [PubMed] [Google Scholar]
- 53.Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35(5):893–899. doi: 10.1016/j.jcrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS PharmSciTech. 2006;7(3):57. doi: 10.1208/pt070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brindley GS. The Bunsen-Roscoe law for the human eye at very short durations. J Physiol. 1952;118(1):135–139. doi: 10.1113/jphysiol.1952.sp004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52(12):9048–9052. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 57.Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54(2):1176–1180. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 58.Hammer A, Richoz O, Arba Mosquera S, Tabibian D, Hoogewoud F, Hafezi F. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest Ophthalmol Vis Sci. 2014;55(5):2881–2884. doi: 10.1167/iovs.13-13748. [DOI] [PubMed] [Google Scholar]
- 59.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(4):691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 60.Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg. 2012;28(11):769–776. doi: 10.3928/1081597X-20121016-01. [DOI] [PubMed] [Google Scholar]