Abstract
4-1BB (CD137) is a strong enhancer of the proliferation of CD8+ T cells. Since these cells require increased production of energy and biomass to support their proliferation, we hypothesized that 4-1BB signaling activated glucose and fatty acid metabolism. We found that treatment with agonistic anti-4-1BB mAb promoted the proliferation of CD8+ T cells in vitro, increasing their size and granularity. Studies with a glycolysis inhibitor and a fatty acid oxidation inhibitor revealed that CD8+ T cell proliferation required both glucose and fatty acid metabolism. Anti-4-1BB treatment increased glucose transporter 1 expression and activated the liver kinase B1 (LKB1)-AMP-activated protein kinase (AMPK)-acetyl-CoA carboxylase (ACC) signaling pathway, which may be responsible for activating the metabolism of glucose and fatty acids. We also examined whether blocking glucose or fatty acid metabolism affected cell cycle progression and the anti-apoptotic effect of 4-1BB signaling. The increase of anti-apoptotic factors and cyclins in response to anti-4-1BB treatment was completely prevented by treating CD8+ T cells with the fatty acid oxidation inhibitor, etomoxir, but not with the glycolysis inhibitor, 2-deoxy-D-glucose. We conclude that anti-4-1BB treatment activates glucose and fatty acid metabolism thus supporting the increased demand for energy and biomass, and that fatty acid metabolism plays a crucial role in enhancing the cell cycle progression of anti-CD3-activated CD8+ T cells in vitro and the anti-apoptotic effects of 4-1BB signaling on these cells.
Keywords: AMPK, CD8+ T cells, 4-1BB (CD137), LKB1, metabolism
Introduction
4-1BB (CD137), an inducible co-stimulatory molecule, is expressed on various types of immune cells including activated T cells, natural killer (NK)/natural killer T (NKT) cells, CD4+CD25+ regulatory T cells, and dendritic cells.1, 2, 3 4-1BB signaling is known to preferentially activate CD8+ T cells in vitro and in vivo, prevent activation-induced cell death (AICD) of CD8+ T cells, and selectively induce Th1-type cytokines such as IFN-γ and TNF-α.4 Accumulating evidence suggests that 4-1BB–4-1BBL interactions have more complex roles in sculpting adaptive immunity than previously appreciated. Recent studies demonstrate that in vivo 4-1BB signaling has immune-regulatory roles, as well as immune-stimulatory ones, and hyper-responsiveness of T cells was induced in 4-1BB-deficient mice in vivo.4, 5, 6
Many recent studies indicate that cellular metabolism is important in regulating the differentiation and memory formation of CD8+ T cells.7, 8 Naïve and memory CD8+ T cells use fatty acid oxidation (FAO) as a primary energy source to sustain their survival,7, 8, 9, 10 while activated effector CD8+ T cells prefer glucose uptake.11 Notably, recent study suggests that asymmetric segregation of mitochondrial mass during cell division determines the fate of CD8+ T cells.12 It seems that larger mitochondrial mass increases FAO by increasing the mitochondrial spare respiratory capacity, leading to differentiation into memory CD8+ T cells. Less mitochondrial mass results in the differentiation into effector CD8+ T cells due to increased dependency on aerobic glycolysis. Indeed, formation of memory CD8+ T cells in vivo can be enhanced by activating AMP-activated protein kinase (AMPK) signaling and lipid metabolism with metformin treatment,13 inhibiting mammalian target-of-rapamycin (mTOR) activity with rapamycin,14 or blocking glycolysis with low dose of glycolysis inhibitor, 2-deoxy-D-glucose (2-DG).15
The currently proposed mechanisms, by which 4-1BB signaling enhances CD8+ T cell responses, include the prevention of AICD and acceleration of the cell cycle.16, 17 Thus, it can be reasoned that to enhance CD8+ T cell responses, 4-1BB signaling activates metabolic pathways to meet the increased energy and biomass demands required for cell proliferation. To corroborate this hypothesis, we tested whether 4-1BB signaling stimulates glucose and fatty acid metabolism by obstructing these metabolic pathways with their respective inhibitors. We found that 4-1BB signaling enhanced both glucose metabolism and FAO with delayed kinetics, and that fatty acid metabolism plays a crucial role in promoting CD8+ T cell survival and cell cycle progression activated by 4-1BB. These results reveal that 4-1BB is involved in activating metabolic pathways, in parallel with its well-established functions in cell cycle progression and anti-apoptosis, thereby maximizing CD8+ T cell proliferation.
Materials and Methods
Mice, reagents and antibodies
Six- to eight-week-old C57BL/6 mice were purchased from OrientBio (Gapyeong, Korea). All animal experiments were reviewed and approved by the Animal Care and Use Committee of the National Cancer Center (NCC-10-080), and were performed in accordance with the Guide for the Care and Use of Laboratory Animals. Anti-CD3 monoclonal antibody (mAb, clone 145-2C11) was purchased from BD Pharmingen (San Diego, CA, USA) and CD8-microbeads from Miltenyi Biotech (Auburn, CA, USA). Agonistic anti-4-1BB mAb (3E1) was a kind gift from Dr Robert Mittler (Emory University, Atlanta, GA, USA). 2-DG was purchased from Acros Organics (New Jersey, NJ, USA), etomoxir (ETX) and compound C were from Sigma (St Louis, MO, USA), STO-609 from Calbiochem (San Diego, CA, USA) and carboxyfluorescein diacetate succinimidyl ester (CFSE) from Molecular Probes (Eugene, OR, USA). All antibodies for Western blots including glucose transporter 1 (GLUT1), p-liver kinase B1 (LKB1), p-AMPK, p-acetyl-CoA carboxylase (ACC) (Ser79), p-p70S6K, Bcl-2, Bcl-XL, Bax, cyclin A, cyclin B1, cyclin E and β-actin were purchased from Cell Signaling (Danvers, MA, USA), except anti-β-catenin mAb (Santa Cruz Biotechnology, CA, USA).
Purification of CD8+ T cells
To isolate CD8+ T cells, lymphocytes from the spleens and lymph nodes (LNs) of wild-type C57BL/6 mice were suspended in phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS) at a concentration of 108 cells/ml, incubated with CD8-microbeads at 4 °C for 15 min and loaded on LS columns. The purified CD8+ T cells were routinely >95% pure as determined by flow cytometry.
CFSE dilution assay
Purified CD8+ T cells were suspended in 1 × PBS at 1 × 107 cells/ml and stained with 10 μM CFSE at 37 °C for 5 min. Next, they were incubated with ice-cold FBS for 1 min, washed three times with RPMI1640 medium containing 10% FBS (RPMI10), and resuspended in RPMI10 medium. CFSE-labeled T cells were plated at 4 × 105 cells/well in 96-well round-bottom microplates and stimulated with 0.1 μg/ml anti-CD3 for 16 h, followed by 5 μg/ml anti-4-1BB or rat IgG for another 48 h. In some experiments, the activated CD8+ T cells were incubated with inhibitors for 1 h before treatment with rat IgG or anti-4-1BB mAb. The dilution of CFSE used was determined by flow cytometry.
RT-PCR
Total RNA was extracted from CD8+ T cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA), and first-strand cDNA was synthesized with 0.5 μg total RNA using a reverse transcription system kit (Promega, Madison, WI, USA) and oligo (dT)12-18. PCR was performed with the cDNA and specific primer sets. The PCR primers used were as follows: GAPDH, forward 5′-ACATGGCCTCCAAGGAGTAA-3′, reverse 5′-GGTCTGGGATGGAAATTGTG-3′ GLUT1, forward 5′-TCTCTGTCGGCCTCTTTGTT-3′, reverse 5′-AGAGGCCACAAGTCTGCAT-3′ and HK2, forward 5′-CCGTGGTGGACAAGATAAGAGAGAACC-3′, reverse 5′-GGACACGTCACATTTCGGAGCCAG-3′.
Western blotting
Various conditions of CD8+ T cells were lysed with a gentle lysis buffer (10 mM Tris pH 7.5, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride and protease inhibitors) and extracts were prepared by removing cell debris by centrifugation. Extracted proteins were separated on 10–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes. Proteins of interest were detected with primary antibodies and secondary horseradish peroxidase-labeled antibody. Bound antibody was detected by enhanced chemiluminescence (Intron, Seoul, Korea).
Lactate production and fatty acid uptake by CD8+ T cells
CD8+ T cells activated with anti-CD3 mAb for 16 h were stimulated with 5 μg/ml rat IgG or anti-4-1BB mAb. To measure lactate production by the cells, the culture media were mixed with the same volume of 50% polyethylene glycol solution and incubated for 30 min on ice. The samples were centrifuged at 12 000 rpm for 5 min and 50 μl of serially diluted supernatants were incubated with 50 μl of L-Lactate Assay Solution (Biomedical Research Service Center, Buffalo, NY, USA) for 30 min at 37 °C. The reactions were stopped by adding 50 μl of 3% acetic acid and the absorbances at 492 nm were measured. To measure fatty acid uptake, activated CD8+ T cells were stimulated with 5 μg/ml of rat IgG or anti-4-1BB mAb for 24 h, and further incubated with 1 μM BoDipy FL C16 (Molecular Probes) for 30 min. BoDipy uptake was quenched by adding ice-cold PBS with 5% FBS and subsequently analyzed by FACSCalibur (BD Bioscience, San Jose, CA, USA).
Statistical analysis
The mean densities of gel bands were determined using Image J (NIH, Bethesda, MD, USA). The relative expression levels of p-LKB1, p-AMPK, p-ACC, Bcl-2, Bcl-XL and Bax were calculated by dividing the densitometry reading of each protein by the value of β-actin. All data were analyzed with the statistical program, Prism 4.0 GraphPad (San Diego, CA, USA). Student’s t-test was used to determine the statistical significance of differences between groups.
Results
4-1BB signaling increases the size and granularity of activated CD8+ T cells
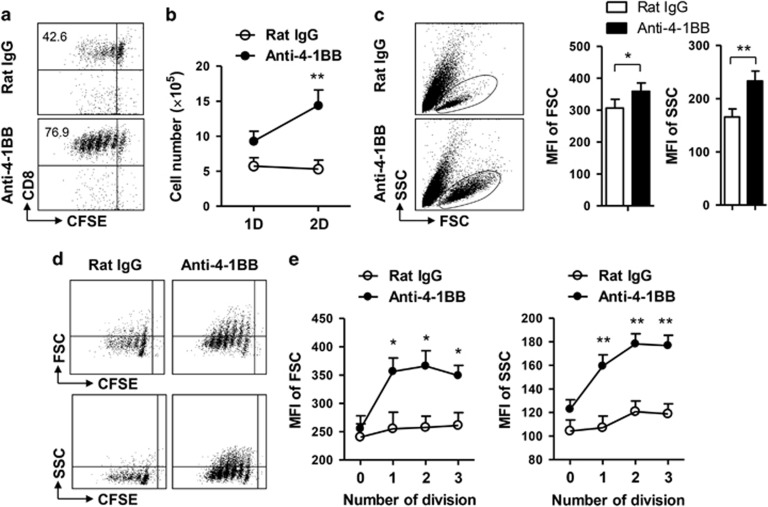
4-1BB signaling is known to enhance the proliferation of CD8+ T cells by preventing AICD and promoting cell cycle progression.16, 18 In addition we found recently that 4-1BB triggering using agonistic anti-4-1BB mAb appeared to enhance the proliferation of CD8+ T cells by preventing AICD rather than by stimulating cell cycling.19 However, it is not clear whether 4-1BB signaling promotes the synthesis of cellular components to enhance CD8+ T cell division. Therefore, we examined whether the enhanced cell division in response to 4-1BB signaling increases CD8+ T cell size and granularity. The CD8+ T cells isolated from C57BL/6 mice were labeled with CFSE to assess the division rate, stimulated with anti-CD3 mAb for 16 h to induce 4-1BB expression on their surface, and further treated with anti-4-1BB mAb or rat IgG for another 48 h. Flow cytometric analysis indicated that the anti-4-1BB treatment increased CFSElow CD8+ T cells, which demonstrated the occurrence of increased cell division (Figure 1a). Moreover, when we counted live cells 1 or 2 days after treatment with rat IgG or anti-4-1BB mAb, live cells were significantly increased in anti-4-1BB-treated CD8+ T cells, but not in rat IgG-treated CD8+ T cells (Figure 1b).
Figure 1.
4-1BB signaling increases the volume of CD8+ T cells. CD8+ T cells were isolated from the LNs and spleen of C57BL/6 mice, labeled with 10 μM CFSE and cultured in 96-well round-bottom plates at a density of 4 × 105 cells/well in the presence of 0.1 μg/ml anti-CD3 mAb. After 16 h incubation, some of anti-CD3-activated CD8+ T cells were further treated with 5 μg/ml anti-4-1BB mAb for 1 or 2 days. (a) CFSE dilution of CD8+ T cells was analyzed by FACSCalibur (BD Bioscience) 2 days after anti-4-1BB treatment. (b) Live CD8+ T cells were counted 1 or 2 days after anti-4-1BB treatment using an ADAM MC Automated Mammalian Cell Counter (Bulldog Bio Inc., Rochester, NY, USA). (c) After two days of anti-4-1BB treatment, total CD8+ T cells were analyzed by FACSCalibur and plotted as FSC vs. SSC. MFIs of FSC and SSC were calculated from five independent experiments using BD CellQuest Software (BD Bioscience). (d) Live cells were gated and plotted as CFSE vs. FSC or vs. SSC after two days of anti-4-1BB treatment. (e) Each division peak of CD8+ T cells was gated and the MFIs of FSC or SSC were calculated. Data representative are of three independent experiments. Results in b and e are means±s.d. (*P<0.05; **P<0.01). Abbreviations: FSC, forward scatter; LNs, lymph nodes; MFI, mean fluorescence intensity; SCC, side scatter.
Under the same conditions, we examined the size and granularity of CD8+ T cells by flow cytometry, 2 days after 4-1BB treatment. Intensities of forward scatter (FSC; a relative measure of cell size) and side scatter (SSC; a relative measure of cytoplasmic complexity) were higher in the anti-4-1BB-treated live CD8+ T cells than in the rat IgG-treated cells (Figure 1c). We further determined the cell size and granularity of each dividing CD8+ T cell population by plotting CFSE vs. FSC or vs. SSC (Figure 1d). The data showed that anti-4-1BB treatment increased the cell size and granularity of dividing CD8+ T cells, particularly in the CD8+ T cells undergoing 1–3 rounds of cell division (Figure 1e). Together, these results indicate that anti-4-1BB treatment increases the size and granularity of actively dividing CD8+ T cells.
4-1BB signaling activates glucose metabolism to enhance CD8+ T cell proliferation
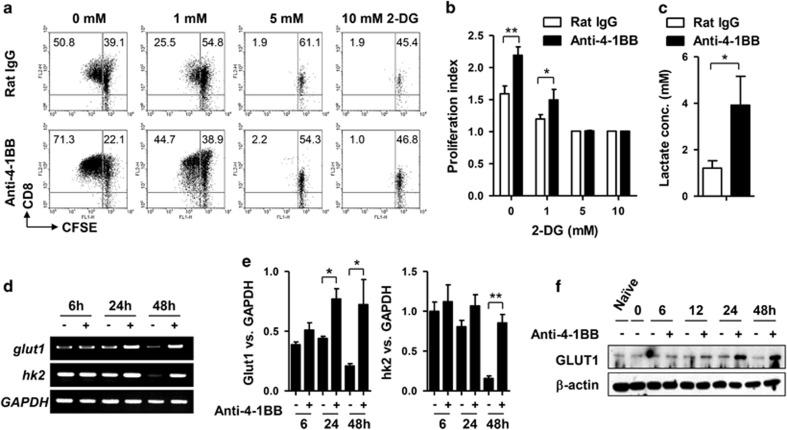
Since 4-1BB signaling increased the cell size and granularity of dividing CD8+ T cells, we hypothesized that it might activate metabolic signaling pathways responsible for generating energy and cellular building blocks. Glucose, the simplest form of carbohydrate, is metabolized by nearly all known organisms to produce energy, nucleotides, lipids and amino acids. We first examined whether glucose was required by 4-1BB signaling to enhance CD8+ T cell proliferation. CFSE-labeled and anti-CD3-activated CD8+ T cells were pre-incubated with the indicated doses of 2-DG (a glycolytic inhibitor) to block glycolysis, and stimulated with anti-4-1BB mAb or rat IgG for 48 h. CFSE dilution indicated that CD8+ T cell division was partially obstructed by 1 mM 2-DG, and completely inhibited by >5 mM 2-DG, independent of 4-1BB signaling (Figures 2a and b). Interestingly, although 2-DG abolished CD8+ T cell division, treatment with anti-4-1BB mAb increased the number of live and non-dividing CD8+ T cells compared with treatment with rat IgG (Figure 2a). To see whether 4-1BB stimulation increased glucose consumption by the CD8+ T cells, lactate was measured in the culture medium of CD8+ T cells after 48 h treatment with rat IgG or anti-4-1BB. We found that 4-1BB stimulation significantly increased lactate production (Figure 2c).
Figure 2.
Glucose is essential for enhancing the proliferation of CD8+ T cells by 4-1BB signals. CFSE-labeled CD8+ T cells were activated with 0.1 μg/ml anti-CD3 mAb for 16 h. Then the activated CD8+ T cells were pre-incubated with the indicated concentration of 2-DG for 1 h and further treated with 5 μg/ml anti-4-1BB mAb for 2 days. (a) The CD8+ T cells were stained with anti-CD8-PE-Cy5 and CFSE dilution of CD8+ T cells were analyzed by FACSCalibur. (b) Proliferation index was calculated using Modfit LT software (Verity Software House, Topsham, ME, USA) from a. (c) Medium concentrations of lactic acid 48 h after rat IgG or anti-4-1BB treatment. (d–f) Anti-CD3-activated CD8+ T cells were cultured in the presence of anti-4-1BB mAb and harvested at the indicated time points for total RNA or protein extraction. First-strand cDNA was synthesized from the total RNA. Transcript levels of GLUT1, HK2 and GAPDH were assessed using specific primer sets (d). Relative expression levels were calculated by dividing the densitometer reading of each band by the value of GAPDH (e). Western blots performed with antibodies specific for GLUT1 and β-actin (f). Naïve indicates freshly isolated CD8+ T cells. Data are representative of three independent experiments. Results in b are means±SDs (*P<0.05; **P<0.01). Abbreviation: 2-DG, 2-deoxy-D-glucose.
Since GLUT1 and HK2 are key genes affecting glucose metabolism,20 their transcript levels were assessed using total RNA from the CD8+ T cells, after 6, 24 or 48 h treatment with anti-4-1BB mAb or rat IgG. GLUT1 transcripts increased in CD8+ T cells 24 and 48 h after anti-4-1BB treatment, while HK2 transcripts increased only 48 h after treatment (Figures 2c and d). Western blots indicated that GLUT1 protein increased 24 h after anti-4-1BB treatment (Figure 2e). These results indicate that anti-CD3 activation alone transiently induces the transcription of GLUT1 and HK2, and 4-1BB signaling sustains or enhances the transcription and translation of these genes after a delay, so stimulating division of the CD8+ T cells.
Fatty acid oxidation is essential for the enhanced proliferation of CD8+ T cells by 4-1BB signaling
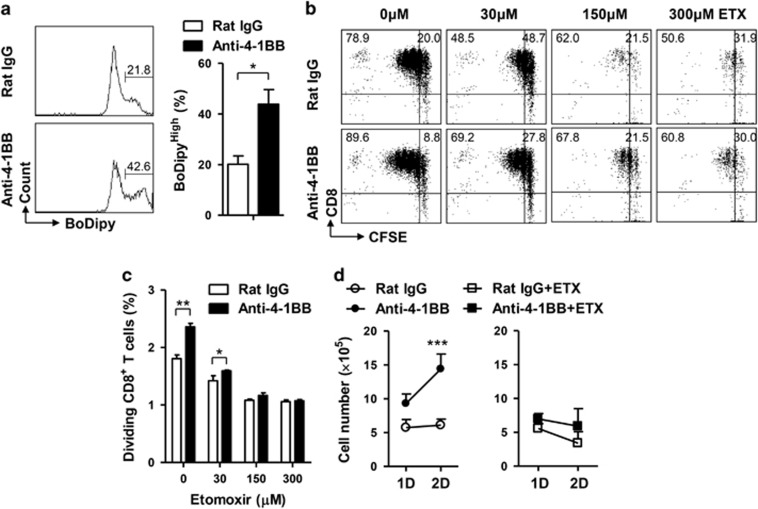
To examine whether 4-1BB stimulation increased fatty acid uptake by the activated CD8+ T cells, CD8+ T cells activated with anti-CD3 mAb for 16 h were stimulated with rat IgG or anti-4-1BB mAb for 24 h and further incubated with BoDipy FL C16 for the last 30 min. Flow cytometric analysis indicated that numbers of BoDipyHigh CD8+ T cells were significantly increased by the anti-4-1BB treatment (Figure 3a).
Figure 3.
ETX prevents the enhanced proliferation of CD8+ T cell by 4-1BB signals. (a) BoDipy FL C16 uptake by the activated CD8+ T cells 24 h after rat IgG or anti-4-1BB treatment. (b and c) CFSE-labeled and anti-CD3-activated CD8+ T cells were pre-incubated with the indicated concentration of ETX for 1 h and further treated with 5 μg/ml anti-4-1BB mAb for 2 days. The CD8+ T cells were stained with anti-CD8-PE-Cy5 and CFSE dilution of CD8+ T cells was subsequently analyzed by FACSCalibur (b). Proliferation index was calculated using Modfit LT software from b (c). (d) Anti-CD3-activated CD8+ T cells were pre-incubated with 150 μM ETX for 1 h and treated with rat IgG or anti-4-1BB mAb. Live cells were counted 1 or 2 days after anti-4-1BB treatment using an Automated Mammalian Cell Counter. Data are representative of three independent experiments. Results in a, c and d are means±s.d. (**P<0.01; ***P<0.001). Abbreviation: ETX, etomoxir.
Since FAO is another key metabolic process generating energy and cellular building blocks, we next examined whether FAO was involved in the proliferation of CD8+ T cells after anti-4-1BB treatment. Conversion of acyl-CoA to carnitine palmitoyltransferase 1 (CPT1) is the rate limiting step in beta-oxidation21 and thus, ETX (inhibitor of CPT1) was used to block FAO. CFSE-labeled and anti-CD3-activated CD8+ T cells were pre-incubated with the indicated doses of ETX for 1 h, and then stimulated with anti-4-1BB mAb or rat IgG for 48 h. Again, the frequency of dividing cells was significantly higher among the anti-4-1BB-treated CD8+ T cells (Figure 3b) and ETX treatment reduced the proliferation of rat IgG- or anti-4-1BB-treated CD8+ T cells in a dose-dependent manner (Figures 3b and c). We also counted numbers of live CD8+ T cells after 1 or 2 days’ treatment with anti-4-1BB mAb or rat IgG, and found that 150 μM ETX completely blocked the increase of CD8+ T cells (Figure 3d). ETX is less effective in inhibiting CTP1 in the presence of glucose.22 Since the CD8+ T cells were cultured in RPMI1640 medium containing 2 g/l glucose, ETX seemed to be effective at >100 μM. Nevertheless, ETX caused a dose-dependent reduction of the division of the activated CD8+ T cells independent of anti-4-1BB treatment, indicating that FAO is required to support the increased demand for energy and building blocks.
4-1BB signaling activates acetyl-CoA carboxylase through the LKB1–AMPK signaling pathway
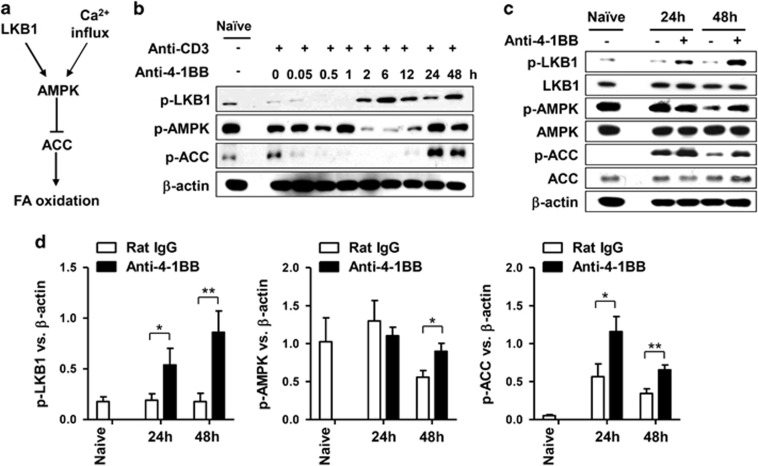
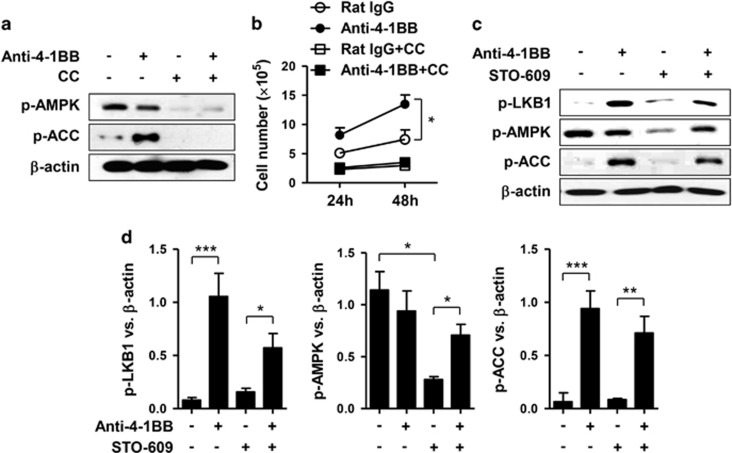
AMPK functions as a metabolic master switch that regulates energy homeostasis in cells, including cellular glucose uptake and β-oxidation of fatty acids,23, 24 while LKB1 is a direct activator of the phosphorylation of AMPK.25 Since AMPK promotes FAO through phosphorylation and subsequent inactivation of ACC (Figure 4a),26 we asked whether 4-1BB signaling induced the phosphorylation of LKB1, AMPK and ACC in CD8+ T cells. When anti-CD3-activated CD8+ T cells were stimulated with anti-4-1BB mAb, LKB1 phosphorylation was induced after about 2 h of anti-4-1BB treatment, and ACC phosphorylation after 24 h of anti-4-1BB treatment (Figure 4b). As for AMPK, its phosphorylation transiently decreased after 2–12 h of anti-4-1BB treatment, and then recovered to the original level after 24 h (Figure 4b). The phosphorylation of AMPK and ACC was confirmed by treating anti-CD3-activated CD8+ T cells with rat IgG or anti-4-1BB mAb for 24 or 48 h. 4-1BB signaling induced LKB1 phosphorylation, and sustained the phosphorylation of AMPK and ACC that had been transiently activated by CD3 stimulation (Figure 4c). Statistical analysis indicated that the phosphorylation of LKB1 and ACC increased significantly after 24 and 48 h of treatment with anti-4-1BB mAb, while phosphorylation of AMPK increased only after 48 h of treatment (Figure 4d).
Figure 4.
4-1BB signaling activates the LKB1–AMPK–ACC signaling pathway. (a) Signaling diagram for fatty acid oxidation through LKB1–AMPK–ACC. (b) CD8+ T cells activated with 0.1 μg/ml anti-CD3 mAb for 16 h were stimulated with 5 μg/ml anti-4-1BB mAb or rat IgG. The CD8+ T cells were harvested at the indicated time points and Western blots were performed with antibodies specific for phosphorylated (p-) LKB1, AMPK and ACC as well as for β-actin. (c and d) Anti-CD3-activated CD8+ T cells were stimulated with rat IgG or anti-4-1BB mAb for 24 or 48 h. Western blots were performed with antibodies specific for the indicated proteins (c). Relative expression levels were calculated by dividing the densitometer reading of each protein by the value of β-actin (d). Naïve indicates freshly isolated CD8+ T cells. Data representative are of three independent experiments. Results in c are means±s.d. (*P<0.05; **P<0.01). Abbreviation: ACC, acetyl-CoA carboxylase.
Collectively, these results indicate that anti-4-1BB treatment rapidly induces LKB1 phosphorylation and also activates the AMPK–ACC signaling pathway after a delay.
LKB1 primarily mediates the 4-1BB-dependent activation of AMPK-ACC signaling pathway
We next examined whether 4-1BB signaling required activation of the AMPK–ACC signaling pathway to enhance CD8+ T cell proliferation. Anti-CD3-activated CD8+ T cells were pre-treated with AMPK inhibitor compound C for 1 h, and further stimulated with rat IgG or anti-4-1BB mAb. Again, we found that phosphorylation of ACC increased in the CD8+ T cells after 24 h of anti-4-1BB treatment, but levels of AMPK phosphorylation were comparable with those in the rat IgG-treated CD8+ T cells (Figure 5a). Compound C completely inhibited the phosphorylation of AMPK and ACC, after both rat IgG and anti-4-1BB treatment (Figure 5a). Moreover, live cell counts showed that the enhanced proliferation of CD8+ T cells was completely suppressed by the AMPK inhibitor (Figure 5b).
Figure 5.
Calcium signaling-dependent and-independent activation of the LKB1–AMPK–ACC signaling pathway. (a and b) CD8+ T cells activated with anti-CD3 mAb for 16 h were pre-incubated with 7 μM AMPK inhibitor compound C for 1 h and further stimulated with 5 μg/ml anti-4-1BB mAb or rat IgG for 48 h. Western blots were performed with antibodies specific for phosphorylated AMPK and ACC as well as for β-actin (a). Live CD8+ T cells were counted using an ADAM MC Automated Mammalian Cell Counter 24 or 48 h after anti-4-1BB treatment (b). (c and d) Anti-CD3-activated CD8+ T cells were pre-incubated with 7 μM CaMMK inhibitor STO-609 for 1 h and further stimulated with 5 μg/ml anti-4-1BB mAb or rat IgG for 48 h. Western blots were performed with antibodies specific for phosphorylated LKB1, AMPK and ACC as well as for β-actin (c). Relative expression levels were calculated by dividing the densitometer reading of each protein by the value of β-actin (d). Data are representative of three independent experiments. Results in b and d are means±s.d. (*P<0.05; **P<0.01; ***P<0.001). Abbreviation: ACC, acetyl-CoA carboxylase.
It is noteworthy that phosphorylation of LKB1 and ACC was significantly increased after 24 h of anti-4-1BB treatment, whereas phosphorylation of AMPK was not (Figure 4c). AMPK activation can be induced by both LKB1 and Ca2+/calmodulin-activated protein kinase kinase (CaMKK).27, 28 Since the triggering of T cell receptors (TCRs), along with basic co-stimulation, typically induces Ca2+ and mitogenic signals,29 we thought that the AMPK phosphorylation might be induced by LKB1 and CaMKK. Therefore, we pre-treated anti-CD3-activated CD8+ T cells with the CaMKK inhibitor, STO-609 for 1 h, and further stimulated them with rat IgG or anti-4-1BB mAb for 48 h. Again, AMPK phosphorylation was comparable in the rat IgG- and anti-4-1BB-treated CD8+ T cells in the absence of STO-609, but in the presence of STO-609 it was higher in the anti-4-1BB-treated cells than in the rat IgG-treated cells (Figures 5c and d). Moreover, anti-4-1BB treatment still increased the phosphorylation of LKB1 and ACC in the presence of the CaMKK inhibitor (Figures 5c and d).
Since the CaMKK inhibitor decreased AMPK phosphorylation in the rat IgG-treated cells, but not in the anti-4-1BB-treated cells (Figures 5c and d), we conclude that 4-1BB stimulation of the LKB1–AMPK–ACC pathway is not dependent on a Ca2+ signal.
Lipid metabolism is involved in the 4-1BB-mediated regulation of anti-apoptosis and cell cycle of CD8+ T cells
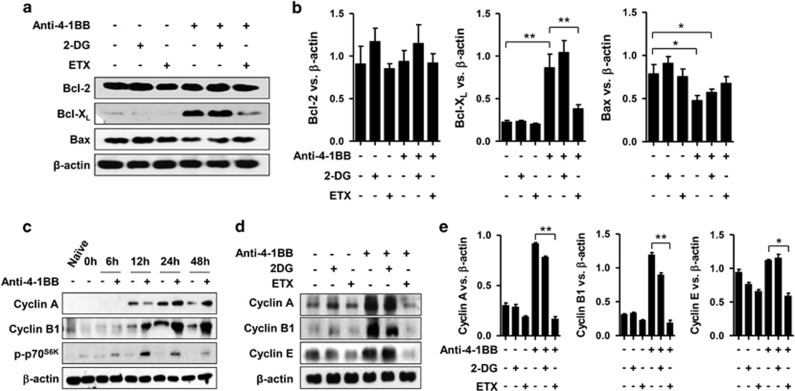
The well-defined effects of 4-1BB signals in activated CD8+ T cells include promoting cell cycle progression and preventing AICD.16, 17, 18 Therefore, we studied whether glucose and lipid metabolism were involved in enhancing anti-apoptosis and cell cycle progression stimulated by anti-4-1BB mAb. We first activated CD8+ T cells with anti-CD3 mAb for 16 h, treated the cells with 2-DG or ETX for 1 h, and further stimulated them with rat IgG or anti-4-1BB mAb for 24 h. Western blots for Bcl-2, Bcl-XL and Bax showed that anti-4-1BB treatment increased the anti-apoptotic molecule Bcl-XL, and decreased the pro-apoptotic molecule Bax (Figure 6a). However, Bcl-2 levels were not further increased by 4-1BB signaling, perhaps because Bcl-2 expression was already maximal in the anti-CD3-activated CD8+ T cells (Figure 6a). Bcl-2 expression was not significantly altered by 2-DG or ETX treatment (Figures 6a and b). The increased Bcl-XL and decreased Bax expression induced by anti-4-1BB treatment were not affected by 2-DG, but were inhibited by ETX treatment (Figures 6a and b). These results indicate that 4-1BB signaling requires lipid metabolism to enhance its anti-apoptotic effect in anti-CD3- activated CD8+ T cells.
Figure 6.
4-1BB signaling regulates apoptosis and the cell cycle of CD8+ T cells by enhancing glucose and lipid metabolism. CD8+ T cells were activated with 0.1 μg/ml anti-CD3 mAb for 16 h and further stimulated with 5 μg/ml anti-4-1BB mAb or rat IgG for 24 h. To block glucose and fatty acid metabolism, 2-DG and ETX, respectively, were incubated with the anti-CD3-activated CD8+ T cells 1 h before anti-4-1BB treatment. (a and b) Western blots were performed with antibodies specific for Bcl-2, Bcl-XL, Bax and β-actin after 24 h of anti-4-1BB treatment (a). Relative expression levels were calculated by dividing the densitometer reading of each protein by the value of β-actin (b). Rat IgG- or anti-4-1BB-treated CD8+ T cells were harvested at the indicated time points. Western blots were made with antibodies specific for cyclin A, cyclin B1, phosphor-p70S6K and β-actin (c). Anti-CD3-activated CD8+ T cells were stimulated with 5 μg/ml anti-4-1BB mAb or rat IgG for 24 h in the presence or absence of 2-DG or ETX. Western blots were made with antibodies specific for cyclin A, cyclin B1, cyclin E and for β-actin after 24 h of anti-4-1BB treatment (d). Relative expression levels were calculated by dividing the densitometer reading of each protein by the value of β-actin (e). Data representative are of three independent experiments. Results in b and e are means±s.d. (*P<0.05; **P<0.01). Abbreviations: 2-DG, 2-deoxy-D-glucose; ETX, etomoxir.
We also examined whether 2-DG or ETX were involved in the cell cycle progression of CD8+ T cells promoted by anti-4-1BB treatment. We first assessed cell cycle progression by treating activated CD8+ T cells with rat IgG or anti-4-1BB mAb for various times, and found that cyclin A (G2 phase), cyclin B1 (G2→M phase) and phospho-p70S6K (G1 phase) were increased after 12 or 24 h of anti-4-1BB treatment (Figure 6c). Therefore, we next examined whether glucose and lipid metabolism were involved in the 4-1BB-mediated cell cycle progression by treating the activated CD8+ T cells with 2-DG or ETX for 24 h. Western blots for cyclin A, B1 and E indicated that 4-1BB-mediated cell cycle progression was significantly inhibited by ETX, but not by 2-DG (Figures 6d and e).
Taken together, these results indicate that lipid metabolism is essential for 4-1BB signaling to promote the survival and proliferation of CD8+ T cells, while glucose metabolism is not.
Discussion
We have shown that 4-1BB signaling activates glucose and fatty acid metabolism and enhances CD8+ T cell proliferation by increasing GLUT1 expression and phosphorylating ACC via the LKB1–AMPK pathway (Figures 2e, 4 and 5). By treating the activated CD8+ T cells with 2-DG or ETX, we have obtained evidence that the glucose and fatty acid metabolism are required to support the proliferation of the anti-CD3-activated CD8+ T cells, as well as the enhanced proliferation of anti-4-1BB-treated CD8+ T cells (Figures 2a and 3a), and even the stimulation of OT-1 CD8+ T cells by OVA-pulsed 4-1BBL+/+ or 4-1BBL−/− dendritic cells (Supplementary Figure 1). Conversely, blocking fatty acid metabolism, but not glucose metabolism, abolished two major effects of 4-1BB signaling on anti-CD3-activated CD8+ T cells: increasing their survival and accelerating their cell cycle progression. These results indicate that 4-1BB signaling modulates at least three biological properties of CD8+ T cells—survival, cell cycle, and metabolism—and, probably by doing so, enhances their proliferation.
Upon activation, naïve T cells go through radical metabolic changes, switching from oxidation of fatty acids to glycolytic metabolism, along with increasing cell size.30, 31 Proliferating T cells, which are probably T cells undergoing clonal expansion, exhibit a high rate of glycolysis even in the presence of an adequate supply of oxygen, while naïve and memory T cells use FAO as their primary source of energy.7, 8, 9, 10 The formation of memory CD8+ T cells is increased by forcing FAO by overexpressing CPT1a12 and activating AMPK with metformin,13 or by moderating inhibition of glycolysis with low dose of 2-DG.15 Anti-4-1BB treatment activated both glucose and fatty acid metabolism in CD8+ T cells (Figures 2 and 4) and increased the size of the cells (Figure 1), and it is known to increase granzyme B and perforin expression.32 Therefore, 4-1BB signaling seems to enhance the characteristics of effector CD8+ T cells by increasing glycolysis, cell size and effector molecules, as well as raising the memory potential of the cells by increasing FAO through activation of the LKB1–AMPK–ACC pathway. However, it is still unclear whether 4-1BB signaling promotes both glucose and fatty acid metabolism in all CD8+ T cells, or enhances each metabolic pathway in separate subpopulations of CD8+ T cells.
A recent report proposed that mitochondrial mass is not equally distributed between the daughter cells when CD8+ T cells divide, and CD8+ T cells which possess greater mitochondrial mass have more chances to become memory T cells.12 If anti-4-1BB treatment increased the size and granularity of CD8+ T cells without any increase in mitochondrial mass, effector T cells might have been the dominant cell type generated from the CD8+ T cells. It is possible that anti-4-1BB treatment increased the mitochondrial mass of CD8+ T cells along with increasing cell size, so leading to more rapid generation of effector and memory T cells through asymmetric division. We suspect that anti-4-1BB treatment would maximize the formation of memory CD8+ T cells when combined with IL-15, a cytokine critical for CD8+ memory T cells that promotes mitochondrial biogenesis and expression of CPT1a.12 Many reports suggest that less-differentiated T cells are required to successfully treat cancer patients,33, 34 and that these can be produced by modulating the metabolic properties of T cells using 2-DG, metformin or rapamycin in vitro.15, 35 To produce such less-differentiated T cells, it seems that their glycolysis needs to be suppressed or their fatty acid metabolism enhanced.35 Since 4-1BB signaling activates both glucose and fatty acid metabolism, it should be possible to use 4-1BB-mediated metabolic modulation to produce less-differentiated T cells for adoptive T cell therapy.
We found that the response of AMPK to anti-4-1BB treatment was delayed, unlike that of LKB1 (Figure 4b). Phosphorylation of AMPK is typically induced by LKB1 and calcium signals. Stimulation of T cells through the CD3 complex not only activates the mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinases (ERK) pathway but also induce Ca2+ influx.36 Therefore, the anti-CD3-mediated Ca2+ signal seems to induce phosphorylation of AMPK before anti-4-1BB triggering does. Given that ATP decreases the phosphorylation of AMPK,37 it appears that 4-1BB signaling not only induces LKB1 phosphorylation but also somehow transiently enhances ATP production by the CD8+ T cells and thus delays the activation of the AMPK–ACC pathway. The LKB1–AMPK pathway was transiently activated by TCR/CD3 in CD8+ T cells after a delay, and it was further stimulated by 4-1BB signaling (Figure 4c). mTOR is one of the major downstream signaling pathways regulated by AMPK. It is inhibited by AMPK activation through phosphorylation of tuberous sclerosis complex (TCS)1/2 or raptor.38, 39, 40 4-1BB signaling not only activates the LKB1–AMPK signaling pathway (Figures 4b and c), but also induces mTOR signaling in CD8+ T cells via IL-2-dependent activation of the PI3K/AKT pathway.16 Since AMPK and AKT compete to inhibit and activate mTOR signaling, respectively, anti-4-1BB treatment may skew AMPK signaling by some unknown mechanism so as to activate ATP-producing pathways without inhibiting mTOR signaling and thus maximize the proliferation of CD8+ T cells.
Since 4-1BB triggering using anti-4-1BB mAb activated glucose and fatty acid metabolism after a delay, we wondered whether such an indirect effect of 4-1BB signaling might be mediated by some intervening molecules. IL-2 not only plays a crucial role in enhancing CD8+ T cell proliferation but also activates glycolytic transcriptional program.41 4-1BB triggering typically promotes the production of IL-2 from activated CD8+ T cells.19 Therefore, it is possible that 4-1BB triggering enhances the glucose metabolism by increasing IL-2 production. However, when CD8+ T cells were stimulated with high dose of anti-CD3 mAb (>1 μg/ml), anti-CD3 triggering alone was sufficient to increase GLUT1 expression in CD8+ T cells (data not shown). In general, 4-1BB triggering results in the rapid acidification of culture medium and the activation of CD8+ T cells with high dose of anti-CD3 mAb also swiftly acidify the medium. Given that lactate production through aerobic glycolysis, which is known as the ‘Warburg effect’, may be the primary cause of such acidification, it is not clear whether the increase of GLUT1 expression in CD8+ T cells was due to the intervening molecule like IL-2 or merely the decrease of glucose in culture medium. When rat IgG- or anti-4-1BB-treated CD8+ T cells were cultured in RPMI10 medium supplemented with 2, 4 or 6 g/l glucose, glucose dose-dependently increased GLUT1 expression in the anti-4-1BB-treated CD8+ T cells but hardly at all in the rat IgG-treated CD8+ T cells (Supplementary Figure 2), while HK2 expression was not significantly altered by the increase of glucose (Supplementary Figure 2). Moreover, blockade of IL-2/IL-2R signaling using anti-CD25 mAb minimally affected GLUT1 expression in the rat IgG- and anti-4-1BB-treated CD8+ T cells (data not shown). These results suggest that the increased GLUT1 expression is not simply due to the decrease of glucose level, and that IL-2 may not be the major factor responsible for inducing GLUT1.
In conclusion, our findings suggest that 4-1BB signaling enhances the proliferation of CD8+ T cells by activating metabolic pathways in order to satisfy increasing demands for energy and biomass. These observations may serve as stepping stones to further elucidating the roles of 4-1BB signaling in immune cell metabolism. In addition, costimulatory molecules play crucial roles in the proliferation and differentiation of T cells and thus in determining their fates. Since the metabolic changes are likely to be essential for the proliferation of T cells and all the differentiation pathways taken by these cells—although employed differently to control the different pathways—we suspect that all costimulatory molecules will be found to be linked to metabolic pathways.
Acknowledgments
This work was funded by grants from the National Cancer Center of Korea (NCC-1310430), the National Research Foundation of Korea (NRF-2005-0093837, NRF-2013R1A1A2008703), the Korea Drug Development Fund (KDDF-201408-11) and the Ministry of Trade, Industry and Energy of Korea (GLOBAL R&D PROJECT, N0000901).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Hurtado JC, Kim SH, Pollok KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J Immunol 1995; 155: 3360–3367. [PubMed] [Google Scholar]
- Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol 2004; 75: 785–791. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell Mol Immunol 2011; 8: 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229: 192–215. [DOI] [PubMed] [Google Scholar]
- Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol 2005; 174: 6803–6808. [DOI] [PubMed] [Google Scholar]
- Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol 2002; 168: 5483–5490. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 2012; 249: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol 2012; 33: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol 2012; 13: 907–915. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham CM, Driessens G, O'Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol 2008; 38: 2438–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012; 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS et al. Enhancing CD8 T-Cell memory by modulating fatty acid metabolism. Nature 2009; 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF et al. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 2013; 123: 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Nam KO, Park SJ, Kwon BS. 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur J Immunol 2003; 33: 2133–2141. [DOI] [PubMed] [Google Scholar]
- Lee HW, Nam KO, Seo SK, Kim YH, Kang H, Kwon BS. 4-1BB cross-linking enhances the survival and cell cycle progression of CD4 T lymphocytes. Cell Immunol 2003; 223: 143–150. [DOI] [PubMed] [Google Scholar]
- Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol 1997; 158: 2600–2609. [PubMed] [Google Scholar]
- Oh HS, Choi BK, Kim YH, Lee DG, Hwang S, Lee MJ et al. 4-1BB signaling enhances primary and secondary population expansion of CD8+ T cells by maximizing autocrine IL-2/IL-2 receptor signaling. PLoS One 2015; 10: e0126765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol 2011; 187: 4187–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler HA, Lupas D, Tan Y, Dai J, Kelzer MS, Martin GG et al. Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyl transferase I. Mol Cell Biochem 2011; 355: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep 2011; 1: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Grossman AB, Korbonits M. The role of AMP-activated protein kinase in obesity. Front Horm Res 2008; 36: 198–211. [DOI] [PubMed] [Google Scholar]
- O'Neill HM. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab J 2013; 37: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 2009; 9: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001; 291: 2613–2616. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005; 2: 9–19. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR et al. Ca2þ/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005; 2: 21–33. [DOI] [PubMed] [Google Scholar]
- Premack BA, Gardner P. Signal transduction by T-cell receptors: mobilization of Ca and regulation of Ca-dependent effector molecules. Am J Physiol 1992; 263: C1119–C1140. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell 2000; 6: 683–692. [DOI] [PubMed] [Google Scholar]
- Krauss S, Brand MD, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity 2001; 15: 497–502. [DOI] [PubMed] [Google Scholar]
- Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One 2010; 5: e11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki O, Hovav E, Ziporen Y, Levy D, Kubi A, Zikich D et al. Establishment and large-scale expansion of minimally cultured "young" tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother 2011; 34: 212–220. [DOI] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015; 21: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med 2009; 1: 11ps12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C, Thomas ML. Recent advances in lymphocyte signaling and regulation. Front Biosci 1997; 2: d207–d221. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011; 472: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 2004; 18: 1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 2004; 6: 91–99. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011; 13: 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol 2014; 15: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.