In this issue of Nature Immunology, Choi and colleagues1 identify a key function of the T cell-specific protein thymocyte-expressed molecule involved in selection (THEMIS) in T cell development. They demonstrate that the two structural CABIT (cysteine-containing all beta in THEMIS) modules contained in this protein are able to directly bind to the phosphotyrosine-phosphatase (PTP) domain of SHP-1 (Src homology region 2 domain-containing phosphatase-1) and inhibit its phosphatase activity by increasing the susceptibility of its catalytic site to oxidation. By keeping SHP-1 under control, THEMIS enhances the T cell receptor signaling response to low-affinity self-peptides (self-p) presented as a complex with the major histocompatibility complex (MHC) by stromal cells of the thymus. These findings highlight a key role for THEMIS in the positive selection of thymocytes.
T cells originate from hematopoietic stem cells in the bone marrow as lymphoid progenitors, which then migrate to populate the thymus, where they expand to generate a large population of immature thymocytes.2 A series of steps then induces the differentiation of thymocytes to mature T cells.3 These steps are defined by the expression of the co-receptors CD4 and CD8, with thymocytes transiting from the CD4−/CD8− double negative stage to the CD4+/CD8+ double positive (DP) stage and finally to the CD4+ or CD8+ single positive (SP) stage.3 This complex developmental program is regulated by signals triggered by immature or mature forms of the T cell receptor. An interesting paradox is that TCR-driven responses elicit two diametrically opposed outcomes on DP thymocytes, that is, negative selection and subsequent death by apoptosis if they express TCRs that fail to bind or that bind with high affinity to self-p/MHCs on thymic antigen-presenting cells, and positive selection, which allows them to proceed along the differentiation pathway if their TCRs bind with low-affinity to self-p/MHCs.4 Hence, the strength of TCR binding to self-p/MHCs during the selection process controls the intensity and the duration of the TCR-driven signaling response, ultimately leading to the differential activation of downstream signaling pathways and transcriptional responses that dictate cell fate.4, 5 The intriguing question is how the signaling machinery of differentiating thymocytes interprets different TCR signal intensities and durations, responding with distinct gene expression patterns. Thymocytes at the DP stage are more responsive to low-affinity peptides compared with mature T cells, supporting the notion that their TCRs are endowed with a higher sensitivity to self-p/MHCs compared with TCRs that are expressed by mature T cells.6
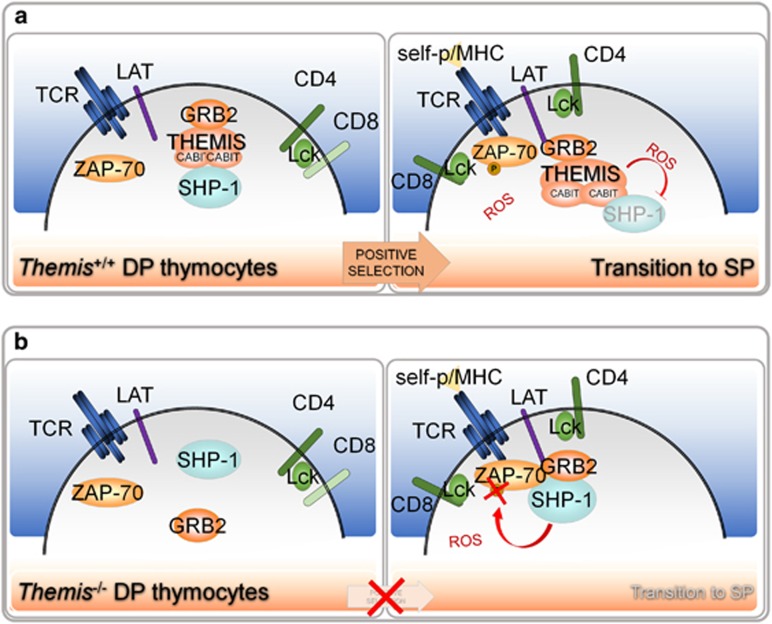
A report by Choi and colleagues provides new evidence of the involvement of THEMIS, an adapter molecule that is selectively expressed in the T cell lineage, particularly at the DP stage of T cell development, in the enhanced sensitivity of preselection thymocytes to self-p/MHCs. The unique domain structure of THEMIS makes it an interesting candidate for this function. THEMIS consists of two CABIT globular tandem modules of unknown function and a C-terminal proline-rich sequence that mediates its binding to the molecular adapter growth factor receptor-bound 2 and its subsequent recruitment to the adapter LAT (linker for activation of T cells)7 (Figure 1a). Once bound to LAT, THEMIS becomes closely associated with both ZAP-70 (zeta chain of T cell receptor-associated protein kinase 70 kDa) and Lck (lymphocyte-specific protein tyrosine kinase), which are responsible for its tyrosine phosphorylation.7 Using a Themis−/− mouse, the authors corroborate the literature by showing that, despite a normal thymocyte maturation up to the DP stage, Themis−/− mice have markedly reduced numbers of SP thymocytes, paralleled by the accumulation of DP thymocytes and low numbers of mature T cells in the periphery. This demonstrates that thymocyte development is blocked at the DP-to-SP transition1, 8, 9 and suggests a positive role for THEMIS in TCR-dependent signaling. However, studies of the TCR signaling cascade in THEMIS-deficient thymocytes failed to reveal any clear signaling defects after stimulation of the TCR by antibody crosslinking.8, 9, 10
Figure 1.
Expression of THEMIS in preselection thymocytes promotes TCR-dependent signaling by attenuating the phosphatase activity of SHP-1. (a) Binding of self-p/MHCs to low-affinity TCRs expressed on the surface of CD4+/CD8+ DP thymocytes leads to the recruitment to phosphorylated LAT to a multimolecular complex containing THEMIS, growth factor receptor-bound 2 and SHP-1. THEMIS inhibits SHP-1 activity by increasing its susceptibility to ROS produced following T-cell activation, leading to the stage-specific enhancement of TCR signaling and allowing thymocytes to proceed along the differentiation process. (b) In stimulated Themis−/− thymocytes, SHP-1 recruited to LAT is in its active state. The subsequent dephosphorylation of initiating kinases ZAP-70 and Lck dampens TCR signaling, impairing positive selection.
To find interactors that could help THEMIS in the modulation of TCR signaling, the authors used a mass-spectrometry approach that resulted in the identification of the tyrosine phosphatase SHP-1, a known negative regulator of phosphotyrosine-based signaling cascades that had been suggested as a mediator of THEMIS functions in developing T cells.8, 10 Choi and colleagues added several tiles to this puzzle by generating various truncated versions of both THEMIS and SHP-1, which they used in pull-down assays and immunoprecipitation experiments on HEK-293 transfectants. They found that the PTP domain of SHP-1 directly interacts with the CABIT modules of THEMIS, an interaction that seems to be potentiated by growth factor receptor-bound 2.
In contrast to other reports, in which THEMIS has mainly been proposed as a recruiter and activator of SHP-1,8, 10 the authors demonstrate that the interaction of THEMIS with SHP-1 elicits a direct inhibitory effect on the phosphatase activity of SHP-1. Using an in vitro phosphatase assay, they indeed showed that the PTP activity of SHP-1 decreases in the presence of THEMIS and mapped this function to the CABIT modules. Hence, THEMIS directly neutralizes one of the most important negative regulators of proximal TCR signaling.
To address the outcome of this function on thymocyte development, the authors set up an in vitro differentiation assay that reproduces the initial stages of positive selection in vivo, testing immature DP thymocytes isolated either from Themis−/−Ptpn6fl/flCD4-Cre mice, in which a T cell-specific deletion of SHP-1 occurs mainly at the DP stage, or from their Themis−/−Ptpn6+/+CD4-Cre counterparts, which express normal levels of SHP-1. They found that the defect in thymocyte development that is caused by the lack of THEMIS in Themis−/−Ptpn6fl/flCD4-Cre mouse thymocytes is corrected by the loss of SHP-1 expression. Interestingly, the defect of Themis−/− DP thymocytes was also reversed when the in vitro differentiation was performed in the presence of sodium stibogluconate, a selective inhibitor of SHP-1 activity, formally demonstrating that the developmental defect of Themis−/− thymocytes is due to the enhanced PTP activity of SHP-1 (Figure 1).
As with other PTPs, SHP-1 is inhibited by the oxidation of a critical cysteine residue in its active site.6 Using a monoclonal antibody that recognizes the oxidized cysteine, the authors observed that the SHP-1 oxidation that is induced by the phosphatase inhibitor pervanadate or by the oxidant H2O2 is enhanced in vitro in the presence of THEMIS. However, by labeling catalytically active SHP-1 with a specific probe, they found that SHP-1 is much less susceptible to oxidation by pervanadate in Themis−/− than in wild-type thymocytes. Hence, THEMIS acts by directly regulating the redox state of SHP-1.
Of note, phosphorylation of SHP-1 on tyrosine residues is not regulated by the same pathway that modulates its catalytic activity. SHP-1 phosphorylation was indeed lower in Themis−/− thymocytes than in wild-type thymocytes, confirming previous reports that the phosphorylation of SHP-1 is not required for its PTP catalytic activity.10 Nonetheless, the treatment of thymocytes with pervanadate or H2O2 increases SHP-1 phosphorylation, leading the authors to conclude that active SHP-1 in Themis−/− thymocytes dephosphorylates itself.11
An important point to be resolved is whether the inhibitory activity of THEMIS on SHP-1 potentiates or inhibits TCR-dependent signaling. To study the signaling pathway elicited by TCR stimulation, the authors reproduced in vitro the stimulatory environment to which preselection thymocytes are subjected, reasoning that, while ROS are generated in mature T cells following TCR activation, this ROS generation does not occur when thymocytes are stimulated via the TCR.12 Hence, the authors stimulated the TCR of Themis−/− or wild-type thymocytes in the presence of the oxidant H2O2 or, alternatively, used the lectin concanavalin A, which engages multiple cell-surface molecules in addition to the TCR, increasing the intracellular ROS concentration.12 Under these conditions, the phosphorylation of key components of the TCR signaling pathway, such as ZAP-70 and Lck, was found to be dramatically decreased in the absence of THEMIS, indicating that THEMIS plays a central role in proximal TCR signaling by promoting the ROS-mediated oxidation of SHP-1 (Figure 1).
What now remains to be clarified is how the two CABIT modules of THEMIS promote SHP-1 oxidation. According to the two interesting hypotheses suggested by the authors, binding of the CABIT modules of THEMIS to the PTP domain of SHP-1 might (i) prevent reducing agents from reaching the oxidized catalytic cysteine of SHP-1 and (ii) stabilize the PTP domain of SHP-1 in an unfolded state, which would expose the catalytic cysteine to oxidation by ROS. These alternative scenarios now need to be addressed experimentally.
The expression levels of THEMIS increase during the maturation to DP thymocytes while decreasing during the subsequent DP-to-SP step.8 On the basis of the results reported by Choi and colleagues, the stage-specific expression of THEMIS may represent a mechanism for the transient and selective attenuation of SHP-1 activity, which determines the unusual sensitivity of DP thymocytes to TCR stimulation essential for positive selection. Interestingly, the origin of ROS in the microenvironment of the thymus is not known. ROS could be physiologically generated within thymocytes or acquired from extrinsic sources, such as thymic epithelial cells or other cellular components of the thymic stroma. What is clear is that ROS are not produced in response to TCR stimulation,12 at least at the DP stage, suggesting an additional role for the thymic stroma in assisting the process of positive selection.
Footnotes
The authors declare no conflict of interest.
References
- Choi S, Warzecha C, Zvezdova E, Lee J, Argenty J, Lesourne R et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol 2017; 18: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev 2006; 209: 47–57. [DOI] [PubMed] [Google Scholar]
- Gascoigne NRJ, Palmer E. Signaling in thymic selection. Curr Opin Immunol 2011; 23: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol 2012; 30: 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AJ. Theories and quantification of thymic selection. Front Immunol 2014; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med 1998; 188: 1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster W, Brockmeyer C, Fu G, Simister PC, de Wet B, Martinez-Riaño A et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J Immunol 2013; 190: 3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne NR, Acuto O. THEMIS: a critical TCR signal regulator for ligand discrimination. Curr Opin Immunol 2015; 33: 86–92. [DOI] [PubMed] [Google Scholar]
- Lesourne R, Uehara S, Lee J, Song K, Li L, Pinkhasov J et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol 2009; 10: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Vallée S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013; 504: 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol 2007; 25: 473–523. [DOI] [PubMed] [Google Scholar]
- Pani G, Colavitti R, Borrello S, Galeotti T. Endogenous oxygen radicals modulate protein tyrosine phosphorylation and JNK-1 activation in lectin-stimulated thymocytes. Biochem J 2000; 347: 173–181. [PMC free article] [PubMed] [Google Scholar]