Type 1 regulatory T (Tr1) cells are Foxp3-negative regulatory CD4+ T cells that develop outside of the thymus and mediate regulatory functions in large part by producing the immunosuppressive cytokine IL-10. They have been shown to play an important role in controlling unwanted immune reactions driven by effector CD4+ and CD8+ T cells, such as those in certain autoimmune diseases and graft-versus-host disease (reviewed in1). These promising results have prompted scientists to test the immunomodulatory potential of these cells in clinical settings by adoptively transferring them into patients who receive hematopoietic stem cells.2 However, although Tr1 cells were first described several decades ago and represent a powerful tool for cell therapies, it is still not resolved whether they represent a distinct T-helper subset or lineage and how they develop. Tr1 cells, at least in vitro, can be induced by several cytokines. In addition to IL-10 itself,3 type 1 interferons4, 5 and IL-276, 7, 8 have been described as potent Tr1 cell inducers. Thus, Tr1 cells may represent another example of apparent T-helper cell plasticity, which may contrast with the existence of a ‘master switch’ TF that regulates their differentiation. Regardless of the Tr1 cell priming cytokine, they all induce IL-10 release, which points to a common or similar transcriptional network that controls their differentiation and remains to be elucidated.
In a recent publication in Nature Immunology, Karwacz et al.,9 sought to identify ‘pioneering factors’ that are induced upon IL-27 stimulation and shape the epigenetic landscape to increase chromatin accessibility and allow the recruitment of additional transcription factors (TFs) during Tr1 cell differentiation.9
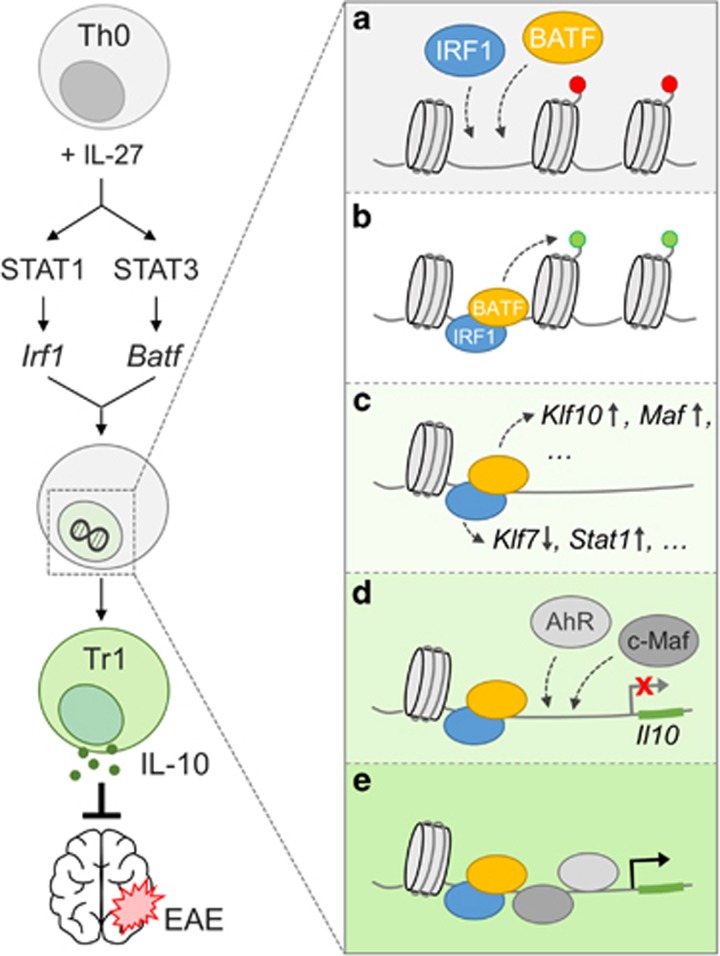
By performing global gene expression analyses, they could show that the expression of two known TFs, IRF1 and BATF, could be rapidly induced during the IL-27-mediated differentiation of naive murine CD4+ T cells into Tr1 cells. Furthermore, both TFs are indispensable for Tr1 cell differentiation and IL-10 production. It is known that BATF-dependent regulation of chromatin accessibility is important for T-helper 17 (Th17) and effector CD8+ T cell differentiation, but in those processes BATF cooperates with IRF4 instead of IRF1.10 IL-27 signaling involves the phosphorylation and activation of both STAT1 and STAT3. Using STAT1- and STAT3-deficient cells, they established that STAT1 controls IRF1 induction, whereas STAT3 is required for BATF expression upon IL-27 stimulation. IRF1 appears to directly control Il10 expression, and thus Tr1 cell functionality, as IRF1 overexpression enhances Il10 transcription in Tr1 cells. This was not observed for BATF overexpression, which instead enforced the transcription of Il21 and Maf, in accord with previous findings.11, 12 Indeed, IRF1 plays a major role in driving regulatory immune responses as IRF1-deficient mice show exacerbated disease in MOG peptide-induced experimental autoimmune encephalomyelitis (EAE). This was accompanied by increased IL-17A and IFN-γ production by MOG-reactive T cells. By contrast, BATF-deficient mice are resistant to EAE induction and do not develop an antigen-reactive Th17 cell signature, which is also consistent with its role in Th17 cell differentiation.11 Karwacz et al. also report that IRF1 deficiency is associated with locally decreased Il10 mRNA expression in the central nervous system (CNS) and draining lymph nodes during disease resolution. Notably, in the CNS the differences are only minor trends that do not reach significance. In addition, it remains to be determined whether the differences in Il10 transcription are indeed due to differences in Tr1 cell differentiation because no intracellular IL-10 staining in T cells was performed. However, IRF1 and BATF seem to be important for the differentiation of functional Tr1 cells because the transfer of in vitro differentiated cells from either Irf1−/− or Batf−/− mice cannot ameliorate EAE, which is in contrast to Tr1 cells from wild-type mice. While Batf−/− Tr1 cells showed no regulatory activity at all, the transfer of Irf1−/− Tr1 cells even induced disease exacerbation. The authors then showed that abrogation of Tr1 cell functionality is most likely a consequence of an altered transcription profile in Irf1−/− and Batf−/− Tr1 cells. In subsequent experiments, the authors investigated how IRF1 and BATF control the Il10 locus and transcription in Tr1 cells. Chromatin immunoprecipitation experiments revealed the direct binding of IRF1 and BATF to conserved non-coding sequences (CNS) and hypersensitivity (HSS) sites in the Il10 locus. Further analyses showed that the binding sites for the two TFs overlap and co-occupy two particular sites: one HSS and one CNS. The binding of IRF1 and BATF to the CNS and HSS sites induced epigenetic changes within the Il10 locus, as knockout cells exhibited reduced recruitment of activating (for example, H3K9Ac) and increased repressive (H3K27me3) histone marks. However, IRF1 and BATF differentially affected the Il10 promoter because only IRF1 could directly transactivate the Il10 promoter in luciferase reporter assays, which could be further enhanced by c-Maf. The expression of c-Maf is controlled by BATF. Furthermore, AhR exhibited diminished binding to the XRE1 binding site within the Il10 locus in both IRF1- and BATF-deficient cells. Consequently, the authors assessed chromatin accessibility using assays for transposase accessible chromatin-sequencing in BATF-, IRF1-, c-Maf- and AhR-deficient Th0 and Tr1 cells. BATF deficiency caused the most dramatic and distinct effect on chromatin accessibility at 72 h of Tr1 cell differentiation. Furthermore, the authors state that deficiency in the other TFs revealed a similar pattern of chromatin landscape changes. However, it became apparent that IRF1 deficiency also induces alterations, although they are less pronounced when compared to wild-type and c-Maf- or AhR-deficient cells. This finding was further supported by the quantification of TF-dependent accessible peaks per transcription factor, which revealed increased numbers in IRF1- and, especially, BATF-deficient cells. Finally, as IRF1- and BATF-deficient cells displayed unique chromatin accessibility under Tr1 cell conditions, the authors investigated the transcriptional program of these cells. In accordance with the previously described findings, BATF deficiency caused a more dramatic and distinct alteration of the transcription profile compared to IRF1 deficiency. IRF1 negatively controls the expression of Klf7 and positively regulates the expression of Stat1, Il10, Ccr5 and Prf1 (perforin). By contrast, the BATF-dependent Tr1 cell network appears to be more complex and regulates the transcription of genes such as Klf10, Runx2, Prdm1 and Hif1a.
Altogether, although they do not function as Tr1 cell ‘master switch’ TFs, IRF1 and, especially, BATF seem to carry out ‘pioneering’ functions on the Il10 locus by inducing the expression of TFs and triggering chromatin remodeling that is necessary for further TF binding, Il10 transcription and, ultimately, Tr1 cell differentiation (Figure 1).
Figure 1.
Roles of the pioneering factors IRF1 and BATF during IL-27-mediated Tr1 cell differentiation. Addition of IL-27 during the activation of naive CD4+ T cells induces IRF1 and BATF transcription in a STAT1- and STAT3-dependent manner. Both TFs are required for IL-10 production, Tr1 cell differentiation and in vivo functionality. The simultaneous binding of IRF1 and BATF to specific conserved non-coding sequences and hypersensitivity sites of the Il10 locus (a) induces epigenetic changes (b) and chromatin remodeling. In addition, by modifying the expression of additional TFs such as Klf7, Klf10 and Maf, IRF1 and BATF further support Tr1 cell differentiation (c). Finally, binding of c-Maf and AhR to the accessible promoter (d) induces Il10 transcription during Tr1 cell differentiation (e), which is protective in settings such as EAE. TFs, transcription factors; Tr1, type 1 regulatory cells; EAE, experimental autoimmune encephalomyelitis.
Since the first description of Tr1 cells by the group of Roncarolo in 1997,3 it has become evident that peripherally induced IL-10-producing Tr1 cells play an important role in regulating immune responses. However, it has remained unclear how the differentiation of these cells and expression of IL-10 is controlled. The study by Karwacz et al. has provided important novel findings on the role of two ‘pioneering TFs’, BATF and IRF1, in controlling Il10 transcription and Tr1 cell in vivo functionality. It remains to be investigated whether these roles are specific to the IL-27-mediated Tr1 cell differentiation of murine T cells or whether they represent a general prerequisite for IL-10 induction in Tr1 cells.
Footnotes
The authors declare no conflict of interest.
References
- Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015; 12: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Lucarelli B, Sartirana C, Gregori S, Lupo Stanghellini MT, Miqueu P et al. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front Immunol 2014; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389: 737–742. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1T regulatory cells. J Immunol 2001; 166: 5530–5539. [DOI] [PubMed] [Google Scholar]
- McRae BL, Semnani RT, Hayes MP, van Seventer GA. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J Immunol 1998; 160: 4298–4304. [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 2007; 8: 1380–1389. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 2007; 179: 3268–3275. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F et al. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett 2011; 136: 21–28. [DOI] [PubMed] [Google Scholar]
- Karwacz K, Miraldi ER, Pokrovskii M, Madi A, Yosef N, Wortman I et al. Critical role of IRF1 and BATF in forming chromatin landscape during type 1 regulatory cell differentiation. Nat Immunol 2017; 18: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F et al. A validated regulatory network for Th17 cell specification. Cell 2012; 151: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature 2009; 460: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol 2011; 12: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]