γδ T cells are often placed at the interface between innate and adaptive immunity. These cells share T-cell receptor (TCR) rearrangements and memory functions1 in common with their αβ T-cell counterparts but differ in terms of their response kinetics and mechanisms of target recognition (Figure 1). Indeed, γδ T cells provide fast responses against infected or transformed cells in a major histocompatibility complex-independent manner, thus participating in the first line of defense, which gives the organism time to mount antigen-specific αβ T-cell responses.2 Although the four TCR loci were discovered and characterized almost simultaneously,3 our knowledge of the mechanisms underlying γδ T-cell responses remains insufficient. However, an increasing amount of evidence has demonstrated that γδ T cells recognize self-antigens on the surface of target cells; the expression of these self-antigens is known or expected to increase upon stress, infection or transformation in a TCR-dependent manner, making them an attractive source for cell-based immunotherapies.4 This response is noted in the case of BTN3A associated with phosphoantigens,5 lipid-presenting CD1 molecules,6 endotelial protein C receptor7 and Annexin A2.8 However, these molecules constitute only a small fraction of the ligands recognized by γδ T cells. In addition, the mechanism by which the γδ TCR repertoire is shaped under physiological conditions and how (much) it changes in response to pathogenic challenge remain poorly understood.
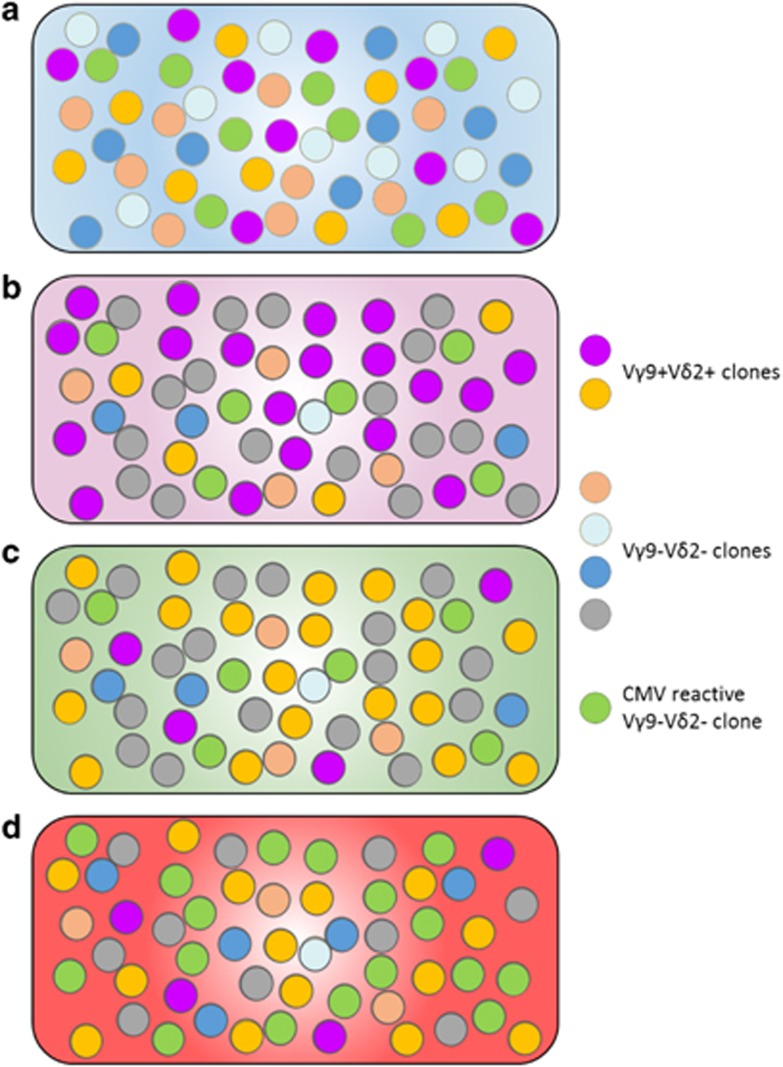
Figure 1.
Graphical representation of the dynamics of the human γδ T-cell repertoire. (a) Prenatal generation of γδ T cells is unfocused and unbiased. At birth, the human γδ repertoire in blood is variable, exhibiting Vγ2, Vγ3, Vγ4, Vγ5, Vδ1, Vδ3 and Vδ5 chain usage and no bias toward Vγ9 and Vδ2. (b) Up to 40% of the adult repertoire is composed of the 20 most abundant clones. The focus toward the Vγ9Vδ2 chain rearrangement (purple circles) is driven by postnatal proliferation in response to pathogen- or tumor-derived phosphoantigens. (c) AlloHSCT strongly perturbs the adult γδ repertoire. However, 60 days after alloHSCT, the repertoire is fully reconstituted and qualitatively comparable to the host repertoires before transplantation but displays very different clonotypes compared with the donor. (d) CMV reactivation occurs 25–60 days after alloHSCT. The γδ T-cell repertoire recovery observed in alloHSCT is perturbed by the proliferation of a few, mainly Vγ9–Vδ2, clones that comprise 20–75% of the repertoire.
In a recent issue of Nature Immunology,9 Ravens and colleagues analyzed the largest available cohort of γδ T-cell repertoires, including more than 2 × 107 rearranged TCR sequences. This prospective longitudinal cohort study was possible thanks to recent technical advances in the comprehensive analysis of TCR repertoires using next-generation sequencing. Moreover, this study was designed to monitor the regeneration of T cell receptor gamma and delta repertoires in allogeneic hematopoietic stem cell transplantation (alloHSCT) patients over 180 days. The study also included not only blood samples from alloHSCT patients who experienced cytomegalovirus (CMV) reactivation, which is a major complication of transplantation associated with γδ T-cell expansion,10 but also cord blood samples as important controls to provide information on the ontogeny and dynamics of the γδ T-cell repertoire.
The newly published data confirmed that the human adult blood γδ TCR repertoire is dominated by the usage of Vγ9 and Vδ2 TCR chains with high prevalence of the Vγ9–Jγ1.2 Vδ2 rearrangement.11 However, the cord blood control group exhibited a less pronounced bias toward Vγ9 and Vδ2 and an increase in Vγ2, Vγ3, Vγ4, Vγ5, Vδ1, Vδ3 and Vδ5 chain usage, confirming the previously reported preponderance of Vγ9-negative γδ T cells in neonates.1 This finding indicates that the bias toward Vγ9Vδ2 rearrangements is driven by postnatal proliferation likely in response to pathogen-derived phosphoantigens, which are well established and specific agonists of Vγ9Vδ2 TCR.12
Consistent with these findings, the 20 most abundant clones of the analyzed adult samples constituted ~40% of the repertoire. Conversely, the 20 most abundant clones in cord blood represented only ~10–20% of the entire repertoire. In addition to the reduction in diversity observed with age, Raven and colleagues pinpointed clear differences between the γ and δ chain repertoires. Greater clonal diversity was observed in rearranged T cell receptor delta genes in both cord blood and adult samples. Moreover, unrelated donors (cord blood or adult) shared TCRγ chain sequences (interestingly only Vγ9JP sequences in adults), making them ‘public’. By contrast, TCRδ chain repertoires were mostly ‘private’, especially in the Vδ2− T-cell compartment. These results are consistent with the long, variable lengths of TCRδ CDR3 compared with the short, constrained TCRγ CDR3, in addition to the presence of three D gene segments in the TCRδ locus that are absent from the TCRγ locus.13 Beyond these insights into the γδ TCR repertoire ontogeny, Ravens and colleagues also documented that established adult γδ T-cell repertoires are stable over time (within a window of 90 days) if no major immunological events, such as alloHSCT or CMV reactivation, occur. Strikingly, even after alloHSCT, stable γδ T-cell repertoires were rapidly reconstituted between 30 and 60 days after transplantation in the absence of CMV reactivation. The new repertoires after transplantation were qualitatively comparable to the host repertoires before transplantation but displayed very different clonotypes compared with both the donor and host repertoires, indicating that the new repertoires were successfully generated from the donor stem cells and differentiated de novo in the host thymus. Whether the γδ T-cell population either recognizes the same ligands and thus restores functionally similar clones or the clones are randomly selected remains to be established.
In the examined cohort, CMV reactivation occurred between 25 and 60 days after transplantation. The γδ T-cell repertoire recovery observed in 10 alloHSCT patients undergoing CMV reactivation was perturbed by a massive proliferation of a few individual clones that comprised 20–75% of the repertoire, with an important variability among patients. These results hence provide interesting details of the previously reported oligoclonal expansion of non-Vγ9Vδ2 γδ T cells responding to CMV in humans10, 14 and mice.15, 16 These clones expressed different Vδ and Vγ chains of diverse clonotypes; thus, their nucleotide (and amino acid) sequences were not shared among different donors, indicating that CMV reactivation did not induce the clonal expansion of public clones. Nevertheless, several Vδ1+ clones shared substantial homology in the CDR3 region, including a common amino-acid sequence composed of a tryptophan, glycin and isoleucine (WGI) preceded by one or more tyrosine(s). Interestingly, a similar amino-acid sequence was found in the CDR3 of Vδ1+ γδ T cells responding to CMV in kidney transplant recipients,10 suggesting structural constraints for recognition of CMV-related antigens by these TCRs. Single-cell analysis of TCRs further allowed studies of Vγ and Vδ chain pairing and confirmed clonal expansion of non-Vγ9Vδ2 γδ T cells of different clonotypes that can express diverse Vγ and Vδ pairings.
These findings strengthen the idea that non-Vγ9Vδ2 γδ T cells undergo continuous and extra-thymic selection of a few TCR clonotypes, thus making their repertoire oligoclonal. Similar to Vγ9Vδ2+ γδ T cells, the presence of ligands for some specific non-Vγ9Vδ2 γδ T cells in the periphery would be responsible for this selection and skewing. Unfortunately, the majority of these ligands remain unknown, but this study raises the interesting issue of the diversity of the antigens recognized by such impressively selected and expanded non-Vγ9Vδ2 γδ T cell clones. Does CMV infection generate as many antigens as the different private γδ TCRs or are a limited number of antigens recognized by many different γδ TCRs, as is the case for CMV-derived peptides recognized by αβ TCRs? The localization of these antigens is also an important issue to address for these populations of non-Vγ9Vδ2 γδ T cells normally residing in tissues. Nevertheless, this study demonstrated that less numerous clones undergo strong clonal selection and expansion when challenged, as in the case of CMV reactivation, which aligns human non-Vγ9Vδ2 γδ T cells with adaptive immunity. Previous data supporting peripheral selection and oligoclonal expansion were derived from the recognition of endotelial protein C receptor by a γδ T cell clone (named LES) bearing a Vγ4Vδ5 TCR.7 This LES clone represented ~25% of the circulating T cells in the patient in which it was found.
This evidence highlights how the γδ T-cell subset mounts an adaptive-like immune response that is independent of canonical major histocompatibility complex presentation but relies on clonal expansion of reactive clones. However, what these reactive clones recognize remains unclear. Some reports suggest that these targets are self-proteins (related or not to major histocompatibility complex) whose expression, membrane localization or tertiary structure is modified in response to cellular stress, such as transformation and bacterial or viral infection. These clones arise from an unfocused repertoire that becomes more focused and more public throughout life (for Vγ9Vδ2 γδ T cells). Adult γδ T-cell repertoires contained variable numbers of highly proliferative oligoclonal sequences, whereas neonatal γδ T-cell repertoires were more diverse and less focused. Each individual γδ T-cell repertoire thus represents a singular individual immunological history. This TCR repertoire skewing allows γδ T cells to be rapidly, but specifically, generated, thereby blurring the boundaries between innate and adaptive immunity.
Acknowledgments
We thank Fundação para a Ciência e Tecnologia (PD/BD/105880/2014 to BDL; and PTDC/DTP-PIC/4931/2014 to BS-S) and the Ligue Contre le Cancer and Fondation pour la Recherche Médicale (to JD-M) for funding.
Footnotes
The authors declare no conflict of interest.
References
- De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA et al. Ontogeny of gamma delta T cells in humans. J Immunol 2004; 172: 1637–1645. [DOI] [PubMed] [Google Scholar]
- Carding SR, Egan PJ. γδ T Cells: functional plasticity and heterogeneity. Nat Rev Immunol 2002; 2: 336–345. [DOI] [PubMed] [Google Scholar]
- Pardoll DM, Fowlkes BJ, Bluestone JA, Kruisbeek A, Maloy WL, Coligan JE et al. Differential expression of two distinct T-cell receptors during thymocyte development. Nature 1987; 326: 79–81. [DOI] [PubMed] [Google Scholar]
- Legut M, Cole DK, Sewell AK. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell Mol Immunol 2015; 12: 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, Peigné CM, Mönkkönen H, Mönkkönen J et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012; 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 2013; 39: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13: 872–879. [DOI] [PubMed] [Google Scholar]
- Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci USA 2017; 114: 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdörfer L et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol 2017; 18: 393–401. [DOI] [PubMed] [Google Scholar]
- Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 1999; 103: 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Faure F, Graziani M, Jitsukawa S, Lefranc MP, Hercend T. A unique V-J-C-rearranged gene encodes a gamma protein expressed on the majority of CD3+ T cell receptor-alpha/beta- circulating lymphocytes. J Exp Med 1988; 167: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M et al. Stimulation of human gamma Delta T cells by nonpeptidic mycobacterial ligands. Science 1994; 264: 267–270. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med 1994; 179: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M et al. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J Exp Med 2010; 207: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S, Dietz M, Schneider A, Holtappels R, Mach M, Winkler TH. Control of murine cytomegalovirus infection by γδ T cells. PLoS Pathog 2015; 11: e1004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah C, Netzer S, Villacreces A, Juzan M, Rousseau B, Dulanto S et al. γδ T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog 2015; 11: e1004702. [DOI] [PMC free article] [PubMed] [Google Scholar]