Once tumour cells have overcome the molecular mechanisms that prevent their uncontrolled proliferation, the only enemy they need to deal with is the immune system, which is able to recognize and destroy transformed cells. Paradoxically, one of the evasion mechanisms put in place by the tumour is made by the suppressive arm of the immune system itself, which has therefore the double capacity of promoting and suppressing cancer cell growth and dissemination. B lymphocytes reflect this duality of the immune system.
Classically considered as positive regulators of the immune response, given their involvement in optimal T-cell activation and as mediators of humoral immunity, B cells started to be viewed from a new perspective following the description of regulatory B cells (Bregs), a functional subset that dampens the immune response in the context of autoimmunity and chronic inflammation in mice and humans.1 IL-10 is an undisputed marker of Bregs, and membrane-bound molecules associated with the Breg phenotype, such as CD1d and CD5, have been correlated with the B cell’s capacity to produce this immunosuppressive cytokine.2, 3 As a natural consequence, the understanding of how to modulate the differentiation and effector functions of these Bregs during tumor development and progression was promptly considered an exciting frontier in cancer immunotherapy, and works describing the role of different Breg subsets in murine and human tumors are steadily increasing (Figure 1).4 The broad array of cytokines (IL-10, IL-35, and TGF-β) and suppressive membrane-bound molecules (PD-L1, PD-1, CD80, CD86, Fas-L, CD40L, and OX40L) expressed by Bregs and involved in the regulation of anti-tumor immunity underline the complexity of elucidating the nature and specific function of the diverse tumor-evoked Breg subsets, which vary according to both the specific tumor setting analyzed and the stage of cancer.5, 6, 7, 8, 9
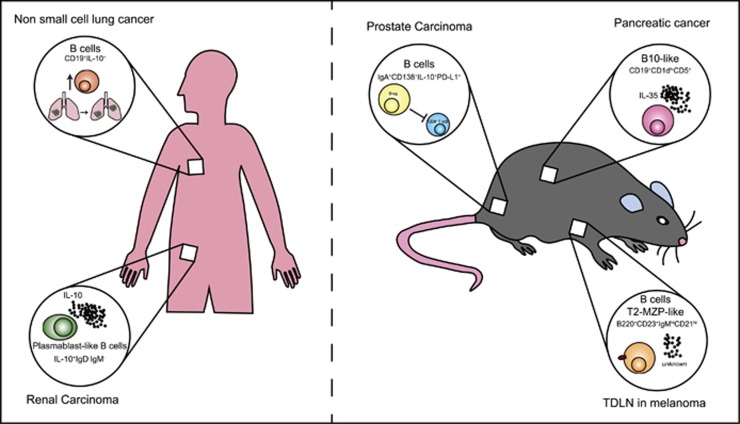
Figure 1.
The phenotype and effector weapon of Bregs vary depending on the specific tumor setting. Reported in the figure are some examples of the Breg subsets described in the contexts of murine and human cancers. Roles for the two most popular phenotype subsets of B cells that exert immunosuppressive functions in the murine system, CD19+CD1dhighCD5+ cells and transitional 2 marginal-zone precursor (T2-MZP) cells, have been shown in models of pancreatic cancer8 and melanoma,7 respectively. Regulatory B cells with a plasma cell-like phenotype play a role in autochthonous transgenic adenocarcinoma in a mouse prostate (TRAMP) model of metastatic prostate cancer9 and human renal cell carcinoma.6 In humans, an upregulation of IL-10-producing B cells was associated with tumor progression in non-small cell lung carcinoma patients.5
In a recent issue of Nature Communications, Ouyang et al.10 showed that in the context of human hepatocellular carcinoma (HCC), the most frequent malignant tumor of the liver, a strong accumulation of activated FcγRIIlow/− B cells could be observed at the invasive margin of tumors. The family of Fc receptors (FcRs) for IgG (FcγRs) is widely expressed on the surface of leukocytes. In B cells, the levels of FcγRII are indicative of the activation state of the cell. The peculiarity of this identified B cell subset is that FcγRIIlow/− B cells isolated from HCC tumors spontaneously produced IL-10 ex vivo, without requiring additional stimulation. Moreover, among all tumor B cells, FcγRIIlow/− B cells were the major producers of this immunosuppressive cytokine.10 Although IL-10 is undisputedly related to a regulatory phenotype, the regulation of IL-10 production by B lymphocytes is still a debated issue, and the idea that any B cell might potentially become a Breg after receiving a specific environmental stimulation is increasingly gaining ground.1 Ouyang and co-workers elegantly demonstrated that the in vivo priming responsible for B-cell activation toward the FcγRIIlow/− IL-10-producing phenotype is derived from the tumor microenvironment and, more specifically, from a subset of semimature dendritic cells (DCs). During tumor onset, there is a general impairment in the differentiation of several immune cell populations of the myeloid lineage, such as macrophages and DCs. In a previous work, the authors of this Nature Communications issue demonstrated that supernatants derived from the culture of solid tumor cell lines, including the human liver cancer cell line HepG2, drove the differentiation of human monocytes into tolerogenic semimature DCs (TDCs), which were characterized by increased expression levels of HLA-DR and the co-stimulatory molecules CD83 and CD86 and by a IL-12lowIL-10high cytokine production profile. Among the soluble factors released by the tested tumor cell lines, hyaluronan (HA) fragments were identified as a common driving force for the induction of the semimature phenotype of DCs.11 Ouyang and co-workers showed a positive correlation between the number of TDCs and the proportion of FcγRIIlow/− in HCC tumors and demonstrated that TDCs isolated from HCC tumors can drive autologous blood B-cell activation toward the FcγRIIlow/− IL-10-producing phenotype ex vivo. Similar to TDCs, tumor-associated macrophages (TAMs) also accumulate in HCC peritumoral stroma and correlate with a worse prognosis. However, TAMs did not elicit B-cell activation or IL-10 production.10 To date, several immune cell types, including DCs, invariant natural killer T cells and mast cells, regulate the expansion and differentiation of the regulatory IL-10-producing B-cell population under homeostatic conditions.12, 13, 14 Ouyang’s observation that in the HCC context, TDCs but not TAMs activate B cells toward the FcγRIIlow/− IL-10-producing phenotype highlights the relevancy of understanding the specific nature of the signals provided by the tumor microenvironment and their impact on the phenotype and function of the diverse immunological B cell partners.
The effect of Bregs suppressive activity has been demonstrated to markedly affect T-cell plasticity. Indeed, the analysis of several pathological settings characterized by an imbalanced immune response revealed that Bregs inhibit Th1 and Th17 cell differentiation while promoting Treg-cell expansion.1 Ouyang et al.10 evaluated whether FcγRIIlow/− B cells affected T-cell immunity in HCC and showed that they were able to suppress the expression of cytotoxic granzyme B and perforin and the proinflammatory cytokines TNF-α and IFN-γ in autologous tumor-derived CD8+ cytotoxic T cells through the release of IL-10. The result that TDC-elicited activation of B cells ultimately leads to the generation of a microenvironment that favors tumor growth obviously opens a new window of opportunity for targeted anticancer immunotherapies. Ouyang’s evidence that the CD95-CD95L interaction participates in the TDC-mediated generation of FcγRIIlow/− IL-10-producing B cells (Figure 2) leads the reader to focus on the possible applications of anti-CD95-related cancer therapies, which are increasingly considered important tools to fight cancer.15
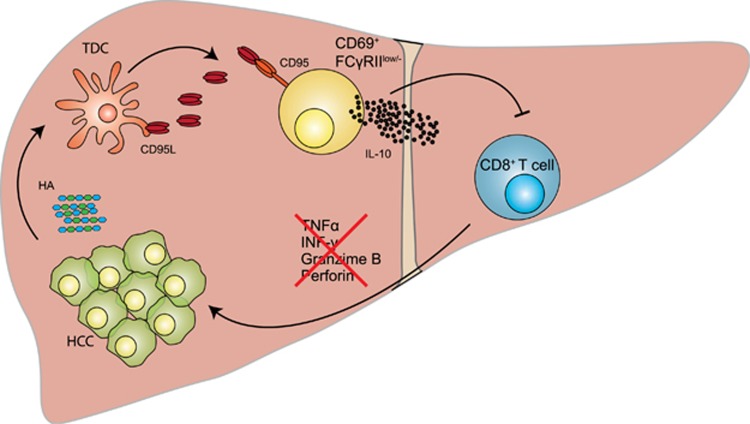
Figure 2.
Schematic recapitulation of TDC-mediated generation of FcγRIIlow/− IL-10-producing B cells in HCC. In this issue of Nature Communications, Ouyang et al. describe the mechanism leading to the expansion of a B-cell population with regulatory potential in hepatocellular carcinoma (HCC). Under the effect of soluble tumor-derived factors and in particular hyaluronan (HA) fragments, human monocytes differentiate into tolerogenic semimature DCs (TDCs), which are responsible for the generation of FcγRIIlow/−-activated B cells. CD95-CD95L interactions play a major role in this activation process. By producing IL-10, FcγRIIlow/−-activated B cells suppressed cytotoxic T-cell immunity and may therefore favor tumor progression.10
Overall, this Nature Communications issue provides new insights into how to modulate the regulatory potential of B lymphocytes in the context of HCC. A novel human Breg phenotype has been characterized as a tumor-educated IL-10-releasing B-cell population that favors tumor escape from the immune system. Moreover, this study highlights the relevance of analyzing how changes in the tumor microenvironment affect B-cell biology to better define the role of these cells in cancer immunology.
Acknowledgments
Authors are supported by the Associazione Italiana Ricerca sul Cancro (AIRC), Progetti di Ricerca di Interesse Nazionale (PRIN), and Associazione Italiana Mastocitosi (ASIMAS).
Footnotes
The authors declare no conflict of interest.
References
- Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015; 42: 607–612. [DOI] [PubMed] [Google Scholar]
- Garaud S, Taher TE, Debant M, Burgos M, Melayah S, Berthou C et al. CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes. Cell Mol Immunol 2016; e-pub ahead of print 8 August 2016; doi:10.1038/cmi.2016.42. [DOI] [PMC free article] [PubMed]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002; 16: 219–230. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer 2016; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Yu Q, Zheng S, Jiang Y, Liu Y et al. Aberrant frequency of IL-10-producing B cells and its association with Treg and MDSC cells in non small cell lung carcinoma patients. Hum Immunol 2016; 77: 84–89. [DOI] [PubMed] [Google Scholar]
- Cai C, Zhang J, Li M, Wu ZJ, Song KH, Zhan TW et al. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumour Biol 2016; 37: 8209–8218. [DOI] [PubMed] [Google Scholar]
- Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep 2015; 5: 12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB et al. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Dis 2016; 6: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015; 521: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang FZ, Wu RQ, Wei Y, Liu RX, Yang D, Xiao X et al. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat Commun 2016; 7: 13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang DM, Zhao Q, Xu J, Yun JP, Wu C, Zheng L. Tumor-educated tolerogenic dendritic cells induce CD3epsilon down-regulation and apoptosis of T cells through oxygen-dependent pathways. J Immunol 2008; 181: 3089–3098. [DOI] [PubMed] [Google Scholar]
- Vomhof-DeKrey EE, Yates J, Hagglof T, Lanthier P, Amiel E, Veerapen N et al. Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells. Proc Natl Acad Sci USA 2015; 112: 12474–12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion F, D’Inca F, Danelli L, Toffoletto B, Guarnotta C, Frossi B et al. Mast cells control the expansion and differentiation of IL-10-competent B cells. J Immunol 2014; 193: 4568–4579. [DOI] [PubMed] [Google Scholar]
- Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L et al. Regulatory dendritic cells program B cells to differentiate into CD19hiFcgammaIIbhi regulatory B cells through IFN-beta and CD40L. Blood 2012; 120: 581–591. [DOI] [PubMed] [Google Scholar]
- Villa-Morales M, Fernandez-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16: 85–101. [DOI] [PubMed] [Google Scholar]