Highlights
-
•
There is no standard therapy for locally advanced TNBC showing disease progression during NAC.
-
•
We presented a case of locally advanced TNBC showing pCR to weekly paclitaxel with bevacizumab treatment showing PD during standard NAC.
-
•
This report suggests the potential of bevacizumab in response-guided sequential therapy and this finding should be confirmed in clinical trial.
Abbreviations: NAC, neoadjuvant chemotherapy; MRI, magnetic resonance imaging; PET-CT, positron emission tomography computed tomography; CNB, core needle biopsy; TNBC, triple negative breast cancer; cCR, clinical complete response; pCR, pathological complete response
Keywords: Case report, Locally advanced breast cancer, Triple negative, Weekly paclitaxel with bevacizumab, Disease progression, Neoadjuvant chemotherapy
Abstract
Introduction
Neoadjuvant chemotherapy (NAC) is the standard of care for locally advanced triple negative breast cancer, however, approximately 5% of cases show disease progression during NAC. Although downstaging is essential to create an opportunity for curative surgery and to improve the local control outcome in such a case, no additional line of chemotherapy has been established.
Case presentation
A 60-year-old woman was referred to our hospital for an axillary mass presenting three weeks ago and was diagnosed as having right locally advanced (T2N2M0, stage IIIA) triple negative breast cancer. After two courses of epirubicine and cyclophosphamide as NAC, disease progression was recognized and curative resection was considered impossible due to enlarged axillary lymph nodes showing invasion to surrounding tissue. As second-line chemotherapy, weekly paclitaxel with bevacizumab treatment was initiated and significant shrinkage was immediately obtained. A clinically complete response was diagnosed after four courses of weekly paclitaxel with bevacizumab and she underwent a right breast mastectomy with axillary lymph node dissection without major complications. Histopathological examination of surgical specimens showed no residual invasive or noninvasive disease and she was diagnosed as having a pathological complete response.
Conclusions
Although the addition of bevacizumab to standard adjuvant chemotherapy is not recommended in unselected triple negative breast cancer, the potent effect on tumor shrinkage should be considered in the treatment of locally advanced triple negative breast cancer showing disease progression during standard NAC.
1. Introduction
Neoadjuvant chemotherapy (NAC) has become the standard-of-care for non-metastatic breast cancer with results from randomized trials showing equivalent long-term survival for NAC compared with survival for adjuvant chemotherapy [1]. Anthracycline- and taxane-based chemotherapy are recommended as standard NAC [2], [3], [4] among patients with triple-negative breast cancer (TNBC), however, approximately 5% of the cases show disease progression during NAC [5], [6], [7]. If patients have inoperable disease, next-line chemotherapy is considered to create the opportunity for curative surgery and local control. The selection of an additional chemotherapy regimen is done at the discretion of physicians because there are no clinical trials that have evaluated the efficacy and safety of treatments in patients who showed disease progression during NAC.
In this case report, we describe a case of locally advanced triple negative breast cancer showing a pathological complete response to weekly paclitaxel with bevacizumab treatment following disease progression during anthracycline-based neoadjuvant chemotherapy. This case report has been reported in line with the SCARE criteria [8].
2. Case presentation
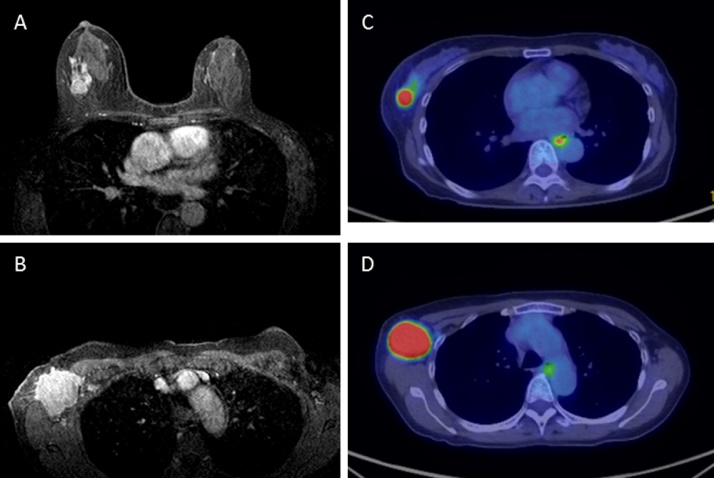
A 60-year-old post-menopausal woman presented at a local clinic with a right axillary mass that had appeared three weeks previous. Right breast cancer with axillary lymph node metastases was suspected and she was referred to our hospital. Physical examination showed a right breast mass at the C area and fixed and enlarged axillary lymph nodes. In her past history, she underwent total gastrectomy and received adjuvant doxifluridine therapy for gastric cancer 15 years ago with no sign of recurrence. She had no family history of breast or ovarian cancer. An abdominal scar for a gastrectomy and emaciation (BMI = 17.6) was recognized. Laboratory results showed a normal range for the tumor markers. Mammography showed focal asymmetric density with clustered pleomorphic calcification, indicating BI-RAD category 4. Ultrasonography confirmed an irregular mass in the right breast and ipsilateral multiple axillary lymph nodes that were enlarged and fixed to one another. Magnetic resonance imaging (MRI) also showed a right breast mass of 3 cm diameter, and multiple enlarged and fixed axillary lymph nodes. Positron emission tomography computed tomography (PET-CT) showed a right breast mass with SUVmax 19.73 and multiple axillary lymph node swelling with a SUVmax range from 4.74 to 25.2 (Fig. 1). No definitive finding of distant metastases was recognized with these modalities. Histopathological examination of core needle biopsy (CNB) of the right breast tumor showed invasive ductal carcinoma. Immunohistochemical examination and fluorescence in situ hybridization of the tumor cells showed negative results for estrogen receptor, progesterone receptor and HER2, indicating that her breast tumor was a triple negative breast cancer (TNBC) subtype. The nuclear grade 3 was recognized and the Ki67 index was 53.39%. With these findings, she was diagnosed as having right locally advanced triple negative breast cancer.
Fig. 1.
Magnetic resonance imaging showed right breast mass with 3 cm diameter in the upper outer portion of right breast and, multiple enlarged and fixed axillary lymph nodes with edematous change of surrounding soft tissue (A, B). Positron emission tomography computed tomography (PET-CT) showed right breast mass with SUVmax 19.73 and multiple axillary lymph node swelling with SUVmax range from 4.74 to 25.2 (C, D).
Because curative surgery is thought to be difficult due to the matted and fixed axillary metastases, NAC was initiated to reduce the tumor burden and to allow for complete resection and local control. Tri-weekly epirubicine 90 mg/m2 and cyclophosphamide 600 mg/m2 were initiated. After two courses of chemotherapy, she complained about enlargement of a right breast mass. CT showed progressive disease of a right breast tumor and axillary lymph nodes (25% increment in the sum of breast tumor and axillary lymph node) and her disease was diagnosed as remaining inoperable. Considering the high response rate and the low frequency of disease progression, the addition of bevacizumab or carboplatin to a taxane-based neoadjuvant chemotherapy regimen was suggested. Because increments of hematologic and gastrointestinal toxicities associated with the addition of carboplatin were considered crucial for her condition after total gastrectomy, weekly paclitaxel 90 mg/m2 with biweekly bevacizumab 10 mg/kg was selected. After initiation of weekly paclitaxel with bevacizumab, there was significant shrinkage of the breast tumor and axillary lymph nodes. Bevacizumab was discontinued at the 4th cycle to reduce the risk of operative complications. After completion of 4 cycles of a weekly paclitaxel with bevacizumab regimen, clinical complete response (cCR) was recognized by CT and MRI findings (Fig. 2). No serious or unknown adverse event occurred during NAC. The weekly paclitaxel with bevacizumab was well tolerated and no dose-reduction or delayed administration was required. She underwent right breast mastectomy and axillary lymph node dissection (level I and level II) as a standard procedure by a doctor in charge. Intraoperative findings showed a remarkable scar in the axillar and no mass was found in breast or axilla. There were no major complications, including bleeding, thrombosis or delay of wound healing, and she left the hospital eight days after the operation. Histopathological examination of surgical specimens showed pathological complete response (pCR) with no residual disease in the breast and dissected axillary nodes (Fig. 3). The patient was satisfied with the tumor shrinkage and complete resection of the disease without any significant complications. Postoperative radiation therapy to her chest wall and regional lymph node was performed as adjuvant therapy, and she was ambulatory followed without any recurrence at a 6-month follow-up.
Fig. 2.
Computed tomography before NAC (A, B), after 2 cycles of EC (C, D), and after 4 cycles of weekly paclitaxel with bevacizumab (E, F). After 2 cycles of EC, progressive disease was recognized with 25% increment in the sum of breast tumor and axillary lymph node. Clinical complete response was recognized after completion of 4 cycles of weekly paclitaxel with bevacizumab (E, F). Last computed tomography was performed without contrast media because of patient allergic reaction.
Fig. 3.
Histological findings of the right breast (A) and axillary lymph nodes (B) (H&E stain X20). There was scarring with invasion of lymphocytes, histiocyte and multinucleated giant cell. No residual breast cancer cells were recognized showing a pathological complete response (Grade 3).
3. Discussion
Because TNBC have a higher risk of relapse compared with other subtypes and are not sensitive to endocrine or HER2-targeting therapy, adjuvant chemotherapy is recommended for most patients with TNBC. For patients with TNBC, NAC is a reasonable alternative to adjuvant chemotherapy and significantly more patients with TNBC were treated with NAC compared with a luminal type [9]. NAC is administered not only to downstage the tumor for less extensive surgery or for improved cosmetic outcome, but also to permit an early evaluation of chemotherapy, which can facilitate response-guided adjuvant treatment. A recently published phase III trial showed that adjuvant capecitabine therapy improved long-term prognosis among HER2-negative breast cancer patients who had residual invasive disease after standard anthracycline and/or taxane-based NAC [10]; the rate of disease-free survival was 69.8% in the capecitabine group as compared with 56.1% in the control group, and the overall survival rate was 78.8% versus 70.3% among patients with triple negative disease. With these findings, more patients with TNBC are expected to receive NAC.
Although a large proportion of TNBC shows a response to NAC, more than 5% of cases show progressive disease during standard NAC [5], [7]. Because the residual tumor burden after NAC is associated with disease recurrence [11], tumor shrinkage is crucial not only for cosmetic outcome and local control, but also to improve long-term survival in such cases. Although no clinical trials have evaluated the efficacy and safety of an additional-line of chemotherapy in patients who showed disease progression during NAC, it is reasonable to select regimens that show a substantial antitumor effect with a high response rate and low frequency of progressive disease. In patients with TNBC, the addition of bevacizumab or carboplatin to anthracycline- and taxane-based chemotherapy can be the most potent regimen in regards to achieving tumor shrinkage [5], [12], [13], [14]. Large randomized clinical trials have demonstrated a significantly higher response rate and pCR rates with the addition of bevacizumab or carboplatin to anthracycline- and taxane-based neoadjuvant NAC. In CALGB 40603, adding either agent significantly increased the rate of the pCR in breast; and 60% of the carboplatin group achieved pCR breast compared with 46% in the control group, and 59% of the bevacizumab group compared with 48% in the control group. In addition, 6 of 108 patients who started standard NAC stopped treatment for progressive disease, and only 1 of 113 patients assigned to the bevacizumab group showed disease progression during NAC [5]. Although the addition of these agents to NAC is not recommended in general due to a lack of long-term survival benefit and an increased rate of adverse events, the potent effect on tumor shrinkage should be considered in selected situations where disease progression is recognized during standard NAC.
In this case report, we administered weekly paclitaxel with bevacizumab to the patient. The weekly paclitaxel with biweekly bevacizumab was well tolerated and no dose reduction or delay of schedule was recognized despite of the patient’s status of emaciation after total gastrectomy. After initiation of weekly paclitaxel with bevacizumab, immediate tumor shrinkage and relief of tenderness of her breast was obtained, and cCR was achieved after 4 cycle treatment. She underwent a standard mastectomy and axillary lymph node dissection with no major surgical and post-operative complications and pCR was recognized at both breast and lymph nodes. She was satisfied with the course of treatment and was ambulatory without any complications.
Of course, the significant effect of weekly paclitaxel with bevacizumab in this case could be by chance, however, the response-guided sequential addition of bevacizumab has shown advantages in clinical trial. In an AVATAXHER trial, patients with HER2-positive breast cancer that did not show a metabolic response after two cycles of neoadjuvant docetaxel and trastuzumab treatment were randomly assigned to receive four cycles of docetaxel and trastuzumab plus bevacizumab or continued on docetaxel plus trastuzumab alone [15]. Patients assigned to the addition of bevacizumab showed a significantly higher pCR rate compared with those assigned to continue the same regimen; pCR was noted at a rate of 43.8% in the bevacizumab group and 24.0% in the control group. This finding of response-guided sequential therapy suggests the potential of bevacizumab in the treatment of breast cancer, and this hypothesis should be confirmed in further clinical trials targeting patients with TNBC showing disease progression during standard NAC.
4. Conclusion
In this case report, the incorporation of bevacizumab showed significant tumor shrinkage of TNBC showing disease progression during standard NAC, and facilitated complete resection with less extensive surgery. Although the addition of bevacizumab to standard NAC is not recommended in the treatment of breast cancer, this case report showed the potential of bevacizumab in response-guided sequential therapy of TNBC. The incorporation of bevacizumab to chemotherapy should be evaluated in further clinical trials targeting patients with TNBC showing disease progression during standard NAC.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This case report was approved by the Kure Medical Center review board (28–79).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Authors’ contribution
HS designed the study, conducted the investigation, and wrote the manuscript. SO performed the surgery and supervised the study. DY supervised the study. TH administered the neoadjuvant chemotherapy and supervised the work. All of the authors contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Guarantor
Hideo Shigematsu,
Department of Breast Surgery, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Kure-City, Hiroshima, Japan.
References
- 1.Mauri D., Pavlidis N., Ioannidis J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J. Natl. Cancer Inst. 2005;97(February (3)):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 2.von Minckwitz G., Raab G., Caputo A. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J. Clin. Oncol. 2005;23(April (12)):2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi P., Anderson S.J., Bear H.D. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008;26(February (5)):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative G, Peto R., Davies C. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(February (9814)):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sikov W.M., Berry D.A., Perou C.M. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J. Clin. Oncol. 2015;33(January (1)):13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toi M., Nakamura S., Kuroi K. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res. Treat. 2008;110(August (3)):531–539. doi: 10.1007/s10549-007-9744-z. [DOI] [PubMed] [Google Scholar]
- 7.Iwata H., Sato N., Masuda N. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn. J. Clin. Oncol. 2011;41(July (7)):867–875. doi: 10.1093/jjco/hyr081. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saeta A. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34(October):180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Graham P.J., Brar M.S., Foster T. Neoadjuvant chemotherapy for Breast cancer, is practice changing? A population-based review of current surgical trends. Ann. Surg. Oncol. 2015;22(October (10)):3376–3382. doi: 10.1245/s10434-015-4714-x. [DOI] [PubMed] [Google Scholar]
- 10.Masuda N., Lee S.J., Ohtani S. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017;376(June (22)):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke C., Mazouni C., Hess K.R. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26(March (8)):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 12.Bear H.D., Tang G., Rastogi P. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 2012;366(January (4)):310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Minckwitz G., Eidtmann H., Rezai M. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 2012;366(4):299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G., Schneeweiss A., Loibl S. eoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(June (7)):747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 15.Coudert B., Pierga J.Y., Mouret-Reynier M.A. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. 2014;15(December (13)):1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]