Abstract
Coronary artery disease (CAD) is a critical cardiovascular disease and a cause of high morbidity and mortality in this world. Hyperhomocysteinemia (HHcy) has been suggested as a risk factor for CAD. In addition, SIRT1 (sirtuin 1) has been reported to play a protective role in a variety of diseases, especially in the cardiovascular system. The main purpose of this study was to investigate the effects of exercise training on apoptosis and inflammation in HHcy animals. We also tested whether exercise protected against Hhcy-induced dysfunction of endothelium through modulation of SIRT1. C57BL mice (8 in each group) were fed with or without 1% L-methionine (w/w) in water for 4 months to induce HHcy. We found that Hhcy repressed SIRT1 and AMPK expression and increased NADPH oxidase activity. Plasma MDA, endothelium LOX-1 and p-p38 were up-regulated by Hhcy induction. NF-κB and it downstream molecules were activated under Hhcy situation, thereby promoting pro-inflammatory responses. Moreover, we also reported that Hhcy caused endothelium apoptosis involving Akt inhibition and mitochondria-dependent apoptotic pathways. Exercise training significantly protected against endothelium from Hhcy caused oxidative injuries. In addition, EX527 (SIRT1 inhibitor) reduced the therapeutic effects by exercise. Our results had indicated that exercise training prevent the development of atherosclerosis through SIRT1 activation and oxidative stress inhibition under Hhcy situation.
Abbreviations: HHcy, hyperhomocysteinemia; Hcy, homocysteine; LOX-1, lectin-like oxidized low density lipoprotein receptor-1; PKC, protein kinase C; SIRT1, sirtuin 1; CAD, coronary artery disease; SOD, superoxidase dismutase; GPx, glutathione peroxidase; and AMPK, AMP-activated protein kinase
Keywords: Sirtuin 1, Coronary artery disease, Hyperhomocysteinemia, Oxidative stress
Highlights
-
•
Exercise reversed Hyperhomocysteinemia(HHcy)-mitigated expression levels of SIRT1 and AMPK.
-
•
Exercise reduced Hyperhomocysteinemia(HHcy)-induced oxidative stress by NADPH oxidase inhibition in the aortic endothelium.
-
•
Exercise reduced the HHcy-induced activation of LOX-1 signaling.
-
•
Exercise reduces HHcy-induced apoptosis and inflammation in the aortic endothelium.
1. Introduction
Coronary artery disease (CAD) is a critical cardiovascular disease and a leading cause of morbidity and mortality all over the world [1]. Hyperhomocysteinemia (HHcy) is caused by dysfunction in enzymes associated with blood homocysteine (Hcy) metabolism. Plasma homocysteine levels above 15.0 μmol/L are classified as abnormal plasma homocysteine levels or hyperhomocysteinemia [2]. Previous studies have suggested that there is an association between high homocysteine concentrations in human plasma and the risk of CAD. The Boushey group first reported the correlation between Hcy and CAD via a meta-analysis. This study reported that higher levels of plasma Hcy are associated with a relatively higher risk of developing atherosclerotic disease in the coronary, cerebral, and peripheral circulatory systems. In addition, the significance of plasma Hcy up-regulation was comparable to the CAD risk of a 19 mg/dL increase in plasma cholesterol [3].
Coronary artery luminal obstructions and atherosclerotic plaque ruptures are characteristic of CAD, which is identified by the presence of endothelial injury and lipid accretion as well as the formation of atherosclerotic plaques [4]. Apoptosis and necrosis of endothelial vessels followed by excessive inflammation are key sources of vascular injury in CAD [5]. Therefore, agents that mitigate endothelial vessel death and control pro-inflammatory events during CAD are potential therapeutic modalities for ameliorating the clinical prognosis of subjects with CAD.
The idea of oxidative stress has been acquainted for study in redox biology and medicine in 1985 [6]. Oxidative stress is one situation that occurs when the formation of reactive oxygen species (ROS) has surpassed the free radical scavenging capacity of antioxidant enzymes [7]. Oxidative stress is characterized as one critical regulator of the development of cardiovascular diseases. For example, previous reports have indicated that hyperlipidemia and diabetes mellitus (DM) are both correlated with increased oxidative stress, which may affect the development of atherosclerotic injury and CAD [8]. Hcy causes endothelial oxidative injury by increasing LOX-1/NADPH oxidase activity and antioxidant enzyme inhibition [9]. Under normal physical conditions, mammalian cells are protected from oxidative stress by antioxidant enzymes such as superoxidase dismutase (SOD), glutathione peroxidase (GPx), and catalase [10]. SOD quickly catalyzes the chelation of O- to H2O2, which is converted to O2 and H2O by catalase or GPx [11]. Therefore, antioxidant enzyme activity is important for normal redox balance in humans.
SIRT1 (sirtuin 1) is thought to play a critical function in modulating cellular physiological processes, such as metabolism, cell degeneration, cell growth and cell survival. In human endothelial cells, SIRT1 controls anti-aging in endothelial cells and protects against endothelial inflammation [12], [13]. SIRT1 is associated with many advantageous influences, including lengthening of the lifespan and postponing the beginning of age-related diseases such as CVD, neurodegenerative diseases and DM [14]. SIRT1 has also been shown to promote antioxidant enzyme activity and protect against ROS-mediated oxidative damage through suppressing NADPH oxidase activation [15], [16]. A previous study reported that the expression level and activity of SIRT1 were reduced in inflammatory endothelial cells [17]. Our group also found that Hcy treatment causes endothelial apoptosis by repressing SIRT1 expression [9]. In addition, SIRT1 represses the uptake of oxLDL by inhibiting the expression level of LOX-1 and the NF-κB signaling pathway [18]. Thus, SIRT1 activation has been considered as a novel therapeutic approach in the management of clinical cardiovascular diseases.
Exercise training has been used as an important therapeutic intervention against cardiovascular diseases [19], and regular exercise has been shown to have beneficial effects on the cardiovascular system [20]. Claudio et al. suggested that regular exercise protects against ovariectomy-induced coronary endothelial dysfunction by repressing oxidative stress [21]. Our group previously found that the activation of SIRT1 function protected against Hcy-induced endothelial apoptosis and oxidative stress [9]. Exercise training has been confirmed to promote SIRT1 activity in aged animals, thereby increasing Mn-SOD and catalase levels in both the heart and adipose tissues [22]. This study was designed to understand whether exercise intervention could reduce HHcy-induced apoptosis and inflammation in mouse vessels through the modulation of SIRT1 function.
2. Materials and methods
2.1. Animals
A total of 32 C57BL mice (8 in each group) were used in this study. The animals were fed with or without 1% L-methionine (w/w) in water for 4 months to induce HHcy. At week 2, half of the mice had access to a motor treadmill for involuntary exercise training. All of the animal studies followed the guidelines that were required for the care and use of laboratory animals and were approved by the animal center of the National Cheng Kung University in Tainan, Taiwan. The Hcy levels in the serum were tested to confirm that HHcy was induced.
2.2. Exercise protocol
The exercise protocol is considered as an involuntary exercise. This protocol had been reported to mitigate pro-inflammatory cytokines in rats fed a high-fat diet and to improve cardiovascular functions in aged animals [23], [24]. The speed of the motor treadmill was 10 m/min for 60 min. The exercised mice were further divided into two groups: Group 1, exercise only; and Group 2, for which 1.2 mM EX527 was prepared in phosphate-buffered saline for a single intravenous (5 μl) injection at 2 h before exercise. The exercise groups were trained for 60 min/day and 5 days/week for a total of 14 weeks. Prior to the training protocol, the animals in the exercise groups were adapted to the training procedure at a speed of 6 m/min for the first week. Running time was extended by 15 min/day until a running time of 60 min/day was reached. During the training period, the running speed was gradually elevated to 10 m/min. This intensity reflects a moderate exercise intensity for humans. No electrical stimulation was used in this study. The sedentary (control) animals were placed on the treadmill without exercise intervention to expose them to the same environmental conditions.
2.3. SOD and MDA levels
The SOD and MDA levels were tested using kits (Sigma). Plasma was obtained through blood collection. The SOD (19160 Sigma) and MDA (MAG085 Sigma) levels in the plasma were tested via an enzymatic assay method using a commercial kit according to the manufacturer's instructions.
2.4. Resting blood pressure
All animals were loosely restrained when measuring the resting blood pressure. A tail-cuff pressure meter system was used to measure the systolic/diastolic blood pressure (LE5001, Panlab, Wood Dale, IL).
2.5. Western blot
RIPA buffer, which was purchased from Millipore, was used to extract total protein. Thoracic aortic endothelial tissue extracts were collected via homogenization at 4 °C in RIPA buffer supplemented with protease and phosphatase inhibitors (Roche Applied Science, Mannheim, Germany). The homogenates were centrifuged at 13,000×g for 30 min, and the supernatant was collected. The proteins were transferred to a polyvinylidene difluoride (PVDF) membrane after the proteins were separated by electrophoresis on an SDS-polyacrylamide gel. The membranes were blocked by buffer for 1 h at 37 °C. Then, the membranes were incubated with primary antibodies overnight at 4 °C followed by hybridization with HRP-conjugated secondary antibodies for one hour. The intensities were quantified by densitometric analysis.
2.6. Antibodies
Anti-LOX-1, anti-β-actin, anti-p-AMPK, anti-p-PKCβ anti-gp91, anti-p22phox, anti-NOX-1, anti-p-p38, anti-p-Akt and anti-SIRT1 were purchased from Santa Cruz Biotechnology (CA, USA). Anti-p-ERK, anti-ICAM-1, anti-VCAM-1, anti-Bax, anti-Bcl-2, anti-cleaved caspase-3 and anti-NF-κBp65 were obtained from Cell Signaling (MA, USA). Anti-Rac-1 and anti-p47phox were obtained from BD Biosciences (NJ, USA).
2.7. Statistical analysis
The data are expressed as the mean± SD. One-way ANOVA followed by Student's t-test was used to analyze the differences between groups. Pearson's correlation coefficients were used to analyze the correlation between Hcy and genes. p< 0.05 was accepted as statistically significant.
3. Results
3.1. Animal characteristics
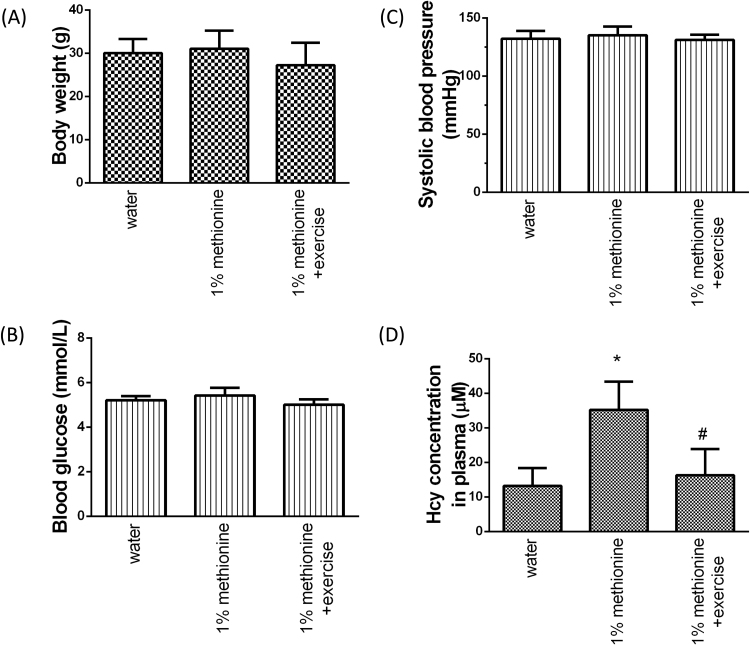
The body weight (BW), systolic blood pressure (SBP), blood glucose (BG) and plasma Hcy concentrations were recorded. As shown in Fig. 1, There was no significant difference in BW, SBP and BG among the three groups. However, the plasma Hcy concentrations in the 1% methionine-fed animals were significantly reduced in the 1% methionine plus exercise group compared with the 1% methionine group.
Fig. 1.
Characteristics of experimental animals. (A) Body weight. (B) Systolic blood pressure. (C) Blood glucose and (D) homocysteine levels. The data are presented as the mean ± SD. *P < 0.05, significant differences compare to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
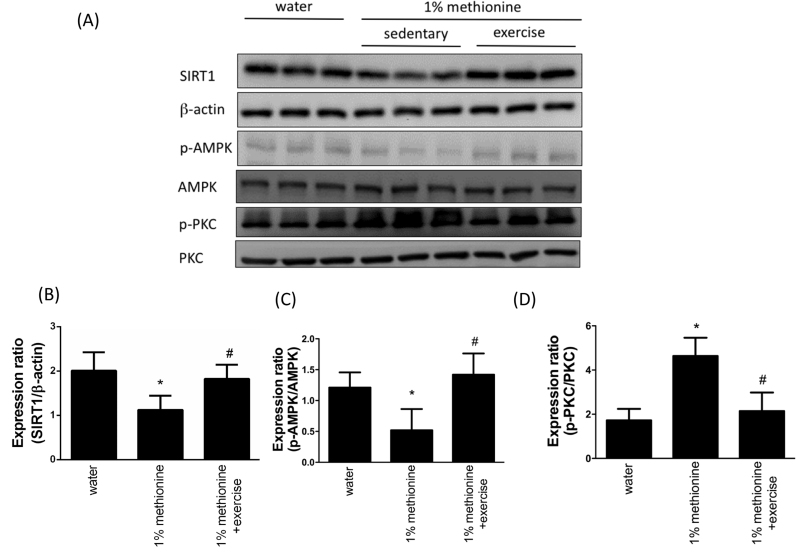
3.2. SIRT1 and the expression of its downstream targets in the aortic endothelium under HHcy conditions
To understand the mechanism of the cardiac SIRT1 signaling in HHcy models with exercise training, the protein levels of SIRT-1, phospho-AMPK and phospho-PKC in the aortic endothelium excised from three groups were investigated using Western blotting. Compared with the control animals, the protein expression levels of SIRT1 and phospho-AMPK were significantly reduced in the 1% methionine group, whereas they were significantly increased in the 1% methionine plus exercise group compared with the 1% methionine group (Fig. 2A-C). In addition, we also found that the protein expression levels of phospho-PKC were significantly increased in the 1% methionine group, whereas they were significantly decreased in the 1% methionine plus exercise group compared with the 1% methionine group (Fig. 2A and D).
Fig. 2.
Exercise training reduced HHcy-induced SIRT1 inhibition. (A) The protein levels of SIRT1, p-AMPK and p-PKC in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D) The bars represent the relative protein levels of SIRT1, p-AMPK and p-PKC, which were normalized to β-actin, and indicate the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
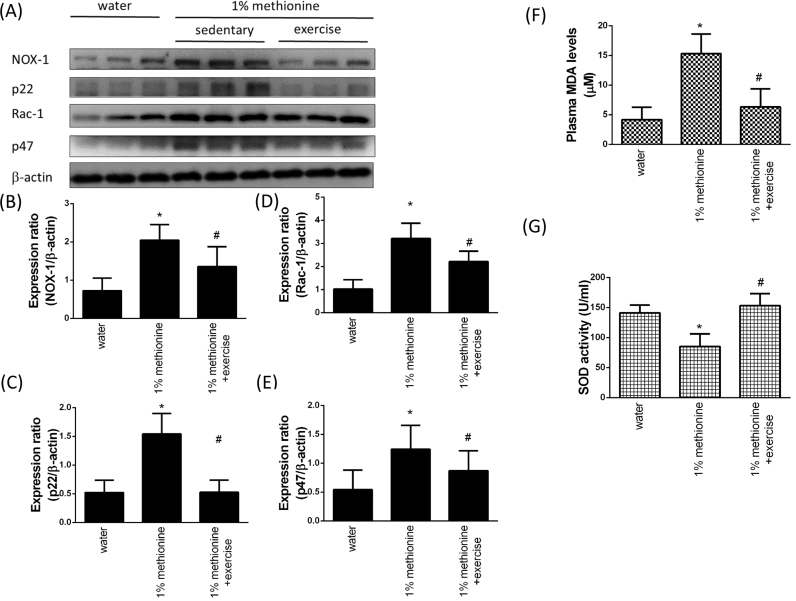
3.3. Exercise reduced HHcy-induced oxidative stress by NADPH oxidase inhibition in the aortic endothelium
NADPH oxidases are multi-subunit enzyme complexes that generate ROS from molecular oxygen using NADPH as the electron donor [25]. NADPH oxidase consists of two membrane subunits, Nox2 (also called gp91) and p22phox, in addition to three cytoplasmic proteins, p47phox, p67phox, and the small GTPase Rac-1. In Fig. 3A-E, we showed that compared with the control animals, the aortic endothelial protein expression levels of NOX-1, p22, Rac-1 and p47 were significantly increased in the 1% methionine group, whereas they were significantly decreased in the 1% methionine plus exercise group compared with the 1% methionine group. In addition, the plasma MDA levels in the 1% methionine group were higher than that in the control group; however, exercise intervention reversed HHcy-induced MDA activation (Fig. 3F). A previous study suggested that plasma total homocystein (tHcy) tended to elevate with more severe coronary atherosclerosis, while SOD activity tended to be down-regulated with CAD severity [26]. We have confirmed that compared with the control animals, the SOD activity in plasma was significantly reduced in the 1% methionine group, whereas it was significantly up-regulated in the 1% methionine plus exercise group compared with the 1% methionine group (Fig. 3G).
Fig. 3.
Exercise training reduced HHcy-induced NADPH oxidase activation. (A) The protein levels of NOX-1, p22, Rac-1 and p47 in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D, E) The bars represent the relative protein levels of NOX-1, p22, Rac-1 and p47, which were normalized to β-actin. (F) MDA levels and (G) SOD activity were measured from plasma. The data are presented as the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
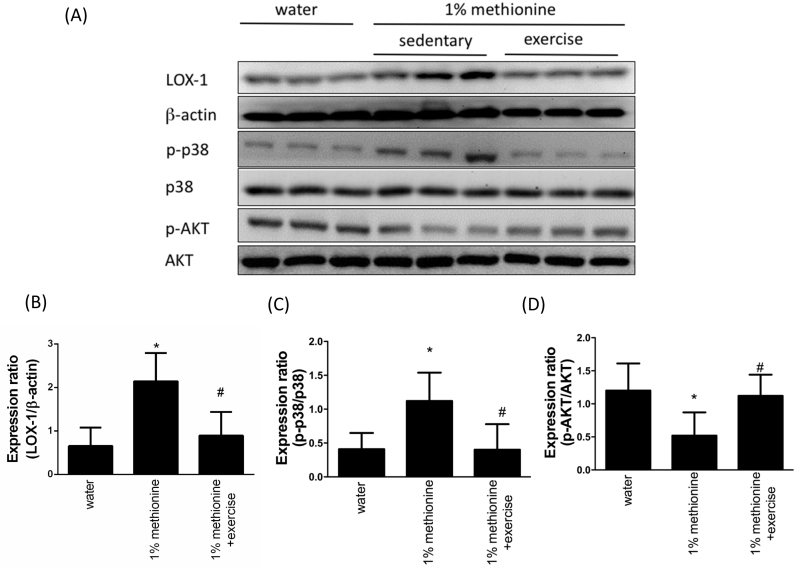
3.4. Exercise reduced the HHcy-induced activation of LOX-1 signaling
LOX-1 is obviously activated in the human cardiovascular system under proatherogenic conditions. LOX-1 activation results in AKT inhibition and the up-regulation of p38MAPK [27]. In Fig. 4A-C, we have found that compared with the control animals, the aortic endothelial protein expression levels of LOX-1 and phospho-p38 were significantly up-regulated in the 1% methionine group, whereas they were significantly repressed in the 1% methionine plus exercise group compared with the 1% methionine group. As expected, phospho-AKT expression levels were reduced in HHcy animals. Exercise intervention effectively reversed HHcy-induced AKT inhibition (Fig. 4A and D).
Fig. 4.
Exercise training repressed HHcy-activated LOX-1 expression. (A) The protein levels of LOX-1, p-p38 and p-Akt in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D) The bars represent the relative protein levels of LOX-1, p-p38 and p-Akt, which were normalized to β-actin, and indicate the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
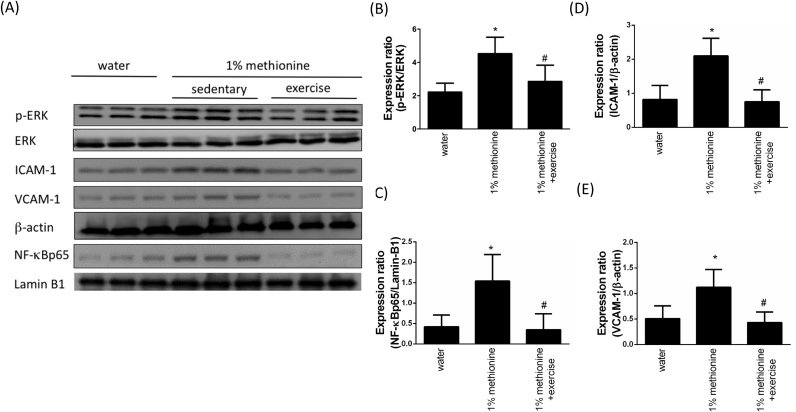
3.5. Exercise repressed HHcy-induced pro-inflammatory events
Previous studies have suggested that p38MAPK leads to the activation of NF-κB, which subsequently triggers downstream pro-inflammatory events [28]. To understand whether exercise protects against HHcy-induced pro-inflammatory responses, we evaluated pro-inflammatory markers using Western blotting. As shown in Fig. 5, we found that HHcy induced phospho-ERK overexpression and NF-κB activation, thereby enhancing ICAM-1 and VCAM-1 expression levels. However, these findings were reversed by exercise intervention.
Fig. 5.
Exercise training repressed HHcy-activated endothelial inflammation. (A) The protein levels of p-ERK, ICAM-1, VCAM-1 in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. Nuclear protein from endothelial tissues was prepared for NF-kBp65 and LaminB1 detection. (B, C, D, E) The bars represent the relative protein levels of p-ERK, ICAM-1 and VCAM-1, which were normalized to β-actin, and the relative protein level of NF-kBp65, which was normalized to Lamin B1. The data are presented as the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
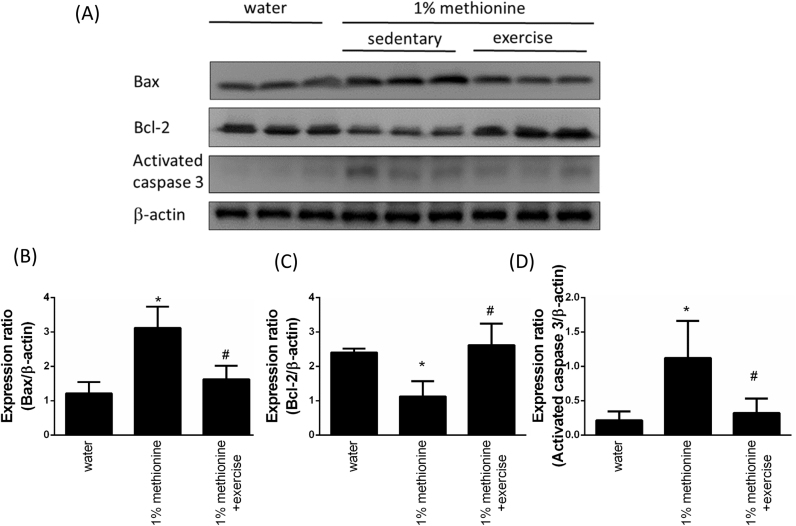
3.6. Exercise reduces HHcy-induced apoptosis in the aortic endothelium
We previously found that exercise protects against HHcy-induced AKT inhibition in the aortic endothelium. AKT plays a key role in maintaining cell survival in response to different stimuli. Next, we investigated the protective effects of HHcy-induced aortic apoptosis. In addition, to further investigate whether exercise intervention reduced upstream components of mitochondria-dependent apoptotic signaling under HHcy conditions, we determined the protein levels of the Bcl-2 family (Bcl-2 and Bax) in the three groups by Western blotting. In Fig. 6A-B, we showed that the levels of pro-apoptotic Bax were higher in the 1% methionine group than the control animals, and Bax levels in the 1% methionine plus exercise animals were lower than that in the 1% methionine group. Bcl-2 expression was reduced in the 1% methionine group compared to the control animals, and Bcl-2 expression in the 1% methionine plus exercise animals was higher than that in the 1% methionine group. In addition, similar results were observed regarding activated-caspase-3 expression, which were reported in Fig. 6A and D.
Fig. 6.
Exercise training repressed HHcy-induced apoptosis. (A) The protein levels of Bax, Bcl-2 and cleaved caspase-3 in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D) The bars represent the relative protein levels of Bax, Bcl-2 and cleaved caspase-3, which were normalized to β-actin, and indicate the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group.
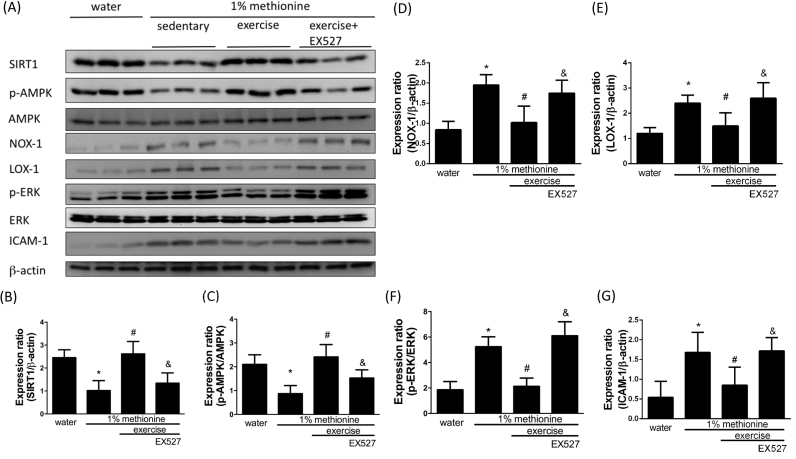
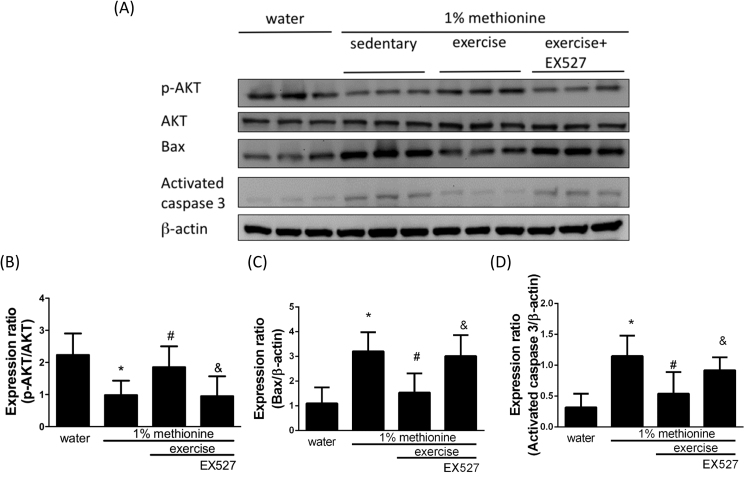
3.7. Exercise protected against HHcy-induced apoptosis and inflammation in the aortic endothelium through the modulation of SIRT1
EX524, a SIRT1 inhibitor, was used to further confirm whether exercise reduces dysfunction of the aortic endothelium through SIRT1 activation. In Fig. 7, we showed that exercise intervention significantly reversed HHcy-induced SIRT1 and phospho-AMPK inhibition to a level similar to that in control animals. Exercise also reduced HHcy-induced NADPH oxidase activation and pro-inflammatory events. SIRT1 and phospho-AMPK expression was reduced to a level similar to that in control animals. Remarkably, EX527 had a powerful inhibitory effect on the exercise-mediated protection against HHcy-induced SIRT1 and phospho-AMPK inhibition (Fig. 7A-C) as well as NOX-1 activation (Fig. 7A and D), LOX-1 up-regulation (Fig. 7A and E) and activation of pro-inflammatory responses (Fig. 7A, F and G). Furthermore, we have also confirmed that EX527 reduced the exercise-mediated protection against HHcy-induced apoptosis in the aortic endothelium (Fig. 8A- D).
Fig. 7.
Exercise training repressed HHcy-activated endothelial inflammation and oxidative stress through the modulation of SIRT1. (A) The protein levels of SIRT1, p-AMPK, NOX-1, LOX-1, p-ERK and ICAM-1 in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D, E, F, G) The bars represent the relative protein levels of SIRT1, p-AMPK, NOX-1, LOX-1, p-ERK and ICAM-1, which were normalized to β-actin, and indicate the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group. &P < 0.05, significant differences compared to the 1% methionine plus exercise group.
Fig. 8.
Exercise training repressed HHcy-activated endothelial apoptosis through the modulation of SIRT1. (A) The protein levels of p-Akt, Bax and cleaved caspase-3 in the aortic endothelium of the control group, the 1% methionine group, and the 1% methionine plus exercise group were investigated by Western blot analysis. (B, C, D) The bars represent the relative protein levels of p-Akt, Bax and cleaved caspase-3, which were normalized to β-actin, and indicate the mean ± SD (n = 8 in each group). *P < 0.05, significant differences compared to the control group. #P < 0.05, significant differences compared to the 1% methionine group. &P < 0.05, significant differences compared to the 1% methionine plus exercise group.
4. Discussion
In summary, our critical discoveries showed that exercise intervention in a HHcy mouse model reduced oxidative stress via SIRT1 activation. In addition, exercise reduced HHcy-induced apoptosis by repressing mitochondria-dependent apoptotic pathways through SIRT1 activation. Exercise also reduced HHcy-induced inflammation in the aortic endothelium. Thus, Hcy might have a critical pathophysiological role, and exercise intervention have a therapeutic effect on vascular dysfunction under Hcy stimulation, which may occur in patients with long-term HHcy.
The initial atherosclerotic injuries arise from a fatty streak within the intimal layer of the human endothelium due to lipid-filled foam cells. Atherosclerotic plaques arise from macrophages that have devoured oxidized low-density lipoprotein (oxLDL) cholesterol molecules. A recent study suggested that dyslipidemia might impact the development of vascular pathologies via high plasma levels of Hcy [29]. Yang et al. have reported that ApoE-/- mice treated with a methionine diet exhibited high levels of blood Hcy and typical atherosclerotic injuries in the thoracic aorta. In vitro investigations further confirmed that Hcy treatment promoted lipid accumulation in the foam cells. They concluded that Hcy promotes the development of atherosclerotic injury and the generation of foam cells, thereby leading to the formation of atherosclerotic plaques [30]. In this present study, we found that animals that received methionine intervention had higher levels of pro-apoptotic and pro-inflammatory events in the aortic endothelium. MDA, which is produced from oxidized lipids, was expressed at higher levels in the plasma of the methionine-fed group. In addition, plasma SOD, an antioxidant enzyme, was also reduced in the methionine-fed group, suggesting that oxidative stress was elevated in the methionine-fed group (Fig. 1).
Pathological endothelium is distinguished by impaired endothelium-dependent vasorelaxation, which symbolizes the initial process in the pathogenesis of atherosclerotic injury [31]. The reported signaling mechanisms of atherosclerotic injury resulted in vascular dysfunction, including impaired eNOS expression by Akt signaling inhibition, elevated oxidative stress and endothelial inflammation [32]. Cardiovascular benefits of SIRT1 have been widely reported; for example, SIRT1 activation reduces oxidative stress, apoptosis and inflammation in response to different stimuli [33]. SIRT1 overexpression protected against high-fat diet-induced atherosclerotic lesions and oxidative stress [34]. SIRT1 inhibition impaired cardiomyocyte ATP production, metabolic flexibility and survival [35]. In addition, Hcy caused the activation of LOX-1 by the regulation of an elaborate mechanism involving PKCβ up-regulation and NADPH oxidase activation, which lead to the degradation of SIRT1 [9]. In this study, through the induction of methionine, our data revealed that SIRT1 and phospho-AMPK expression levels were down-regulated and phospho-PKC expression levels were up-regulated in HHcy animals (Fig. 2).
Previous reports have suggested that exercise training has positive effects on the human cardiovascular system. In addition, exercise intervention has been confirmed to be very helpful for both the delay and management of many diseases, especially cardiovascular diseases [36]. Exercise training enhances SIRT1 activity in aged animals and increases antioxidant capacity [22]. This study also revealed that exercise training significantly reversed SIRT1 inhibition as well as pro-apoptotic and pro-inflammatory events in animals receiving methionine intervention (Figs. 5 and 6). EX527, a pharmacological inhibitor of SIRT1, markedly reduced the therapeutic effects of exercise training, indicating that exercise training protected against HHcy-induced endothelial dysfunction by modulating SIRT1.
The lectin-like oxidized low-density lipoprotein receptor (LOX)-1 is a critical receptor for oxLDL. OxLDL binding to LOX-1 is the first step in the development of atherosclerotic injury [37]. In addition, LOX-1 expression is up-regulated in many pathological scenarios or systemic diseases, such as hypertension, diabetes, and hypercholesterolemia [38]. Up-regulated LOX-1 expression further triggers the formation of free radicals and represses antioxidant capacity, thereby inducing oxidative stress in the cardiovascular system [39]. SIRT1 negatively regulates LOX-1 expression by repressing the LOX-1 promoter [9]. Here, we showed that LOX-1 expression levels were activated in HHcy animals and that this finding was reversed by exercise training through the modulation of SIRT1(Figs. 4 and 7).
The procedure by which the NADPH oxidase enzyme complex is stimulated starts with the phosphorylation of p47phox, which results in the translocation of the p47phox/p67phox complex to the plasma membrane. Subsequently, p47phox collaborates with p22phox, while p67phox acts as a NOX activator via a direct protein–protein interaction. Previous reports have identified that PKC plays a critical role in the up-regulation of NADPH oxidase through the modulation of p47phox in the human cardiovascular system [40], [41]. In this study, we successfully confirmed that exercise reversed HHcy-induced NADPH oxidase activation, MDA up-regulation and SOD repression through SIRT1 activation (Figs. 3 and 7).
Apoptosis and inflammation in terminally differentiated endothelial cells are important pathological mechanisms that induce atherosclerotic injury [42]. Therefore, we set out to confirm whether an understanding of the process of apoptosis and inflammation could allow for the development of new strategies to delay or mitigate atherosclerotic injuries. Exercise training has been suggested to inhibit activated cardiac Fas and mitochondria-dependent apoptotic signaling pathways in hypertensive animal models, DM or metabolic syndromes [43], [44], [45]. In the present study, we further evaluated whether exercise training could protect against endothelial apoptosis in HHcy animals. Our data strongly implied that exercise training did prohibit or mitigate HHcy-induced mitochondria-dependent apoptosis in the endothelium via SIRT1 activation. The current report is the first to suggest that exercise training prevents HHcy-induced mitochondria-dependent apoptosis signaling in the endothelium through the modulation of SIRT1. Moreover, NF-κB, ICAM-1 and VCAM-1 protein expression levels provided further evidence for the anti-inflammatory effects of exercise training (Figs. 7 and 8).
Taken together, the data from the present study suggested that exercise training might be a method for interrupting endothelial apoptosis and preventing the development of atherosclerosis through SIRT1 activation and oxidative stress inhibition under HHcy conditions. Further therapeutic or clinical investigations are required to clarify the potential clinical therapeutic applications.
Conflicts of Interest
None
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology (MOST 105-2311-B-006-008 and 106-2314-B-006-023).
References
- 1.Ladapo J.A., Goldfeld K.S., Douglas P.S. Projected morbidity and mortality from missed diagnoses of coronary artery disease in the United States. Int. J. Cardiol. 2015;195:250–252. doi: 10.1016/j.ijcard.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hankey G.J., Eikelboom J.W. Homocysteine and vascular disease. Lancet. 1999;354(9176):407–413. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- 3.Boushey C.J., Beresford S.A., Omenn G.S., Motulsky A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. J. Am. Med. Assoc. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 4.Yahagi K., Kolodgie F.D., Lutter C., Mori H., Romero M.E., Finn A.V., Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2016 doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orogo A.M., Gustafsson A.B. Cell death in the myocardium: my heart won't go on. IUBMB Life. 2013;65(8):651–656. doi: 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sies H. Academic Press; London: 1985. Oxidative Stress. [Google Scholar]
- 7.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 8.Haidara M.A., Yassin H.Z., Rateb M., Ammar H., Zorkani M.A. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr. Vasc. Pharmacol. 2006;4(3):215–227. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- 9.Hung C.H., Chan S.H., Chu P.M., Tsai K.L. Homocysteine facilitates LOX-1 activation and endothelial death through the PKCbeta and SIRT1/HSF1 mechanism: relevance to human hyperhomocysteinaemia. Clin. Sci. 2015;129(6):477–487. doi: 10.1042/CS20150127. [DOI] [PubMed] [Google Scholar]
- 10.Miller A.A., De Silva T.M., Jackman K.A., Sobey C.G. Effect of gender and sex hormones on vascular oxidative stress. Clin. Exp. Pharmacol. Physiol. 2007;34(10):1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 12.Lei J., Gu X., Ye Z., Shi J., Zheng X. Antiaging effects of simvastatin on vascular endothelial cells. Clin. Appl. Thromb./Hemost.: Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2012 doi: 10.1177/1076029612458967. [DOI] [PubMed] [Google Scholar]
- 13.Orecchia A., Scarponi C., Di Felice F., Cesarini E., Avitabile S., Mai A., Mauro M.L., Sirri V., Zambruno G., Albanesi C., Camilloni G., Failla C.M. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PloS One. 2011;6(9):e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colman R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W., Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarzuelo M.J., Lopez-Sepulveda R., Sanchez M., Romero M., Gomez-Guzman M., Ungvary Z., Perez-Vizcaino F., Jimenez R., Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem. Pharmacol. 2013;85(9):1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Kitada M., Kume S., Imaizumi N., Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60(2):634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao C.L., Chen L.K., Chang Y.L., Yung M.C., Hsu C.C., Chen Y.C., Lo W.L., Chen S.J., Ku H.H., Hwang S.J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010;17(9):970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 18.Gorenne I., Kumar S., Gray K., Figg N., Yu H., Mercer J., Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127(3):386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 19.Fagard R.H. Exercise therapy in hypertensive cardiovascular disease. Prog. Cardiovasc. Dis. 2011;53(6):404–411. doi: 10.1016/j.pcad.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Lavie C.J., Thomas R.J., Squires R.W., Allison T.G., Milani R.V. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin. Proc. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claudio E.R., Almeida S.A., Mengal V., Brasil G.A., Santuzzi C.H., Tiradentes R.V., Gouvea S.A., Bissoli N.S., Santos R.L., Abreu G.R. Swimming training prevents coronary endothelial dysfunction in ovariectomized spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2017;50(1):e5495. doi: 10.1590/1414-431X20165495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N., Rinaldi B., Corbi G., Conti V., Stiuso P., Boccuti S., Rengo G., Rossi F., Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 23.Cho K., Kwon D., Park J., Song Y. Serum levels of appetite-regulating hormones and pro-inflammatory cytokines are ameliorated by a CLA diet and endurance exercise in rats fed a high-fat diet. J. Exerc. Nutr. Biochem. 2015;19(4):303–309. doi: 10.5717/jenb.2015.15120905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Zhao Z., Cai J., Gu B., Lv Y., Zhao L. The frequency-dependent aerobic exercise effects of hypothalamic GABAergic expression and cardiovascular functions in aged rats. Front. Aging Neurosci. 2017;9:212. doi: 10.3389/fnagi.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond G.R., Selemidis S., Griendling K.K., Sobey C.G. Combating oxidative stress in vascular disease: nadph oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011;10(6):453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerkeni M., Added F., Ben Farhat M., Miled A., Trivin F., Maaroufi K. Hyperhomocysteinaemia and parameters of antioxidative defence in Tunisian patients with coronary heart disease. Ann. Clin. Biochem. 2008;45(Pt 2):193–198. doi: 10.1258/acb.2007.007066. [DOI] [PubMed] [Google Scholar]
- 27.Tsai K.L., Chen L.H., Chiou S.H., Chiou G.Y., Chen Y.C., Chou H.Y., Chen L.K., Chen H.Y., Chiu T.H., Tsai C.S., Ou H.C., Kao C.L. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011;55(Suppl 2):S227–S240. doi: 10.1002/mnfr.201100147. [DOI] [PubMed] [Google Scholar]
- 28.Chen X.P., Zhang T.T., Du G.H. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc. Drug Rev. 2007;25(2):146–161. doi: 10.1111/j.1527-3466.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 29.Wierda R.J., Rietveld I.M., van Eggermond M.C., Belien J.A., van Zwet E.W., Lindeman J.H., van den Elsen P.J. Global histone H3 lysine 27 triple methylation levels are reduced in vessels with advanced atherosclerotic plaques. Life Sci. 2015;129:3–9. doi: 10.1016/j.lfs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Xiaoling Y., Li Z., ShuQiang L., Shengchao M., Anning Y., Ning D., Nan L., Yuexia J., Xiaoming Y., Guizhong L., Yideng J. Hyperhomocysteinemia in ApoE-/- mice leads to overexpression of enhancer of Zeste Homolog 2 via miR-92a regulation. PLoS One. 2016;11(12):e0167744. doi: 10.1371/journal.pone.0167744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimbrone M.A., Jr., Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donato A.J., Morgan R.G., Walker A.E., Lesniewski L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015;89(Pt B):122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C., Chen L., Hou X., Li Z., Kabra N., Ma Y., Nemoto S., Finkel T., Gu W., Cress W.D., Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat. Cell Biol. 2006;8(9):1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q.J., Wang Z., Chen H.Z., Zhou S., Zheng W., Liu G., Wei Y.S., Cai H., Liu D.P., Liang C.C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2008;80(2):191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bround M.J., Wambolt R., Luciani D.S., Kulpa J.E., Rodrigues B., Brownsey R.W., Allard M.F., Johnson J.D. Cardiomyocyte ATP production, metabolic flexibility, and survival require calcium flux through cardiac ryanodine receptors in vivo. J. Biol. Chem. 2013;288(26):18975–18986. doi: 10.1074/jbc.M112.427062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti V., Russomanno G., Corbi G., Filippelli A. Exercise training in aging and diseases. Transl. Med. UniSa. 2012;3:74–80. [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal T., Mitra S., Khaidakov M., Wang X., Singla S., Ding Z., Liu S., Mehta J.L. Current Concepts of the Role of Oxidized LDL Receptors in Atherosclerosis. Curr. Atheroscler. Rep. 2012 doi: 10.1007/s11883-012-0228-1. [DOI] [PubMed] [Google Scholar]
- 38.Mehta J.L., Chen J., Hermonat P.L., Romeo F., Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006;69(1):36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Makino J., Asai R., Hashimoto M., Kamiya T., Hara H., Ninomiya M., Koketsu M., Adachi T. Suppression of EC-SOD by oxLDL During Vascular Smooth Muscle Cell Proliferation. J. Cell. Biochem. 2016;117(11):2496–2505. doi: 10.1002/jcb.25542. [DOI] [PubMed] [Google Scholar]
- 40.White C.N., Figtree G.A., Liu C.C., Garcia A., Hamilton E.J., Chia K.K., Rasmussen H.H. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am. J. Physiol. Cell Physiol. 2009;296(4):C693–C700. doi: 10.1152/ajpcell.00648.2008. [DOI] [PubMed] [Google Scholar]
- 41.Ungvari Z., Csiszar A., Huang A., Kaminski P.M., Wolin M.S., Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108(10):1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 42.Tsai K.L., Hung C.H., Chan S.H., Shih J.Y., Cheng Y.H., Tsai Y.J., Lin H.C., Chu P.M. Baicalein protects against oxLDL-caused oxidative stress and inflammation by modulation of AMPK-alpha. Oncotarget. 2016;7(45):72458–72468. doi: 10.18632/oncotarget.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C.Y., Yang A.L., Lin Y.M., Wu F.N., Lin J.A., Chan Y.S., Tsai F.J., Tsai C.H., Kuo C.H., Lee S.D. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J. Appl. Physiol. 1985;112(5) doi: 10.1152/japplphysiol.00605.2011. (883-91) [DOI] [PubMed] [Google Scholar]
- 44.Lee S.D., Shyu W.C., Cheng I.S., Kuo C.H., Chan Y.S., Lin Y.M., Tasi C.Y., Tsai C.H., Ho T.J., Huang C.Y. Effects of exercise training on cardiac apoptosis in obese rats. Nutr. Metab. Cardiovasc. Dis. 2013;23(6):566–573. doi: 10.1016/j.numecd.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S.M., Ho T.J., Yang A.L., Chen I.J., Kao C.L., Wu F.N., Lin J.A., Kuo C.H., Ou H.C., Huang C.Y., Lee S.D. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int. J. Cardiol. 2013;167(2):478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]