Abstract
Objective
A low ankle-brachial index (ABI) is a known predictor for future cardiovascular events and mortality in patients with chronic kidney disease (CKD). While most prior studies have defined CKD as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, recent reports have suggested that the cardiovascular risk may be increased even in early stages of renal insufficiency. We hypothesized that a low ABI may predict future cardiovascular morbidity and mortality in patients with mild impairment of the renal function.
Methods
The IMPACT-ABI study was a retrospective, single-center, cohort study that enrolled and obtained ABI measurements for 3,131 patients hospitalized for cardiovascular disease between January 2005 and December 2012. From this cohort, we identified 1,500 patients with mild renal insufficiency (eGFR =60-89 mL/min/1.73 m2), and stratified them into 2 groups: ABI ≤0.9 (low ABI group; 9.2%) and ABI >0.9 (90.8%). The primary outcome measured was the cumulative incidence of major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, and stroke).
Results
Over a mean follow-up of 5.0 years, 101 MACE occurred. The incidence of MACE was significantly higher in patients with low ABI than in those with ABI >0.9 (30.2% vs. 14.4%, log rank p<0.001). A low ABI was associated with MACE in a univariate Cox proportional hazard analysis. A low ABI remained an independent predictor of MACE in a multivariate analysis adjusted for cardiovascular risk factors (hazard ratio (HR): 2.27; 95% confidence interval (CI): 1.33-3.86; p=0.002).
Conclusion
Low ABI was an independent predictor for MACE in patients with mild renal insufficiency.
Keywords: ankle brachial index, estimated glomerular filtration rate, mild renal insufficiency, peripheral artery disease, prognosis
Introduction
Peripheral artery disease (PAD) is widely known as a predictor of future cardiovascular and cerebrovascular morbidity and mortality (1-3). The ankle-brachial index (ABI) is used to detect PAD with high specificity, and a low ABI (ABI ≤0.9) is regarded as a strong predictor of future cardiovascular events and mortality (4-6). Chronic kidney disease (CKD) is also an established risk factor for cardiovascular disease and all-cause mortality (7,8). It has been demonstrated that PAD and CKD share a number of risk factors, and patients with CKD are at an increased risk of incident PAD (9). It was previously reported that patients with CKD accompanied with PAD had a particularly high risk of cardiovascular and all-cause mortality (10). Furthermore, an ABI <0.9 has been shown to be a significant predictor of mortality in patients with advanced-stage CKD or undergoing hemodialysis (11,12). While most prior studies have defined CKD as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, recent reports have suggested that the cardiovascular risk may start to increase even earlier, at an eGFR of 90 mL/min/1.73 m2 (13-15). However, the combined effect of low ABI and such a mildly reduced kidney function on cardiovascular events and mortality has not been well investigated.
In present study, we assessed whether or not a low ABI was able to predict future cardiovascular events and mortality in patients with mild renal insufficiency.
Materials and Methods
Study populations
This study was a sub-analysis of the impressive predictive value of ankle brachial index for clinical long term outcome in patients with cardiovascular disease examined by ABI (IMPACT-ABI) study. The IMPACT-ABI study was a retrospective, single-center, cohort study that enrolled 3,131 consecutive patients who were admitted to Shinshu University for cardiovascular disease between January 2005 and December 2012. All patients had their ABI measured upon admission, with no exclusion criteria. We obtained the clinical, demographic, and laboratory data from the medical records. Follow-up data were collected from medical records or by contacting patients. This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Shinshu University School of Medicine. As the present study was performed retrospectively without written informed consent, the data were analyzed anonymously. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), as accepted by the International Committee of Medical Journal Editors (UMIN-ID; 000020276).
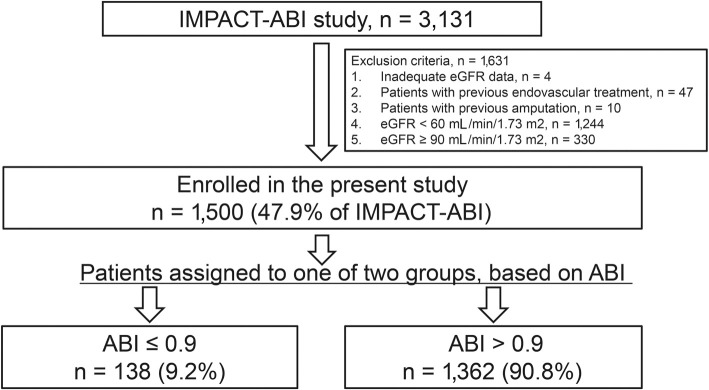
Of the initial 3,131 patients, 4 were excluded due to inadequate eGFR data, 47 for previous endovascular treatment, 10 for previous amputation, 1,244 for eGFR <60 mL/min/1.73 m2, and 330 for eGFR ≥90 mL/min/1.73 m2. We therefore ultimately evaluated the 1,500 patients who exhibited mild renal insufficiency (eGFR 60-89 mL/min/1.73 m2). We divided the study cohort into 2 groups based on the ABI: a low ABI group (ABI ≤0.9), and ABI >0.9 group. We then analyzed the relationship between the ABI and prognosis (Fig. 1).
Figure 1.
Patient flow diagram illustrating the inclusion process and exclusion criteria. The present study selected patients with mild renal insufficiency from among cardiovascular disease patients enrolled in the IMPACT-ABI (Impressive Predictive Value of Ankle Brachial Index for Very Long Term Outcome in Patients with Cardiovascular Disease) study. Mild renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) of 60-89 mL/min/1.73 m2. Patients were stratified into 2 groups based on their ankle-brachial index (ABI) value.
Definitions
The GFR was estimated using the Japanese equation for estimating the kidney function, as follows: eGFR (mL/min/1.73 m2) =194× Serum creatinine-1.094 × Age-0.287 for male patients, with the same calculation multiplied by 0.739 for female patients (16). A patient was defined as having PAD when presenting with ABI values ≤0.9 in either leg (6). All-cause death was defined as any death recorded during the follow-up period. Cardiovascular death was defined as death resulting from acute myocardial infarction (MI), significant cardiac arrhythmia, congestive heart failure (HF), stroke, or other cardiovascular causes (17). MI was defined as a 2-fold rise in serum troponin I or in creatine kinase-MB isoenzyme to least twice the upper normal limits with acute onset of prolonged typical ischemic chest pain, ST-segment elevation of at least 1 mm in 2 contiguous electrocardiogram leads, or ST-segment depression of at least 0.5 mm in 2 contiguous leads (17). Stroke was defined as ischemic stroke that persist for ≥24 hours or evidence of infarction on magnetic resonance imaging (17). Previous HF was defined as a prior diagnosis of HF according to the Framingham criteria (18) or current treatment for HF. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the current use of anti-hypertensive agents. Dyslipidemia was defined as serum total cholesterol levels ≥220 mg/dL, low-density lipoprotein cholesterol levels ≥140 mg/dL, high-density lipoprotein cholesterol levels ≤40 mg/dL, triglyceride levels ≥150 mg/dL, or the use of lipid-lowering agents. Coronary heart disease was defined as a history of angina or previous MI. Diabetes mellitus (DM) was defined as fasting blood glucose levels ≥126 mg/dL, casual plasma glucose levels ≥200 mg/dL, HbA1c ≥6.5%, or the use of insulin or oral hypoglycemic agents. Anemia was defined as hemoglobin levels <13.0 g/dL in men or <12.0 g/dL in women (19). A smoking habit was defined according to the current smoking status, as obtained by an interview. Echocardiographic parameters included the left ventricular ejection fraction (LVEF), as estimated using the Teichholz method.
ABI measurement
The ABI was determined using the form pulse wave velocity (PWV)/ABI (Omron Colin, Tokyo, Japan) with the patient at rest in the supine position for at least 10 minutes. The form PWV/ABI is an automated oscillometric device with four cuffs that can measure the blood pressure in both the upper and lower extremities simultaneously. The ABI was obtained as the ratio of the systolic blood pressure measured in the lower extremity divided by the higher of the two systolic blood pressures measured in the upper extremities. The lower of the two results was used as the patient ABI for all analyses.
Outcome measures
The primary outcome of this study was the cumulative incidence of major adverse cardiovascular events (MACE), including cardiovascular death, MI, and stroke. The secondary outcome was all-cause death, cardiovascular death, MI, and stroke.
Statistical analyses
Continuous variables were presented as the median and interquartile range (25th to 75th percentile), as these variables were non-normally distributed. Normal distribution of data was tested using the Shapiro-Wilk test. Categorical variables were expressed as numbers and percentages. Differences regarding categorical variables were assessed using the chi-squared test, while the Mann-Whitney U test was used to assess differences regarding continuous variables. Cumulative occurrences were estimated using the Kaplan-Meier method, and differences were compared using the log-rank test. For each group, univariate Cox proportional hazard analyses were performed to determine the independence of the predictors of MACE and all-cause death. Variables that were associated with MACE and all-cause death in the univariate analysis (p<0.1) were entered into the multivariable model. The magnitude of the relationship between variables and MACE or all-cause death was expressed as the hazard ratio (HR) and 95% confidence interval (CI). A two-tailed p<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS version 22 software program (SPSS, Chicago, USA).
Results
Characteristics of the study population
The baseline characteristics of our cohort are shown in Table 1. The median age was 68 years, and 70.1% of the patients included in the study were male. The causes of admission among the enrolled patients were acute MI (4.0%), unstable angina pectoris (1.6%), stable angina pectoris (27.3%), other atherosclerotic diseases (28.2%), arrhythmia (21.6%), heart failure (12.8%), and others (3.6%). The patients were stratified into 2 groups as follows: 138 patients (9.2%) had a low ABI (≤0.9), while 1,362 patients (90.8%) had an ABI >0.9. No marked differences between the two groups were observed with respect to dyslipidemia, eGFR, atrial fibrillation, previous HF, coronary heart disease, previous MI, LVEF, or levels of B-type natriuretic peptide (BNP). However, patients with a low ABI were significantly older, more predominantly male smokers, and had a lower body mass index (BMI) and a higher incidence of hypertension, DM, anemia, and previous cerebral infarction than those with an ABI >0.9. The use of antiplatelet agents and angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) was significantly higher in patients with a low ABI, while the use of beta-blockers was significantly higher in patients with an ABI >0.9.
Table 1.
Baseline Characteristics.
| Variables | Overall (N) | ABI≤0.9 | ABI>0.9 | p value |
|---|---|---|---|---|
| 1,500 | 138 | 1,362 | ||
| Age (years) | 68 (59–75) | 73 (65–79) | 67 (59–75) | <0.001 |
| Female sex | 449 (29.9%) | 28 (20.2%) | 421 (30.9%) | 0.009 |
| ABI | 1.12 (1.04–1.17) | 0.68 (0.56–0.80) | 1.13 (1.07–1.18) | <0.001 |
| Body mass index (kg/m2) | 23.3 (21.2–25.5) | 22.2 (20.4–23.9) | 23.4 (21.4–25.7) | <0.001 |
| Hypertension | 834 (55.6%) | 93 (67.4%) | 741 (54.4%) | 0.003 |
| Dyslipidemia | 667 (44.5%) | 64 (46.3%) | 603 (44.2%) | 0.636 |
| Diabetes mellitus | 348 (23.2%) | 48 (34.7%) | 300 (22.0%) | 0.001 |
| Smoking habit | 677 (45.1%) | 92 (66.6%) | 585 (42.9%) | <0.001 |
| eGFR (mL/min/1.73 m2) | 71.8 (65.6–79.0) | 72.5 (65.2–79.0) | 71.8 (65.8–79.0) | 0.929 |
| Atrial fibrillation | 172 (11.4%) | 17 (12.3%) | 155 (11.3%) | 0.749 |
| Anemia | 203 (13.5%) | 28 (20.3%) | 175 (12.8%) | 0.015 |
| Coronary heart disease | 262 (17.4%) | 32 (23.1%) | 230 (16.8%) | 0.063 |
| Previous myocardial infarction | 211 (14.0%) | 15 (10.8%) | 196 (14.3%) | 0.268 |
| Previous cerebral infarction | 83 (5.5%) | 16 (11.5%) | 67 (4.9%) | 0.001 |
| Previous heart failure | 81 (5.4%) | 8 (5.7%) | 73 (5.3%) | 0.829 |
| Left ventricle ejection fraction | 69 (60.6–75.3) | 68.7 (62.2–76.8) | 69.0 (60.6–75.3) | 0.686 |
| BNP (pg/mL) | 39.9 (18.5–93.3) | 47.7 (21.0–95.0) | 39.8 (18.3–95.1) | 0.224 |
| Medication | ||||
| Aspirin | 659 (43.9%) | 77 (55.7%) | 582 (42.7%) | 0.004 |
| Thienopyridines | 352 (23.5%) | 46 (33.3%) | 306 (22.4%) | 0.004 |
| Cilostazol | 82 (5.5%) | 42 (30.4%) | 40 (2.9%) | <0.001 |
| ACEI/ARB | 651 (43.4%) | 75 (54.3%) | 576 (42.2%) | 0.008 |
| Beta-blockers | 355 (23.7%) | 23 (16.6%) | 332 (24.3%) | 0.036 |
| Statin | 599 (37.4%) | 64 (46.3%) | 535 (39.2%) | 0.124 |
| Warfarin | 275 (18.3%) | 25 (18.1%) | 252 (18.5%) | 0.851 |
Data are shown as median (interquartile range), or total number (percentage). ABI: ankle-brachial index, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, BNP: B-type natriuretic peptide, eGFR: estimated glomerular filtration rate
Distribution of ABI values
The distribution of ABI values is illustrated in Fig. 2. The median value (interquartile range) was 1.12 (1.04-1.17), with the most frequent values ranging from 1.10 to 1.19 (40.1%), followed by those ranging from 1.00 to 1.09 (26.3%). The prevalence of a low ABI (ABI ≤0.9) was 9.2%.
Figure 2.
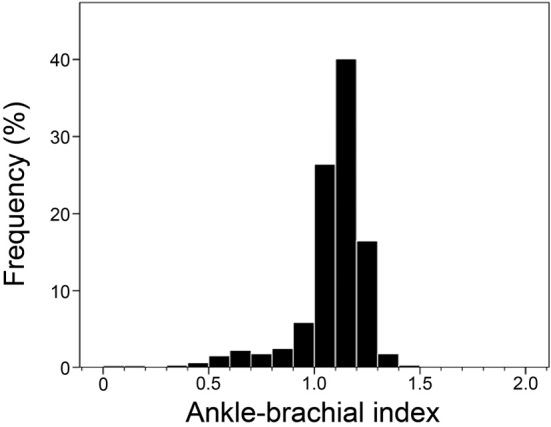
The distribution of the ankle-brachial index values in our cohort.
Incidence of MACE and all-cause death
During the follow-up period (median, 5.0 years), a total of 101 MACE occurred, including 66 cardiovascular deaths, 32 strokes, and 18 MIs. There were 163 all-cause deaths (cardiac, 52; stroke, 14; cancer, 42; respiratory failure, 17; infection, 3; renal failure, 1; decrepitude, 8; other, 26). In the group of patients with a low ABI, a total of 21 MACE (cardiovascular death, 13; stroke, 7; MI, 4) and 29 all-cause deaths occurred. In the group of patients with ABI >0.9, a total of 80 MACE (cardiovascular death, 53; stroke, 25; MI, 14) and 134 all-cause deaths occurred.
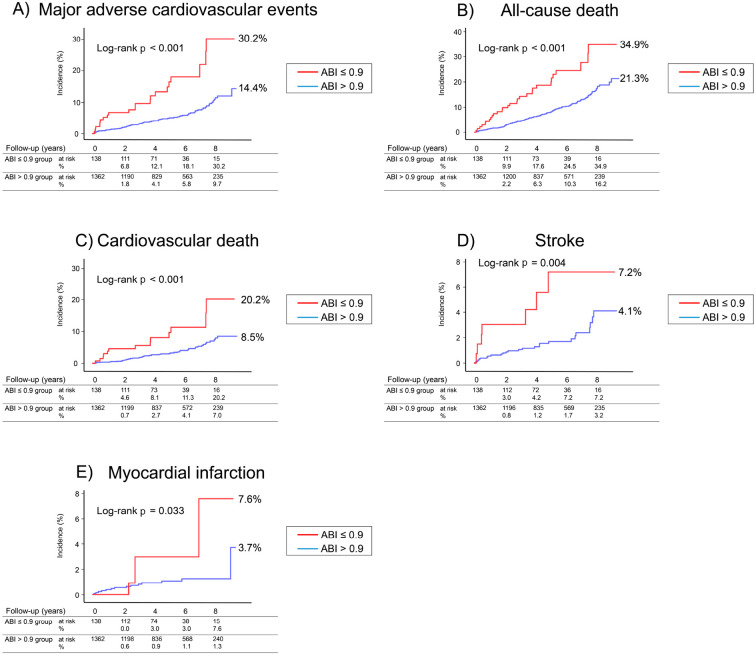
The Kaplan-Meier analysis showed that the cumulative incidence of MACE (Fig. 3A) was markedly higher in patients with a low ABI than in those with ABI >0.9 (30.2% vs. 14.4%, log rank p<0.001). Similarly, the cumulative incidences for all-cause death, cardiovascular death, stroke, and MI (Fig. 3B-E) were significantly higher in the patients with a low ABI than in those with an ABI >0.9 (34.9% vs. 21.3%, log rank p<0.001; 20.2% vs. 8.5%, log rank p<0.001; 7.2% vs. 4.1%, log rank p=0.004; 7.6% vs. 3.7%, log rank p=0.033, respectively).
Figure 3.
A Kaplan-Meier plot for (A) major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke), (B) all-cause death, (C) cardiovascular death, (D) stroke, and (E) myocardial infarction. Two groups of patients were included in the analysis. The patients were assigned to a group according to their ankle-brachial index (ABI) value.
Predictors of MACE and all-cause death
We performed Cox proportional hazard analyses to evaluate the prognostic value of a low ABI for MACE (Table 2). The univariate analysis showed that the HR of low ABI for MACE was 3.08 (95% CI: 1.90-5.00; p<0.001). After adjusting for age, sex, medication, and traditional cardiovascular risk factors, a low ABI remained an independent predictor of MACE (HR: 2.27; 95% CI: 1.33-3.86; p=0.002). Additional independent predictors of MACE were advanced age, reduced LVEF, ACEI and/or ARB use, and a smoking habit.
Table 2.
Cox Proportional Hazard Analysis for Major Adverse Cardiovascular Events (Cardiovascular Death, Myocardial Infarction, and Stroke) during the Follow-up Period.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p value | HR (95% CI) | p value |
| ABI≤0.90 | 3.08 (1.90–5.00) | <0.001 | 2.27 (1.33–3.86) | 0.002 |
| Age (for each 10 year increase) | 1.44 (1.19–1.75) | <0.001 | 1.40 (1.14–1.73) | 0.001 |
| Female sex | 0.43 (0.25–0.73) | 0.002 | 0.84 (0.45–1.58) | 0.607 |
| BMI (for each 1–kg/m2 increase) | 0.99 (0.94–1.05) | 0.862 | ||
| Previous myocardial infarction | 1.48 (0.90–2.42) | 0.114 | ||
| Previous cerebral infarction | 2.28 (1.22–4.27) | 0.010 | 1.71 (0.88–3.34) | 0.113 |
| Hypertension | 1.00 (0.67–1.48) | 0.992 | ||
| Dyslipidemia | 0.57 (0.38–0.87) | 0.010 | 0.60 (0.38–0.94) | 0.029 |
| Diabetes | 1.38 (0.90–2.11) | 0.139 | ||
| Atrial fibrillation | 1.57 (0.92–2.69) | 0.094 | 1.53 (0.85–2.76) | 0.152 |
| LVEF (for each 1% increase) | 0.97 (0.96–0.98) | <0.001 | 0.97 (0.95–0.98) | <0.001 |
| eGFR (mL/min/1.73 m2) | 1.00 (0.97–1.02) | 0.787 | ||
| Smoking habit | 2.09 (1.40–3.13) | <0.001 | 1.68 (1.02–2.77) | 0.041 |
| Anemia | 1.89 (1.17–3.07) | 0.009 | 1.61 (0.93–2.77) | 0.085 |
| BNP (for each 100pg/mL increase) | 1.14 (1.05–1.23) | <0.001 | 0.96 (0.85–1.09) | 0.551 |
| Antiplatelet agents | 1.29 (0.85–1.94) | 0.220 | ||
| ACEI/ARB | 2.12 (1.39–3.23) | <0.001 | 1.68 (1.06–2.65) | 0.026 |
| Beta-blockers | 1.23 (0.78–1.95) | 0.367 | ||
| Statin | 0.99 (0.65–1.49) | 0.960 | ||
ABI: ankle-brachial index, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, BMI: body mass index, BNP: B-type natriuretic peptide, CI: confidence interval, eGFR: estimated glomerular filtration rate, HR: hazard ratio, LVEF: left ventricle ejection fraction
Table 3 contains the results of the univariate and multivariate Cox regression analyses for predictors of all-cause mortality. In the multivariate analysis adjusted for all factors found to be associated with all-cause death in the univariate analysis (i.e. age, sex, BMI, previous cerebral infarction, dyslipidemia, LVEF, smoking habit, anemia, ACEI and/or ARB use, and the levels of BNP), a low ABI was an independent predictor of all-cause death (HR: 1.58; 95% CI: 1.02-2.45; p=0.040).
Table 3.
Cox Proportional Hazard Analysis for All-cause Death during the Follow-up Period.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p value | HR (95% CI) | p value |
| ABI≤0.90 | 2.50 (1.67–3.74) | <0.001 | 1.58 (1.02–2.45) | 0.040 |
| Age (for each 10 year increase) | 1.98 (1.67–2.35) | <0.001 | 1.81 (1.51–2.18) | <0.001 |
| Female sex | 0.55 (0.38–0.81) | 0.003 | 0.77 (0.48–1.24) | 0.288 |
| BMI (for each 1–kg/m2 increase) | 0.93 (0.89–0.98) | 0.005 | 0.97 (0.91–1.02) | 0.282 |
| Previous myocardial infarction | 1.37 (0.92–2.04) | 0.114 | ||
| Previous cerebral infarction | 1.92 (1.13–3.27) | 0.016 | 1.50 (0.86–2.62) | 0.149 |
| Hypertension | 1.11 (0.81–1.52) | 0.483 | ||
| Dyslipidemia | 0.60 (0.44–0.84) | 0.003 | 0.67 (0.47–0.96) | 0.030 |
| Diabetes | 1.21 (0.86–1.71) | 0.269 | ||
| Atrial fibrillation | 1.38 (0.89–2.16) | 0.141 | ||
| LVEF (for each 1% increase) | 0.98 (0.97–0.99) | 0.017 | 0.98 (0.97–1.00) | 0.044 |
| eGFR (mL/min/1.73 m2) | 0.99 (0.97–1.01) | 0.736 | ||
| Smoking habit | 1.67 (1.22–2.28) | 0.001 | 1.50 (1.02–2.20) | 0.038 |
| Anemia | 2.49 (1.75–3.54) | <0.001 | 1.79 (1.21–2.65) | 0.004 |
| BNP (for each 100-pg/mL increase) | 1.13 (1.06–1.20) | <0.001 | 1.01 (0.92–1.10) | 0.785 |
| Antiplatelet agents | 1.08 (0.79–1.49) | 0.607 | ||
| ACEI/ARB | 1.45 (1.05–1.99) | 0.021 | 1.22 (0.87–1.71) | 0.237 |
| Beta-blockers | 0.89 (0.60–1.32) | 0.579 | ||
| Statin | 0.86 (0.62–1.20) | 0.390 | ||
ABI: ankle-brachial index, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, BMI: body mass index, BNP: B-type natriuretic peptide, CI: confidence interval, eGFR: estimated glomerular filtration rate, HR: hazard ratio, LVEF: left ventricle ejection fraction
Predictors of MACE in patients with CKD
In a separate analysis, we also identified 1,244 patients with CKD (eGFR <60 mL/min/1.73 m2). A total of 51 MACE occurred in patients with low ABI (n=279). In patients with ABI >0.9 (n=965), a total of 113 MACE occurred. We next performed a multivariate Cox proportional analysis adjusted for traditional cardiovascular risk factors. As shown in Table 4, low ABI was an independent predictor of MACE in this group (HR: 1.57; 95% CI: 1.08-2.26; p=0.016).
Table 4.
Cox Proportional Hazard Analysis for Major Adverse Cardiovascular Events (Cardiovascular Death, Myocardial Infarction, and Stroke) during the Follow-up Period in Patients with Chronic Kidney Disease.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p value | HR (95% CI) | p value |
| ABI≤0.90 | 2.18 (1.56–3.05) | <0.001 | 1.57 (1.08–2.26) | 0.016 |
| Age (for each 10 year increase) | 1.03 (1.01–1.04) | <0.001 | 1.04 (1.02–1.06) | <0.001 |
| Female sex | 0.87 (0.60–1.25) | 0.470 | ||
| BMI (for each 1–kg/m2 increase) | 0.92 (0.89–0.96) | <0.001 | 0.95 (0.91–0.99) | 0.034 |
| Previous myocardial infarction | 1.16 (0.77–1.73) | 0.458 | ||
| Previous cerebral infarction | 2.09 (1.40–3.10) | <0.001 | 1.87 (1.22–2.86) | 0.004 |
| Hypertension | 1.01 (0.71–1.42) | 0.953 | ||
| Dyslipidemia | 0.68 (0.49–0.93) | 0.019 | 1.01 (0.70–1.48) | 0.927 |
| Diabetes | 1.26 (0.92–1.72) | 0.148 | ||
| Atrial fibrillation | 1.19 (0.81–1.77) | 0.366 | ||
| LVEF (for each 1% increase) | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | 0.003 |
| eGFR (mL/min/1.73 m2) | 0.97 (0.96–0.98) | <0.001 | 0.97 (0.97–0.98) | <0.001 |
| Smoking habit | 0.97 (0.71–1.32) | 0.87 | ||
| Anemia | 1.51 (1.11–2.07) | 0.008 | 0.95 (0.66–1.36) | 0.954 |
| BNP (for each 100pg/mL increase) | 1.07 (1.04–1.10) | <0.001 | 1.03 (1.00–1.07) | 0.019 |
| Antiplatelet agents | 1.13 (0.83–1.54) | 0.426 | ||
| ACEI/ARB | 1.56 (1.13–2.15) | 0.006 | 1.44 (1.01–2.06) | 0.042 |
| Beta-blockers | 1.42 (1.03–1.96) | 0.037 | 1.25 (0.87–1.78) | 0.220 |
| Statin | 0.69 (0.49–0.95) | 0.027 | 0.74 (0.50–1.08) | 0.121 |
ABI: ankle-brachial index, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, BMI: body mass index, BNP: B-type natriuretic peptide, CI: confidence interval, eGFR: estimated glomerular filtration rate, HR: hazard ratio, LVEF: left ventricle ejection fraction
Discussion
To our knowledge, this is the first report describing the predictive power of a low ABI on the prognosis of patients with a mildly reduced kidney function. We demonstrated that the incidence of MACE (cardiovascular death, MI, and stroke) in patients with mild renal insufficiency was markedly higher if the ABI was low (ABI ≤0.9) than if the ABI was >0.9. Furthermore, the all-cause mortality rate during the follow-up period was significantly higher in patients with a low ABI. Another important finding of the current study was that a low ABI was an independent predictor for MACE and all-cause mortality in patients with mild renal insufficiency. Additional independent predictors of MACE were advanced age, reduced LVEF, ACEI or ARB use, and smoking habit. Our results support those obtained in previous randomized studies (19-22). In our cohort of patients with mild renal insufficiency, the group of patients with a low ABI contained more males and tended to be significantly older with more conventional cardiovascular risk factors than the patients with ABI >0.9. However, after adjusting for these risk factors, low ABI was found to be independently associated with MACE and all-cause death.
While most prior studies defined CKD as an eGFR <60 mL/min/1.73 m2, several recent findings have suggested that increased cardiovascular morbidity and mortality are already observed at eGFR levels below 90 mL/min/1.73 m2 (13-15). Despite its poor prognosis, the combined effect of PAD and mild renal insufficiency has not been discussed. It is important to note that the number of individuals with such mildly impaired renal function is estimated to be 63 million among the general Japanese population (23). We showed that the incidence of MACE, all-cause death, cardiovascular death, MI, and stroke in patients with mild renal insufficiency was significantly higher if the ABI was low. Therefore, early screening of such individuals and subsequent surveillance of the identified high-risk patients are essential. At the very least, we should start treatment for these patients based on the latest the American College of Cardiology Foundation/the American Heart Association (ACCF/AHA) guidelines for patients with PAD, including antiplatelet therapy and smoking cessation (6). In particular, smoking prevalence was significantly higher in patients with a low ABI in this study than in those with a higher ABI (Table 1). Furthermore, a smoking habit was an independent predictor for MACE (Table 2). Smoking cessation may be recommended for patients with mild renal insufficiency and a low ABI.
The prevalence of a low ABI in the general Japanese population varies from 1.47% (24) to 2.7% (25). The prevalence of a low ABI in our cohort was 9.2%, which is higher than that in the general Japanese population. However, with respect to the ABI values, the median (interquartile range) in our cohort was 1.12 (1.04-1.17), while most values ranged from 1.10 to 1.19 (40.1%), followed by values ranging from 1.00 to 1.09 (26.3%). This distribution of the ABI values is similar to that reported in prior studies of the Japanese general population (24,25). As PAD is widely regarded to be a clinical manifestation of advanced systemic atherosclerosis (3), it is likely that mild renal impairment is related to manifestations of the initial stages of atherosclerosis.
Luo et al. reported that patients with PAD combined with CKD had significantly higher rates of all-cause and cardiovascular mortality than patients with PAD alone or CKD alone (10). Several authors have shown that, in patients with PAD, a lower eGFR was related to a higher rate of cardiovascular events and mortality (26,27). However, these studies focused on patients with an eGFR of <60 mL/min/1.73 m2. Previous studies have shown that a low ABI is an independent predictor of all-cause and cardiovascular mortality in patients with advanced-stage CKD or patients undergoing hemodialysis (11,12). Consistent with previous studies, low ABI was an independent predictor of MACE in patients with CKD in this study. We further showed that a low ABI was associated with an alarmingly increased incidence of MACE and all-cause mortality, even in patients with mild renal insufficiency. Thus, special attention should be given to patients with mild renal insufficiency that exhibit a low ABI, because this patient population is more likely to experience cardiovascular events in the future than those with higher ABI values. Early detection of low ABI in patients with a mildly reduced renal function and treatment based on the latest ACCF/AHA guidelines for the management of patients with PAD may help prevent the progression of cardiovascular disease.
While our study population was large and we were able to detect a sufficient number of cardiovascular events, our study is limited in several ways. First, it was a retrospective analysis and involved patients who were hospitalized for cardiovascular disease at a single institution in Japan. Thus, our study population may not be representative of the general population. Second, we did not have access to information regarding proteinuria or albuminuria. The combination of proteinuria and a reduced eGFR was found to be associated with a substantial increase in mortality (28), while microalbuminuria was found to be correlated with cardiovascular and non-cardiovascular mortality in the general population (29). We were therefore unable to assess the relationship between a low ABI and mild renal dysfunction after adjusting for proteinuria or albuminuria. Third, we assumed the treatment based on the PAD guidelines to be effective in patients with mild renal insufficiency and a low ABI. Although it is important to note that any risk reduction therapy in these patients is speculative, because there are no prospective randomized controlled trials that have examined the effectiveness of therapy in patients with mild renal insufficiency and a low ABI. Finally, although it is possible that there is a combined effect of mild renal insufficiency and PAD promoting the atherosclerotic process, which in turn contributes to cardiovascular events and mortality, we were unable to completely clarify the pathophysiologic mechanism underlying the mildly impaired renal function in the presence of a low ABI that is associated with the prognosis of cardiovascular disease. Future investigations are warranted in order to determine the pathophysiologic mechanism underlying the combined effect of PAD and mild renal insufficiency that leads to a poor prognosis.
Conclusion
We found that patients with a mildly impaired renal function accompanied with a low ABI had a significantly higher incidence of MACE and all-cause death than patients with an ABI >0.9. Furthermore, a low ABI was an independent predictor of MACE and all-cause death.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors wish to extend their thanks to Minako Aono for her secretarial and technical assistance.
References
- 1. Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol 103: 130-135, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol 57: 294-300, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 326: 381-386, 1992. [DOI] [PubMed] [Google Scholar]
- 4. Dachun X, Jue L, Liling Z, et al. Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: a structured review. Vasc Med 15: 361-369, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. Jama 300: 197-208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline). Vasc Med 16: 452-476, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048-1056, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296-1305, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 18: 629-636, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Luo Y, Li X, Li J, et al. Peripheral arterial disease, chronic kidney disease, and mortality: the Chinese Ankle Brachial Index Cohort Study. Vasc Med 15: 107-112, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Guerrero A, Montes R, Munoz-Terol J, et al. Peripheral arterial disease in patients with stages IV and V chronic renal failure. Nephrol Dial Transplant 21: 3525-3531, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 14: 1591-1598, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 28: 478-483, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375: 2073-2081, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int 62: 1402-1407, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 132: 302-361, 2015. [DOI] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441-1446, 1971. [DOI] [PubMed] [Google Scholar]
- 19. Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 298: 789-794, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation 83: 356-362, 1991. [DOI] [PubMed] [Google Scholar]
- 21. Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee, Stroke Statistics Subcommittee.. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation 133: e38-e360, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. N Engl J Med 312: 1665-1671, 1985. [DOI] [PubMed] [Google Scholar]
- 23. Japanese Society of Nephrology Evidence-based practice guideline for the treatment of CKD. Clinical and Experimental Nephrology 55: 585-860, 2013. [Google Scholar]
- 24. Usui T, Ninomiya T, Nagata M, et al. Albuminuria as a risk factor for peripheral arterial disease in a general population: the Hisayama study. J Atheroscler Thromb 18: 705-712, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara T, Saitoh S, Takagi S, et al. Prevalence of asymptomatic arteriosclerosis obliterans and its relationship with risk factors in inhabitants of rural communities in Japan: Tanno-Sobetsu study. Atherosclerosis 177: 83-88, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Pasqualini L, Schillaci G, Pirro M, et al. Renal dysfunction predicts long-term mortality in patients with lower extremity arterial disease. J Intern Med 262: 668-677, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Mlekusch W, Exner M, Sabeti S, et al. Serum creatinine predicts mortality in patients with peripheral artery disease: influence of diabetes and hypertension. Atherosclerosis 175: 361-367, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Irie F, Iso H, Sairenchi T, et al. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 69: 1264-1271, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777-1782, 2002. [DOI] [PubMed] [Google Scholar]