Abstract
A 58-year-old man with a recent history of generalized myalgia and muscle weakness was transferred to our hospital because of acute progressive dyspnea. The patient underwent left ventricular (LV) assist device (LVAD) implantation due to cardiogenic shock with a LV ejection fraction (LVEF) of 6%. The histological findings obtained from LV apex showed the infiltration of multinucleated giant cells and severe myocardial contusion. Combining this histological finding with our experienced neurologists comments, resulted in a final diagnosis of fulminant giant cell myocarditis associated with polymyositis. A day after LVAD implantation, the patient received corticosteroid and immunosuppressive therapy, and the LVEF recovered to 68%.
Keywords: fulminant giant cell myocarditis, polymyositis, left ventricular assist device, corticosteroid therapy, immunosuppressive therapy
Introduction
Giant cell myocarditis is a rare disease with an extremely poor prognosis (1,2). Without heart transplantation, the rate of recovery is very low. Its diagnosis is commonly achieved using endomyocardial biopsy, which reveals multinucleated giant cells and diffuse cardiomyocyte necrosis. Giant cell myocarditis is also considered an autoimmune disorder because of its association with other autoimmune disorders. We herein report an extremely rare case in which the treatment of fulminant giant cell myocarditis associated with polymyositis using a left ventricular assist device and subsequent corticosteroid and immunosuppressive therapy led to remission.
Case Report
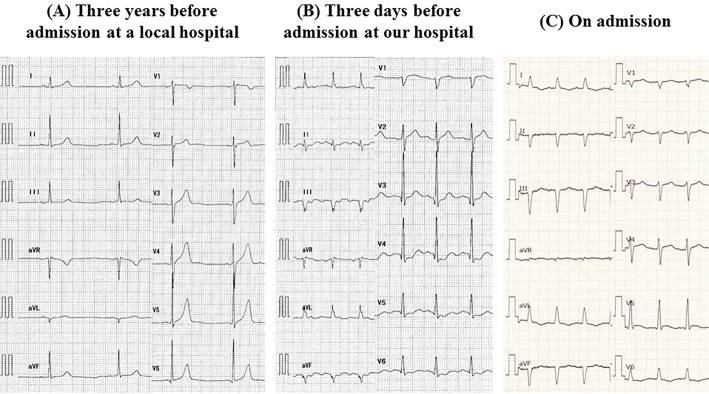
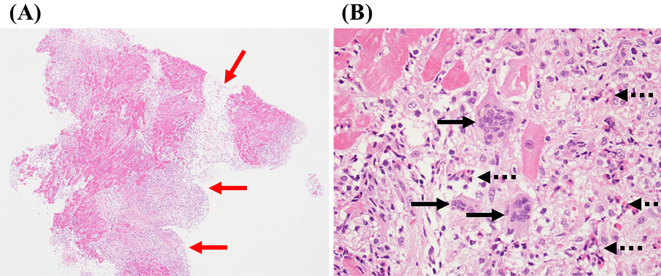
A 58-year-old man, who had been supported by intraaortic balloon pumping, percutaneous cardiopulmonary support and artificial respiration, was transferred to our hospital because of acute progressive dyspnea. One month before presentation, he had been examined at a local hospital for generalized myalgia and muscle weakness as well as for diplopia. On admission, laboratory findings showed enhanced levels of creatine kinase (CK) (4,906 IU/L), CK-MB isoenzyme (117 IU/L), C-reactive protein (12.6 mg/dL), aspartate aminotransferase (1,811 IU/L), alanine aminotransferase (1,171 IU/L), lactate dehydrogenase (4,063 IU/L), brain natriuretic peptide (BNP) (809 pg/mL), and troponin-1 (27.5 ng/mL). Coronary angiography did not show any significant stenosis. An electrocardiogram revealed sinus tachycardia with a heart rate of 110 bpm and first-degree atrioventricular block (Fig. 1). Transthoracic echocardiography revealed severely depressed left ventricular (LV) systolic dysfunction with an LV ejection fraction (LVEF) as low as 6% without pericardial effusion or myocardial edema (Fig. 2, Supplementary material 1). The LV end-diastolic and end-systolic diameters were 51 mm and 50 mm, respectively, without any abnormalities in the mitral and aortic valves. Since fulminant myocarditis was strongly suspected due to the patient's rapidly progressive heart failure, the patient underwent LV assist device (LVAD) implantation. The histological findings of hematoxylin-eosin staining obtained from the LV apex showed the infiltration of multinucleated giant cells and eosinophil cells, and severe myocardial contusion (Fig. 3). In addition, electromyography showed active myogenic changes in the proximal regions, but no waning or waxing pattern in response to repetitive nerve stimulation. On the basis of these specific clinical features and the electromyographic findings, our experienced neurologists made a diagnosis of polymyositis. The diagnosis of polymyositis in this case was also considered to be definite according to Bohan and Peter's criteria (3,4). Combining this diagnosis with the histological findings for the left ventricle, resulted in a final diagnosis of fulminant giant cell myocarditis associated with polymyositis. Moreover, magnetic resonance imaging showed the extraocular muscle to have a hyperintense signal on T2-weighted images, indicating the cause of diplopia to be extraocular myositis.
Figure 1.
Electrocardiograms. (A) three years before admission (at a local hospital), (B) three days before admission (at a local hospital), (C) on admission.
Figure 2.
Two-dimensional transthoracic echocardiography on admission, showing severely depressed left ventricular (LV) systolic dysfunction with an LV ejection fraction (LVEF) as low as 6%.
Figure 3.
Histological findings of Hematoxylin and Eosin staining obtained from the left ventricular apex (A: ×40; B: ×200), showing the infiltration of multinucleated giant cells (black arrow) and eosinophil cells (black dotted arrow), and severe myocardial contusion (red arrow).
Parasternal long-axis view of transthoracic echocardiography on admission
Two-dimensional transthoracic echocardiography on admission, showing severely depressed left ventricular (LV) systolic dysfunction with an LV ejection fraction as low as 6%
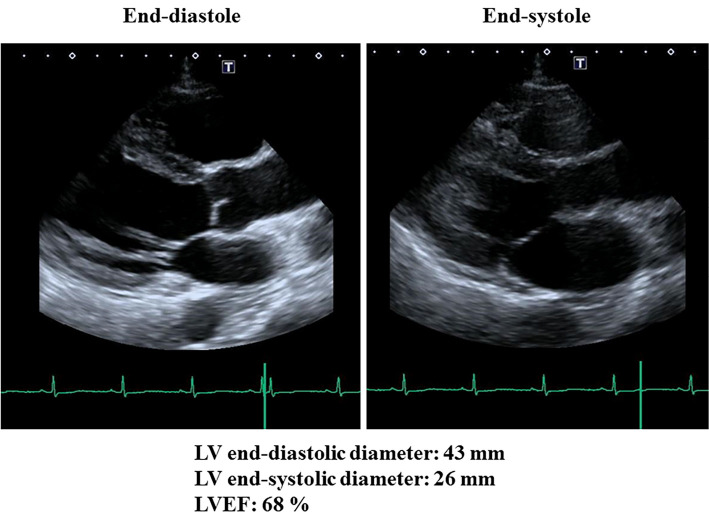
A day after LVAD implantation, the patient received a 3-day course of intravenous methylprednisolone (1,000 mg daily) followed by a maintenance dose of 50 mg prednisolone daily, and immunosuppressive therapy consisting of cyclosporine at a dose of 100 mg daily. Furthermore, the patient also started to receive perindopril and bisoprolol at the third day after the surgery followed by a maintenance dose of 4 mg and 5 mg, respectively. Subsequently, the patient's status including LV dysfunction, generalized myalgia and muscle weakness, and diplopia gradually improved. On the 11th hospital day the LVAD was removed, LVEF had recovered to 51% on the 21st hospital day, and to 68% on the 50th hospital day (Fig. 4, Supplementary material 2). Cardiac magnetic resonance imaging was performed on the 32nd hospital day, and LVEF was 49% and there was no sign of any delayed enhancement without apex (the site of LVAD implantation). The systolic blood pressure was maintained 90 to 100 mmHg, and diastolic blood pressure was maintained 50 to 60 mmHg during hospitalization. The highest values of CK, CK-MB, Troponin I and BNP during the course of the disease were 22,358 IU/L, 348 IU/L, 27.5 ng/mL, and 809 pg/mL, respectively (Table). The course after discharge from our hospital was uneventful, and the patient has been asymptomatic during a follow-up period of 2 months.
Figure 4.
Two-dimensional transesophageal echocardiography on the 50th hospital day, showing that left ventricular (LV) ejection fraction (LVEF) had recovered to 68%.
Table.
Biomarker and LVEF during the Course of the Disease.
| 3 days before admission |
On admission |
10th hospital day |
16th hospital day |
21st hospital day |
28th hospital day |
50th hospital day |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK (IU/L) | 22,358 | 4,906 | 282 | 139 | 21 | |||||||||
| CK-MB (IU/L) | 348 | 117 | 19 | 22 | ||||||||||
| BNP (pg/mL) | 512 | 809 | 105 | |||||||||||
| Troponin I (ng/mL) | 13.9 | 27.5 | ||||||||||||
| LVEF (%) | 45 | 6 | 38 | 43 | 51 | 68 | ||||||||
CK: creatine kinase, CK-MB: CK-MB isoenzyme, BNP: brain natriuretic peptide, LVEF: left ventricular ejection fraction
Parasternal long-axis view of transthoracic echocardiography on the 50th hospital day
Two-dimensional transesophageal echocardiography on the 50th hospital day, showing that left ventricular ejection fraction had recovered to 68%
Discussion
Giant cell myocarditis is a rare disease with a fatal form known as fulminant acute myocarditis, thus leading to an extremely poor prognosis (1,2). The rate of death or cardiac transplantation was 89 percent, with a median survival of 5.5 months from the onset of symptoms to the time of death or transplantation from 63 patients with confirmed giant cell myocarditis (1). Most patients die of congestive heart failure, but some have survived for long periods, often after receiving immunosuppressive treatment (5,6). It was previously reported that the median survival of patients who received no immunosuppressive agents was 3.0 months, whereas, those who received only immunosuppressive agent survived 3.8 months (1). In addition, patients treated with corticosteroids and azathioprine had a longer survival, averaging 11.5 months. More recent reports showed the 5-year survival rate free of heart transplant to range from 52% to 72% with appropriate immunosuppressive therapy (7,8). Furthermore, a combined double- or triple-drug cyclosporine-based therapy reportedly leads to a partial clinical remission in two-thirds of all patients (8). Immunosuppression must be continued long-term as giant cell myocarditis can recur up to 8 years after diagnosis if immunosuppressive therapy is tapered or terminated (8). However, the protocol of immune suppressive therapy in patients with giant cell myocarditis during the late-phase still remains controversial.
Giant cell myocarditis is also considered an autoimmune disorder because of its association with other autoimmune disorders such as systemic lupus erythematosus, vasculitis, Hashimoto's disease, myasthenia gravis, and inflammatory bowel disease. Giant cell myocarditis associated with polymyositis, as in our present case, is even more sporadic. After reviewing the literature, and to the best of our knowledge, this is the first case report of a patient with fulminant giant cell myocarditis associated with polymyositis who achieved remission after LVAD implantation and subsequent combined corticosteroid and immunosuppressive therapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 336: 1860-1866, 1997. [DOI] [PubMed] [Google Scholar]
- 2. Cooper LT Jr, Hare JM, Tazelaar HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 102: 1535-1539, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292: 344-347, 1975. [DOI] [PubMed] [Google Scholar]
- 4. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292: 403-407, 1975. [DOI] [PubMed] [Google Scholar]
- 5. Desjardins V, Pelletier G, Leung TK, Waters D. Successful treatment of severe heart failure caused by idiopathic giant cell myocarditis. Can J Cardiol 8: 788-792, 1992. [PubMed] [Google Scholar]
- 6. Ren H, Poston RS Jr, Hruban RH, Baumgartner WA, Baughman KL, Hutchins GM. Long survival with giant cell myocarditis. Mod Pathol 6: 402-407, 1993. [PubMed] [Google Scholar]
- 7. Maleszewski JJ, Orellana VM, Hodge DO, Kuhl U, Schultheiss HP, Cooper LT. Long-term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol 115: 1733-1738, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Kandolin R, Lehtonen J, Salmenkivi K, Raisanen-Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 6: 15-22, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long-axis view of transthoracic echocardiography on admission
Two-dimensional transthoracic echocardiography on admission, showing severely depressed left ventricular (LV) systolic dysfunction with an LV ejection fraction as low as 6%
Parasternal long-axis view of transthoracic echocardiography on the 50th hospital day
Two-dimensional transesophageal echocardiography on the 50th hospital day, showing that left ventricular ejection fraction had recovered to 68%