Abstract
Purulent pericarditis is a life-threatening disorder, even in the modern antibiotic era. Although diabetes mellitus is known to be associated with an increased risk of multiple types of infections, purulent pericarditis is extremely rare. We herein report an unusual case of pericarditis caused by Salmonella enterica subspecies arizona that was not associated with any evident underlying immunosuppressive disorder apart from uncontrolled type 2 diabetes mellitus. Because a pet snake was suspected as being the source of infection in the present case, patient education and a detailed review of exposure history could play an important role in treating patients with diabetes mellitus.
Keywords: pericarditis, S. enterica subsp. arizona, diabetes mellitus, reptile
Introduction
Purulent pericarditis is rare in the modern antibiotic era and it is commonly caused by Gram-positive bacteria, which tend to directly spread from an intrathoracic focus (1,2). Although Salmonellosis, an infection due to Salmonella bacteria, is common worldwide, Salmonellae are rarely reported as causative agents of purulent pericarditis (3,4).
An altered immune response in patients with uncontrolled diabetes mellitus is recognized to lead to an increased risk of multiple types of infections (5). However, only three reported cases of associated purulent pericarditis have so far been published based on our review of the pertinent literature (3,4). We herein report an unusual case of pericarditis caused by Salmonella enterica subspecies arizona (S. enterica subsp. arizona) associated with poorly controlled type 2 diabetes mellitus.
Case Report
In March 2016, a 36-year-old man visited his primary doctor because of chest pain and respiratory distress. The patient had a history of type 2 diabetes mellitus, which was diagnosed at 29 years of age. Despite being treated with liraglutide, his glycohemoglobin (HbA1c) levels were 9-13% during the preceding 2 years. He had a low-grade fever and sore throat for 2 weeks, but neither any abdominal pain nor diarrhea. A chest X-ray showed cardiomegaly (cardiothoracic ratio =66.8%) (Fig. 1). Because congestive heart failure was suspected based on the patient's symptoms and chest X-ray findings, he was referred to our hospital.
Figure 1.

Erect chest X-ray (posteroanterior projection) showing cardiomegaly.
The patient was 174 cm tall and weighed 88.0 kg (body mass index =29.1 kg/m2). His vital signs were as follows: blood pressure of 132/77 mmHg, pulse rate of 131/min, body temperature of 36.8℃, respiratory rate of 14/min, and a pulse oximetry of 97% on room air. Neither a heart murmur, nor a pericardial friction rub, nor an abnormal breath sound was heard. He had bilateral pitting edema of the lower extremities. A skin examination showed no rashes, tattoos, or cutaneous ulcers.
Laboratory values were as follows: white-blood-cell (WBC) count of 13,900 /μL; neutrophils, 75.1%; C-reactive protein, 7.3 mg/dL; plasma glucose, 422 mg/dL; HbA1c, 12.2%; creatine phosphokinase, 35 U/L; and N-terminal pro-brain natriuretic peptide, 1,939 pg/mL (normal range: <125 pg/mL). The liver, kidney, and thyroid functions were unimpaired. An electrocardiogram showed sinus tachycardia at 128/min, normal QRS width and axis, low voltage, and a normal ST segment. Echocardiography and a chest X-ray computed tomography revealed a large amount of pericardial effusion around the heart with pericardial thickening. Bilateral pleural effusion without pulmonary parenchymal involvement was also noted (Fig. 2).
Figure 2.

Coronary chest X-ray computed tomography (mediastinal window) showing massive pericardial effusion with an increased pericardial thickness (arrowheads).
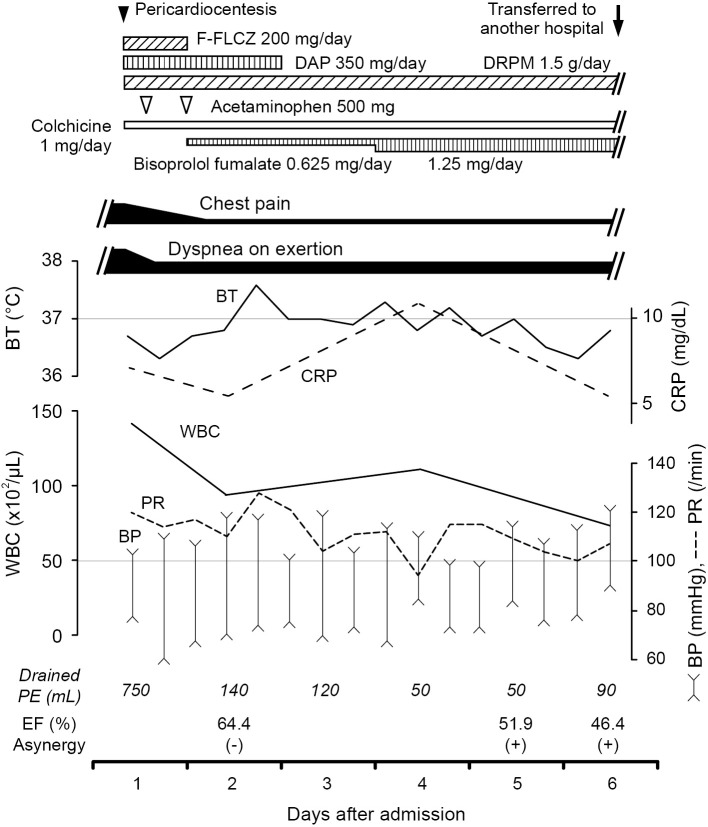
Acute pericarditis was diagnosed based on the presence of chest pain and pericardial effusion (6). The patient was admitted to our hospital for further evaluation, and emergency pericardiocentesis was performed. After 750 mL of brownish-red purulent fluid were drained, chest pain and dyspnea on exertion improved. Because purulent pericarditis was considered, empiric antibiotic treatment with fosfuluconazole (F-FLCZ), daptomycin (DAP) and doripenem (DRPM) were initiated. Colchicine was administered as an anti-inflammatory agent, and bisoprolol fumarate was initiated for tachycardia (Fig. 3). The following day, cultures obtained from the pericardial fluid were reported as growing Gram-negative bacilli. No other obvious primary source of infection was identified, despite an evaluation in the hospital setting.
Figure 3.
Clinical course of the patient. Ejection fraction (EF) and asynergy were evaluated by an echocardiography (Vivid E9; GE-Healthcare, Horten, Norway) on day 2 and 5 or by left ventriculography on day 6 of hospitalization. Brachial blood pressure (BP) and pulse rate (PR) were simultaneously measured by OMRON HEM-7130 (OMRON HEALTHCARE, Japan) in supine position. F-FLCZ: fosfuluconazole, DAP: daptomycin, DRPM: doripenem, BT: body temperature, WBC: white-blood-cell, CRP: C-reactive protein, PE: pericardial effusion
On day 4 of hospitalization, the Gram-negative bacilli were identified as S. enterica subsp. arizona by the fully automated system for rapid identification and antimicrobial susceptibility testing (RAISUS; Nissui Pharmaceutical, Tokyo, Japan). We also analyzed blood cultures on the same day, which were negative for any bacterium including Salmonella species. Detailed history taking revealed that the patient had received a ball python (Python regius) and a Mexican black kingsnake (Lampropeltis getula nigrita) as pets 7 and 4 months before his admission, respectively. Notably, the latter died only 1 month before the patient was referred to our hospital.
Chest pain was gradually relieved, and WBC count returned to within the reference interval on day 6 of hospitalization. However, dyspnea on exertion did not ameliorate despite medical treatment, considering both bacterial susceptibility combined with daily pericardial drainage (Fig. 3). Accordingly, cardiac catheterization was performed on the same day. The examination revealed a normal coronary artery, whereas the ejection fraction was 46.4% with asynergy; pulmonary capillary wedge pressure was 24 mmHg and the cardiac index was 1.29 L/min/m2. Because of an unstable hemodynamic status, the patient was transferred to the department of cardiovascular surgery at another hospital to receive pericardiotomy. At that hospital, constrictive pericarditis secondary to bacterial infection was diagnosed based on the pericardial thickness along with the fibrinous and exudative nature of the effusion. After the procedure, the patient recovered well and was discharged from the hospital. Written informed consent was obtained from the patient for the publication of this case report.
Discussion
Acute pericarditis, the most common disorder of the pericardium, refers to the inflammation of the pericardial sac. In the modern antibiotic era, purulent cases are rare, consisting of fewer than 1% of all cases of acute pericarditis (1,7). Among the wide variety of bacterial organisms reported as causative agents, Gram-positive cocci are the most common causes (6). The mortality rate in purulent pericarditis is relatively high at approximately 10%, and its prognosis depends both on the severity of the pericarditis itself and on the underlying disorders (2). Although no definitive guidelines have been established for this rare infection in contrast to idiopathic pericarditis (6), aggressive treatments, including urgent pericardiotomy and appropriate antibiotic therapy, are thought to be associated with higher rates of recovery (2,6). In the present case, pericardiocentesis was immediately conducted, followed by empiric antibiotic treatment and anti-inflammatory therapy. To guide the duration of medical therapy, we focused on whether the symptoms, vital signs, WBC count, C-reactive protein (CRP), and echocardiographic data changed or not as shown in Fig. 3. Eventually, we decided to transfer the patient to allow him to undergo interventional treatment because of persistent symptoms along with an unstable hemodynamic status, although several therapeutic indices (e.g., chest pain, body temperature, and WBC count) improved.
Salmonellae are motile Gram-negative bacteria that infect or colonize a wide range of mammalian hosts. The genus Salmonella currently comprises two species: S. bongori and S. enterica. The latter includes over 2,500 serovars, which can be categorized into six subspecies, i.e., enterica, arizonae, diarizonae, houtenae, salamae, and indica (8). Most cases of human salmonellosis, an infection caused by Salmonella bacteria, are attributed to S. enterica. subsp. enterica (9). Clinically, salmonellosis has been divided into typhoidal and non-typhoidal types, which are based on host preference and disease manifestations. Enteric fever, also known as typhoid fever, is caused by S. enterica subsp. enterica serovar Typhi or serovar Paratyphi A. Other serotypes, such as S. enterica subsp. enterica serovar Enteritidis or S. enterica subsp. arizona, are collectively known to induce non-typhoidal salmonellosis. Most cases of non-typhoidal salmonellosis are limited to gastroenteritis; however, bacteremia occurs in approximately 5% of infections (10). Importantly, patients with bacteremia do not typically exhibit gastrointestinal symptoms, as the patient reported herein had no antecedent abdominal pain or diarrhea. Furthermore, Brown et al. reported that Salmonella bacteremia without gastrointestinal symptoms was associated with underlying immunosuppression (11).
Some patients with non-typhoidal Salmonella bacteremia further develop focal metastatic infections, including osteomyelitis, infectious endarteritis, and cardiac infections (10). Among the various types of cardiac involvement, purulent pericarditis is extremely rare, as only 20 reports have been published to date according to our review of the literature (3,4). The mortality rate is relatively high in such cases (15%; 3/20 cases). The most common organism was S. enterica subsp. enterica serovar Enteritidis (60%; 12/20 cases), whereas only 2 cases of S. enterica subsp. arizona have been reported. Importantly, 14 out of 20 patients with non-typhoidal Salmonella-induced pericarditis had an identifiable immunosuppressed state, including chronic corticosteroid therapy (35%; 7/20 patients), renal disease managed with hemodialysis (20%; 4/20 patients), and diabetes mellitus (15%, 3/20 patients). In the present case, we conducted detailed examinations, including antinuclear antibody and anti-human immunodeficiency virus antibody testing, which demonstrated no possible causes apart from poorly controlled type 2 diabetes.
Diabetes mellitus is known to be associated with increased rates of infection, which could be partially explained by a decreased immune response (5). In addition, there have been several reported cases of idiopathic pericarditis associated with type 2 diabetes mellitus (12,13). Interestingly, Bennett et al. suggested that dehydration caused by hyperglycemia might contribute to decreased pericardial effusion and subsequent pericardial injury (12). Furthermore, cases of acute dry pleurisy associated with diabetic ketoacidosis have been reported, which completely resolved with rehydration (14). Taken together, decreased pericardial effusion apart from immunosuppressive status caused by uncontrolled diabetes mellitus could have been associated with purulent pericarditis in the present case.
It has been reported that non-typhoidal Salmonella transmission can result from several sources, such as contaminated food products (e.g., poultry and eggs) or contact with pets (e.g., reptiles and birds) (10). Of note, S. enterica subsp. arizona is a common gut inhabitant of reptiles, with snakes serving as the most common reservoir (15). Disseminated human infection is rare, and only two cases of pericardial infection have been reported to date (15,16). Because a Mexican black kingsnake (Lampropeltis getula nigrita) died only 1 month before the patient was admitted to our hospital, S. enterica subsp. arizona transmitted from a snake appeared to have caused bacteremia as well as subsequent pericarditis in the present case.
In conclusion, we herein described an unusual case of purulent pericarditis that was not associated with any identified underlying immunosuppressive disorder apart from poorly controlled type 2 diabetes mellitus. To the best of our knowledge, S. enterica subsp. arizona was isolated from pericardial fluid in a patient with diabetes mellitus for the first time. Purulent pericarditis is rare, but nevertheless life-threatening inflammatory condition. Because it is becoming increasingly common to keep reptiles as pets, patient education and a detailed review of exposure history could play an important role in treating patients with uncontrolled diabetes mellitus.
The authors state that they have no Conflict of Interest (COI).
Ai Suzuki and Takamitsu Tanaka contributed equally to this work.
References
- 1. Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med 351: 2195-2202, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Sagrista-Sauleda J, Barrabes JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol 22: 1661-1665, 1993. [DOI] [PubMed] [Google Scholar]
- 3. Ortiz D, Siegal EM, Kramer C, Khandheria BK, Brauer E. Nontyphoidal cardiac salmonellosis: two case reports and a review of the literature. Tex Heart Inst J 41: 401-406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chand G, Jhaj R, Sanam K, Sinha P, Alexander P. Pericardial salmonella with cardiac tamponade and ventricular wall rupture: A case report. Ann Med Surg (Lond) 7: 83-86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 41: 281-288, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA 314: 1498-1506, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 115: 2739-2744, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Tindall BJ, Grimont PA, Garrity GM, Euzeby JP. Nomenclature and taxonomy of the genus Salmonella. Int J Syst Evol Microbiol 55: 521-524, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Stock I, Wiedemann B. Natural antibiotic susceptibility of Salmonella enterica strains. Int J Antimicrob Agents 16: 211-217, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis 32: 263-269, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Brown M, Eykyn SJ. Non-typhoidal Salmonella bacteraemia without gastroenteritis: a marker of underlying immunosuppression. Review of cases at St. Thomas' Hospital 1970-1999. J Infect 41: 256-259, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Bennett KR, Blake TM. Pseudopericarditis in diabetic ketoacidosis. South Med J 64: 610-612, 1971. [DOI] [PubMed] [Google Scholar]
- 13. Campbell IW, Duncan LJ, CLarke BF. Pericarditis in diabetic ketoacidosis. Br Heart J 39: 110-112, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armanino LP, Ory EM. Acute pleurisy as a dehydration phenomenon in diabetic precoma. Am J Med Sci 211: 597-601, 1946. [PubMed] [Google Scholar]
- 15. Hoag JB, Sessler CN. A comprehensive review of disseminated Salmonella arizona infection with an illustrative case presentation. South Med J 98: 1123-1129, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Salavert M, Navarro V, Roig P. Purulent pericarditis due to Salmonella enterica subsp. arizonae. Enferm Infecc Microbiol Clin 20: 47-49, 2002. [PubMed] [Google Scholar]