Abstract
TAFRO syndrome is a rare systemic inflammatory disease characterized by thrombocytopenia, pleural effusion, fever, renal dysfunction, reticulin fibrosis of the bone marrow, and organomegaly. The clinical course varies significantly among patients. However, the prognosis is usually dismal in patients with severe TAFRO syndrome, and no optimal treatment has yet been established. We herein describe the first case of TAFRO syndrome, which was successfully treated with combination therapy consisting of tocilizumab, prednisone, and cyclophosphamide.
Keywords: TAFRO syndrome, tocilizumab, cyclophosphamide
Introduction
TAFRO syndrome is a systemic inflammatory disease characterized by thrombocytopenia, anasarca including pleural effusion and ascites, fever, renal dysfunction, and organomegaly (1,2). This syndrome sometimes mimics the mixed type of multicentric Castleman disease (MCD), but the clinical characteristics and courses are different between TAFRO syndrome and MCD. The etiology remains largely unknown, leading to the lack of any standard treatments for TAFRO syndrome. While a majority of patients with this syndrome are refractory to treatment and tend to demonstrate a fatal outcome, other patients responding to immunosuppressive drugs, such as glucocorticoids, cyclosporine A, tocilizumab and rituximab have also been reported (3-15). However, there have been no reported case of TAFRO syndrome that were successfully treated with tocilizumab, prednisone, and cyclophosphamide. We herein report a case of TAFRO syndrome, which was successfully treated with tocilizumab, prednisone, and cyclophosphamide, together with a review of the pertinent literature.
Case Report
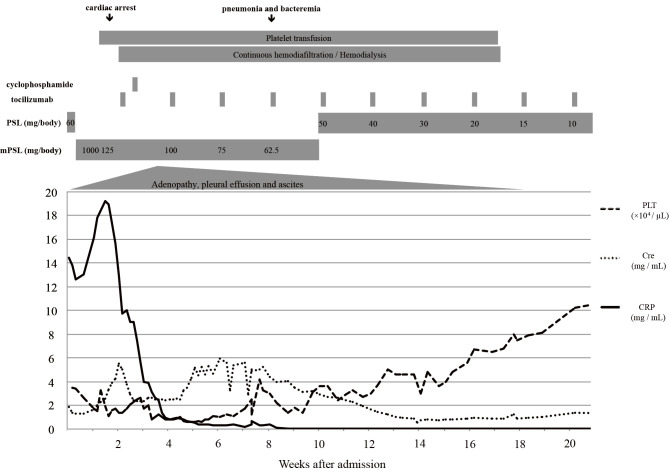
A 72-year-old man was admitted to the hospital for the evaluation of bilateral pleural effusion. He had been well until 4 weeks before this admission when he started complaining of fatigue, appetite loss and dyspnea. Three weeks later, he was pointed out to have bilateral pleural effusion by computed tomography (CT) of the lung. On admission, the patient was alert and oriented. His temprerature was 37.1℃, blood pressure 153/90 mmHg, pulse 89 beats per minute, and oxygen saturation 96% while he was breathing ambient air. There were coarse crackles at the lung bases. Pitting edema was present in the both lower extremities. His superficial lymph nodes were not palpable. Neither purpural nor petechial lesions were not seen. The results of a laboratory examination are shown in Table 1. A complete blood count for peripheral blood showed anemia, lymphopenia, and marked thrombocytopenia. Coagulation tests revealed an elevation of fibrinogen together with fibrinogen degradation product (FDP) and D-dimer. The blood chemistry findings were remarkable for C-reactive protein (CRP), mild renal impairment, hypoalbuminemia and an elevation of soluble IL-2 receptor. Immunoglobulin (Ig) G level elevated, but IgG-4 remained normal. Although anti-Sjögren syndrome (SS)-A antibody was positive, all other antibodies were negative, which were indicative of Sjögren syndrome and other collagen disease. However, the patient denied any symptoms related to dry mouth or dry eye. A whole body CT showed the swelling of bilateral axillary, mediastinal, paraaortic, celiac and pelvic lymph nodes. Splenomegaly with pleural and peritoneal effusion was also recognized (Fig. 1). A bone marrow biopsy revealed a normocellular marrow with mild reticulin fibrosis and about 50% of polyclonal plasma cells. The level of serum IL-6 was 26.2 pg/mL. Blood culture and quantitative polymerase chain reaction (PCR) examinations for human herpes virus type 8 (HHV-8) and human immunodeficiency virus (HIV) antibody were negative. A lymph node biopsy could not be performed due to the patient's rapidly deteriorating condition and transfusion refractory thrombocytopenia. Based on the findings of clinical and laboratory data, and a bone marrow examination, he was diagnosed with TAFRO syndrome. He was initially treated with 60 mg (1 mg/kg) of prednisone, but his general condition and renal dysfunction both worsened. After the first administration of prednisone, mental disturbance, hypovolemic shock and respiratory failure due to excessive vascular permeability developed. Methylprednisolone pulse therapy at a dose of 1,000 mg/day for 3 consecutive days was then administered. In the first day of methylprednisolone, the patient developed temporary cardiac arrest due to severe lactic acidosis, which was immediately reversed by the rapid infusion of sodium bicarbonate. However, his renal function rapidly deteriorated during these episodes, and he was thus placed on continuous hemodiafiltration (CHDF), which was performed intermittently because of his unstable hemodynamic status. The patient subsequently received biweekly injections of tocilizumab (8 mg/kg) on day 16 and a single dose of cyclophosphamide (1,000 mg) on day 20, respectively. After the administration of cyclophosphamide, his hemodynamic status started to stabilize, and CHDF was performed as scheduled. CHDF was eventually stopped after 18 days and hemodialysis was stopped about 3 months after starting tocilizumab. During this period, corynebacterium sepsis and pneumonia developed on day 57, which were all successfully treated with antibiotics. The platelet count started increasing gradually on day 82 and the patient became platelet transfusion independent at 3 months after the administration of cyclophosphamide. Repeated CT scans confirmed a decrease of pleural effusion and ascites, and also a decrease in the size of the lymph nodes on day 228 (Fig. 2). Currently, he is being followed as an outpatient and is free from fever, ascites, pleural effusion, and thrombocytopenia with tocilizumab (8 mg/kg) infusion every 2 weeks and prednisone (8 mg). The clinical course of patient is shown in Fig. 3.
Table 1.
Laboratory Data.
| Complete blood count | Biochemistry | ||||
| White blood cell | 5,300 | /μL | Total protein | 6.2 | g/dL |
| neutrophil | 88 | % | Albumin | 1.9 | g/dL |
| lymphocyte | 8 | % | Total-bilirubin | 1.3 | mg/dL |
| monocyte | 3 | % | Direct-bilirubin | 0.5 | mg/dL |
| basophil | 1 | % | Indirect-bilirubin | 0.8 | mg/dL |
| Red blood cell | 352 | ×106/μL | Blood urea nitrogen | 39.8 | mg/dL |
| Hemoglobin | 10.8 | g/dL | Creatinine | 1.3 | mg/dL |
| Hematocrit | 32.9 | % | Sodium | 137.1 | mEq/L |
| Platelet | 34,000 | /μL | Potassium | 4.9 | mEq/L |
| Reticulocyte | 1.5 | % | Chloride | 107 | mEq/L |
| Calcium | 8.9 | mg/dL | |||
| Coagulation | Glucose | 97 | mg/dL | ||
| APTT | 35.7 | sec | LDH | 270 | U/L |
| PT | 67 | % | AST | 43 | U/L |
| PT-INR | 1.25 | ALT | 19 | U/L | |
| Fibrinogen | 434 | mg/dL | ALP | 599 | U/L |
| FDP | 32.7 | μg/mL | γ-GTP | 41 | U/L |
| D-dimer | 22.5 | μg/mL | Creatine kinase | 39 | U/L |
| CRP | 12.59 | mg/dL | |||
| Immunochemistry | Haptoglobin | 12.59 | mg/dL | ||
| IgG | 1,892 | mg/dL | IL-6 | 26.8 | pg/mL |
| IgG4 | 33 | mg/dL | sIL-2r | 625 | U/mL |
| IgA | 266 | mg/dL | |||
| IgM | 54 | mg/dL | Serology | ||
| Antinuclear Ab | 40 | HBs-Ag | negative | ||
| Anti-ds-DNA Ab | 3.7 | HBs-Ab | negative | ||
| Anti-RNP Ab | <2.0 | HBc-Ab | negative | ||
| Anti-SM Ab | 1 | HCV-Ab | negative | ||
| Anti-SS-A Ab | 953 | HIV-Ab | negative | ||
| Anti-SS-B Ab | 3.8 | HHV-8 DNA | negative | ||
| Anticardiolipin Ab | <8.0 | CMV antigenemia | (0, 0) | ||
| PR3-ANCA | <1.0 | ||||
| MPO-ANCA | <1.0 | ||||
| Anti GBM Ab | <2.0 | ||||
APTT: activated partial thromboplastin time, PT: protrombin time, PT-INR: prothrombin time-international normalized ratio, FDP: fibrinogen degradation product, Ig: immunoglobulin, Ab: antibody, Anti-ds-DNA: anti-double-stranded DNA, Anti-RNP: anti-ribonucleoprotein, Anti-SM: anti-Smith, Anti-SS: anti-Sjögren syndrome, PR3-ANCA: proteinase 3-anti neutrophil cytoplasmic antibody, MPO-ANCA: myeloperoxidase-anti neutrophil cytoplasmic antibody, Anti-GBM: anti-glomerular basement membrane, LDH: lactate dehydrogenase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, CRP: C-reactive protein, IL-6: interleukin-6, sIL-2r: soluble interleukin-2 receptor, HBs-Ag: hepatitis B surfave antigen, HBc: hepatitis B core, HCV: hepatitis C virus, HIV: human immunodeficiency virus, HHV-8: human herpes virus 8, CMV: cytomegalovirus
Figure 1.
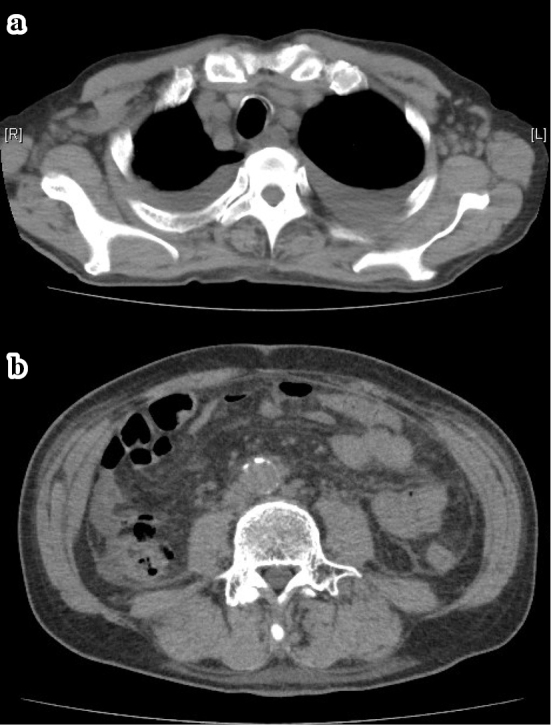
CT of the chest and abdomen on admission showing axillary, mediastinum, paraaortic lymph node swelling, bilateral pleural effusion and ascites.
Figure 2.
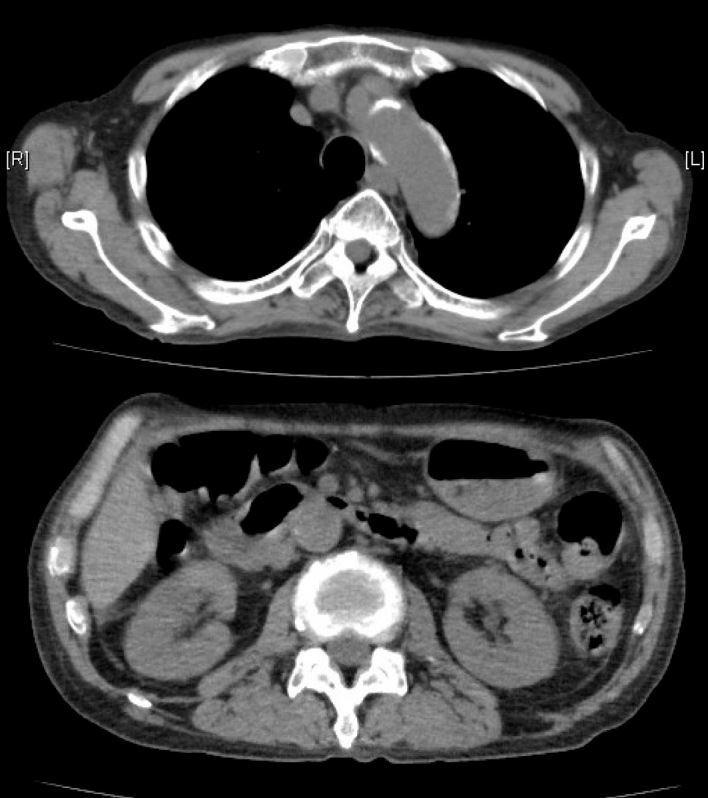
CT of the chest and abdomen 228 days after the treatment showing a marked improvement in lymphadenopathy, pleural effusion and ascites.
Figure 3.
Discussion
TAFRO syndrome was first described by Takai et al. (12). The three reported cases had fever, anasarca, organomegaly, thrombocytopenia, and reticulin fibrosis of the bone marrow. The histological features of lymph nodes are mixed type MCD so that TAFRO syndrome thus appears to be a clinicopathologic variant of MCD (1,2).
Our patient presented with an elevation of CRP, bilateral pleural effusion, ascites, thrombocytopenia, myelofibrosis, and renal dysfunction. He possessed five specific signs and symptoms in TAFRO syndrome. Recently, Masaki et al. proposed the diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome (2). Although a lymph node biopsy is strongly recommended, the histological findings are not required for diagnosing this syndrome and classified thus there are considered to only be a minor criterion. Our patient fulfilled all the major criteria. In some cases TAFRO syndrome progresses rapidly so that the adequate samples may not be obtained. Both an early diagnosis and early intervention are crucial to obtain a better treatment outcome. As a result, we think that these diagnostic criteria are simple and in line with good clinical practice.
According to the disease severity classification, the present case was classified as a very severe (grade 5) (2). In our case, the FDP and D-dimer levels were both increased, which might be due to pleural effusion and ascites. An elevation of the FDP and D-dimer levels has also been described in other case reports (3-6,8,10-15). We also speculate that these data may indicate the severity of TAFRO syndrome. In fact, the administration of steroids, including pulse therapy, was only partially effective in lowering the CRP level and renal dysfunction did not improve. Some cases reported the successful treatment with cyclosporine A (CsA) in steroid-refractory TAFRO syndrome (3-5). However, we considered cyclophosphamide to be the more appropriate choice due to the renal dysfunction observed in our case. The administration of single-dose cyclophosphamide in combination with the tocilizumab and steroid was successful to control disease activity and it also improved the patient's quality of life. Although anti-SS-A antibody was positive, the patient did not have the symptoms of SS. Edahiro et al. also reported a case of TAFRO syndrome with a positive finding for anti-SS-A/B antibody (16). On the other hand, Takasawa et al. reported a case of TAFRO syndrome complicated with SS (17). These phenomena may indicated that immunologic dysregulation is involved in the pathogenesis of TAFRO syndrome.
Twenty-nine case reports on TAFRO syndrome were identified by a PubMed search from 2010 to 2016 (3-25). The clinical characteristics and courses of those cases are summarized in Table 2 together with our case. Their median age of was 49 years of age (range 15 to 77). Pleural effusion was observed in 27 cases and ascites in 25. The median platelet count was 30,000/μL (range 8,000 to 214,000). Median value of CRP and serum creatinine were 13.43 mg/dL (range 1.74 to 31.6) and 1.25 mg/dL (range 0.51 to 2.59), respectively. Initial treatment with steroids alone was performed in 22 cases, but a response was obtained in only 9 cases, suggesting that the efficacy of steroids alone as initial therapy for TAFRO syndrome is limited. Regarding the second treatment, treatments with tocilizumab were performed in 12 out of 18 patients and a complete response was obtained in 6 cases (50% of patients) and a partial response in 1 case. Regarding the subsequent therapies after the second line treatment, cyclophosphamide was used in 6 cases including those who received CHOP chemotherapy, and all achieved either a complete response (n=4) or a partial response (n=2).
Table 2.
Characteristics of Previous Reported Cases with TAFRO Syndrome.
| Reference | Age (years) |
Sex | Anasarca | Platelet (× 104/μL) |
CRP (mg/dL) |
Creatinine (mg/dL) |
1st | 2nd / 3rd / 4th | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Response | Treatment | |||||||||
| 3 | 61 | M | PE, ascites, edema | 1.3 | 17.22 | 2.15 | mPSL | PD | TCZ / CyA | Alive | |
| 4 | 49 | F | PE, ascites, edema | 1.7 | 6.04 | 0.96 | Dex | PR | CyA | Alive | |
| 5,12 | 47 | F | PE, ascites, edema | 1.5 | 18.27 | 0.7 | CHOP | PR but relapse | PSL* | Alive | |
| 5,12 | 56 | M | PE, ascites | 1.9 | 16.35 | 1.9 | IvIG, mPSL | PD | CyA | Alive | |
| 5,12 | 49 | M | PE, ascites, edema | 1 | 16.42 | 0.9 | IvIG, mPSL | PD | - | Dead | |
| 5,12 | 53 | F | Ascites | 3.8 | 31.6 | 0.7 | PSL | PR | Splenectomy, PSL / Cyclophosphamide | Alive | |
| 5,12 | 56 | F | PE, ascites, edema | 4.4 | 6.35 | 1.53 | PSL, CyA | CR | - | Alive | |
| 6 | 49 | F | Ascites | 2 | 21.9 | 2.2 | mPSL | PD | TCZ | Alive | |
| 7 | 15 | M | PE, ascites | 3 | 17.8 | N.D | mPSL | PD | TCZ | Alive | |
| 8 | 50 | M | PE, ascites, edema | 6.7 | 17.19 | 2.26 | mPSL | PD | TCZ | Alive | |
| 9 | 48 | F | PE, edema | 2.3 | 1.74 | 0.72 | IvIG | PD | PSL / Rituximab | Alive | |
| 10 | 48 | M | PE, ascites, edema | 1.6 | 2.5 | 1.5 | mPSL | PD | TCZ, IvIG / Rituximab, TPOR agonist | Alive | |
| 15 | 77 | F | Edema | 4.4 | 9.6 | 0.93 | TCZ | PD | PSL / Rituximab / CyA | Alive | |
| 11 | 29 | F | PE, ascites | 1.9 | 18.9 | 1.94 | mPSL | PD | CHOP** | Alive | |
| 11 | 21 | F | PE, ascites | 5.9 | 28.87 | 0.82 | mPSL | PD | TCZ / CyA / R-CHOP** | Alive | |
| 13 | 72 | M | PE, edema | 5 | 22.26 | 0.88 | PSL | PD | TCZ | Dead | |
| 14 | 57 | F | PE, ascites | 1.3 | 17.73 | 1.34 | IvIG, mPSL | PD | CHOEP | Dead*** | |
| 14 | 73 | M | PE, ascites | 2.4 | 4.17 | 1.45 | PSL | PD | - | Dead | |
| 16 | 46 | F | PE, ascites | 1.9 | 3.2 | 0.51 | mPSL | CR | - | Alive | |
| 17 | 46 | F | PE, ascites | 21.2 | 13.43 | 0.89 | mPSL | PR | CyA | Alive | |
| 18 | 57 | M | PE, ascites, edema | N.D | N.D | N.D | Glucocorticoid | PR | TCZ | Alive | |
| 19 | 66 | M | PE, ascites | 0.8 | N.D | 1.2 | Glucocorticoid | PR | CyA / Glucocorticoid. CyA | Dead | |
| 20 | 56 | M | PE, ascites | 7.6 | 11.7 | 1.43 | PSL | PD | TCZ / Thalidomide | Alive | |
| 21 | 39 | M | PE, ascites | 3.6 | 4.49 | 1.18 | mPSL | CR | - | Alive | |
| 21 | 38 | M | PE, ascites | 20.2 | 11.18 | 2.59 | PSL | CR | - | Alive | |
| 22 | 28 | M | PE | 7.5 | N.D | 2.5 | PSL | PR | Rituximab | Alive | |
| 23 | 21 | F | PE, ascites, edema | 2.9 | 22.2 | 0.8 | mPSL | PD | TCZ / R-CVP | Alive | |
| 24 | 47 | F | PE, ascites, edema | 3.9 | 12.4 | 1.4 | PSL | PD | TCZ | Alive | |
| 25 | 43 | F | PE | 13.5 | 20.1 | 0.86 | PSL, TCZ, Rituximab | CR | - | Alive | |
| Presented case | 72 | M | PE, ascites, edema | 3.4 | 12.59 | 1.3 | mPSL | Not improved | TCZ, Cyclophosphamide | Alive | |
M: male, F: female, PE: pleural effusion, mPSL: methylprednisone, PSL: prednisone, IvIG: intravanous immunoglobulin, CyA: cyclosporine A, TCZ: tocilizimab, PD: progression disease, PR: partial response, CR: complete response, R-CHOP: rituximab, cyclophosphamide, adriamycin, vincristine and prednisone, R-CVP: rituximab, cyclophosphamide, vincristine and prednisone
* The patient relapsed four times with tapering of PSL, but responsed to PSL.
**Cardiomyopathy due to adriamycin was suspected, and CEPP was performed as the alternative regimen.
***The patient died due to septic shock.
In our case, the administration of cyclophosphamide soon after commencing tocilizumab was able to stabilize his hemodynamic status with acceptable toxicities, such as mild myelosuppression. The reported cases together with our case suggested that intensive immunosuppression early in the course of TAFRO syndrome results in a better control of the disease activity. To this end, the use of cyclosporine is another treatment of choice. In fact, cyclosporine was given in 9 cases and 7 cases achieved a complete remission. However, its use may be limited due preexisting renal impairment. Although the number of cases is small, rituximab seems to be effective as six out of seven cases treated with rituximab achieved a complete response.
Based upon the literature review and our experience, it is plausible that the administration of steroids alone is not effective for severe TAFRO syndrome and an earlier introduction of agents other than steroi may improve the transplant outcome. The optimal combination of drugs for each patient remains to be elucidated, and further study is clearly warranted.
In conclusion, this is the first case of TAFRO syndrome which was successfully treated with tocilizumab, prednisone, and cyclophosphamide. Adding cyclophosphamide to tocilizumab may therefore be of value to control the disease activity and it may lead to remission in cases of steroid-refractory TAFRO syndrome, especially in cases of very severe disease. This may also result in a reduction of the steroid dose and thereby allows such patients to avoid the adverse events associated with steroids.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kawabata H, Takai K, Kojima M, et al. Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop 53: 57-61, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Yamaga Y, Tokuyama K, Kato T, et al. Successful treatment with cyclosporin A in tocilizumab-resistant TAFRO syndrome. Intern Med 55: 185-190, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A: a case report. J Clin Exp Hematop 53: 95-99, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N, Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J Clin Exp Hematop 53: 63-68, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Kawashima M, Usui T, Okada H, et al. TAFRO syndrome: 2 cases and review of the literature. Mod Rheumatol 20: 1-5, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Kubokawa I, Yachie A, Hayakawa A, et al. The first report of adolescent TAFRO syndrome, a unique clinicopathologic variant of multicentric Castleman's disease. BMC Pediatr 14: 139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujiwara S, Mochinaga H, Nakata H, et al. Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol 103: 718-723, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Ozawa T, Kosugi S, Kito M, et al. Efficacy of rituximab for TAFRO syndrome, a variant type of multicentric Castleman's disease. Rinsho Ketsueki 55: 350-355, 2014. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10. Hiramatsu S, Ohmura K, Tsuji H, et al. Successful treatment by rituximab in a patient with TAFRO syndrome with cardiomyopathy. Nihon Rinsho Meneki Gakkai Kaishi 39: 64-71, 2016. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 11. Yasuda S, Tanaka K, Ichikawa A, et al. Aggressive TAFRO syndrome with reversible cardiomyopathy successfully treated with combination chemotherapy. Int J Hematol 104: 512-518, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsho Ketsueki 51: 320-325, 2010. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 13. Tadokoro A, Kanaji N, Hara T, et al. An uncharted constellation: TAFRO syndrome. Am J Med 129: 938-941, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Masaki Y, Nakajima A, Iwao H, et al. Japanese variant of multicentric castleman's disease associated with serositis and thrombocytopenia - a report of two cases: is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop 53: 79-85, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Konishi Y, Takahashi S, Nishi K, et al. Successful treatment of TAFRO syndrome, a variant of multicentric Castleman's disease, with cyclosporine A: possible pathogenetic contribution of interleukin-2. Tohoku J Exp Med 236: 289-295, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Edahiro Y, Ichikawa K, Sunami Y, Koike M, Komatsu N. Autoimmune hemolytic anemia in a patient with TAFRO syndrome. Rinsho Ketsueki 56: 2346-2350, 2015. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 17. Takasawa N, Sekiguchi Y, Takahashi T, Muryoi A, Satoh J, Sasaki T. A case of TAFRO syndrome, a variant of multicentric Castleman's disease, successfully treated with corticosteroid and cyclosporine A. Mod Rheumatol 14: 1-5, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Sakashita K, Murata K, Inagaki Y, Oota S, Takamori M. An anterior mediastinal lesion in TAFRO syndrome showing complete remission after glucocorticoid and tocilizumab therapy. Respirol Case Rep 4: e00173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allegra A, Rotondo F, Russo S, et al. Castleman-Kojima disease (TAFRO syndrome) in a Caucasian patient: A rare case report and review of the literature. Blood Cells Mol Dis 55: 206-207, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Tatekawa S, Umemura K, Fukuyama R, et al. Thalidomide for tocilizumab-resistant ascites with TAFRO syndrome. Clin Case Rep 3: 472-478, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawashima M, Usui T, Okada H, et al. TAFRO syndrome: 2 cases and review of the literature. Mod Rheumatol 20: 1-5, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Jain P, Verstovsek S, Loghavi S, et al. Durable remission with rituximab in a patient with an unusual variant of Castleman's disease with myelofibrosis-TAFRO syndrome. Am J Hematol 90: 1091-1092, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tedesco S, Postacchini L, Manfredi L, et al. Successful treatment of a Caucasian case of multifocal Castleman's disease with TAFRO syndrome with a pathophysiology targeted therapy - a case report. Exp Hematol Oncol 4: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawabata H, Kotani S, Matsumura Y, et al. Successful treatment of a patient with multicentric Castleman's disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody. Intern Med 52: 1503-1507, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Iwaki N, Sato Y, Takata K, et al. Atypical hyaline vascular-type castleman's disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J Clin Exp Hematop 53: 87-93, 2013. [DOI] [PubMed] [Google Scholar]