Abstract
A 56-year-old Japanese man diagnosed with acquired immunodeficiency syndrome, Pneumocystis jirovecii pneumonia and cytomegalovirus infection presented with thrombocytopenia after starting antiretroviral therapy, which included dolutegravir (DTG). Although good control of the human immunodeficiency virus and cytomegalovirus infections was achieved, the patient's thrombocytopenia persisted. The patient's platelet count decreased to ≤50,000 /μL even after the cessation of valganciclovir, which can cause bone marrow suppression. At five months after starting antiretroviral therapy, DTG was replaced by ritonavir-boosted darunavir. Soon after, his platelet count improved and was maintained at a level of >100,000 /μL. This is the first reported case of severe thrombocytopenia during DTG-containing antiretroviral therapy.
Keywords: dolutegravir, HIV, thrombocytopenia, platelet, adverse drug reaction
Introduction
Dolutegravir (DTG) is a recently approved, easy-to-take, and highly active integrase strand transfer inhibitor (INSTI) that offers a high genetic barrier to resistance and which has few drug interactions (1). Thus, the usage of DTG as a treatment for human immunodeficiency virus (HIV) infection has been increasing worldwide. As with other INSTIs, DTG has been regarded as a well-tolerated drug; however, there is insufficient information about the incidence and type of adverse drug reactions (ADRs) associated with DTG. The most commonly reported ADRs include headache, nausea, diarrhea, and some biochemical disturbances (e.g. elevation of the creatinine, transaminase and creatine kinase levels); these are typically reported as mild ADRs and do not require the treatment to be discontinued or changed. There are few published reports on the association between DTG and blood cell disorders. We encountered a case of an HIV-infected Japanese man who developed severe thrombocytopenia during DTG-containing antiretroviral therapy. To the best of our knowledge, this is the first report of this ADR. We herein report the case and review the literature on the subject.
Case Report
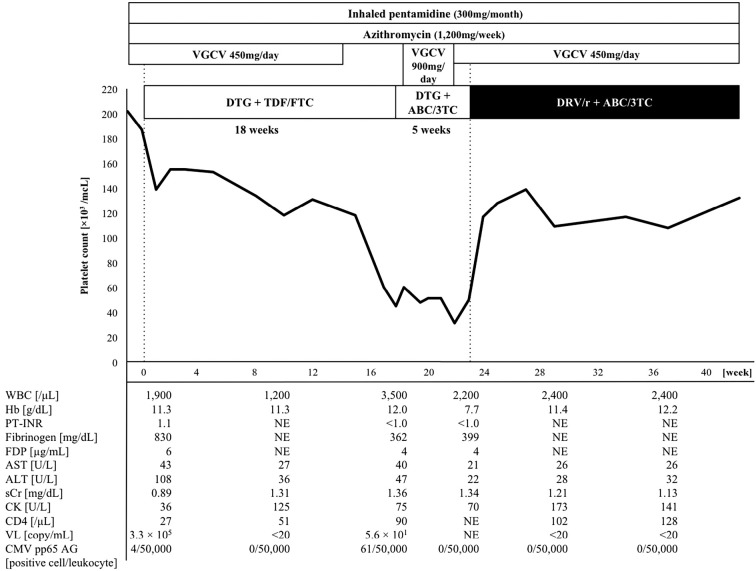
A 56-year-old Japanese man presenting with prolonged fever and dyspnea was admitted to Jikei University Hospital, Tokyo, Japan. He had a comorbidity of hyperuricemia, which was treated with febuxostat. His height and body weight were 1.58 m, and 54 kg, respectively. Chest computed tomography revealed diffuse ground-glass opacity in both lungs. Following detailed examinations, he was diagnosed with acquired immunodeficiency syndrome (AIDS), Pneumocystis jirovecii pneumonia (PCP), and cytomegalovirus (CMV) infection with no evidence of organ involvement. With the exception of PCP and CMV infection, he had no active co-infections (such as hepatitis virus, Helicobacter pylori, or other opportunistic organisms). At the time of his diagnosis, the patient's CD4 cell count was 10 cells/mm3 and the viral load of HIV was 97,000 copies/mL. Induction therapies for PCP and CMV infection were successfully completed, and secondary prevention for each disease was initiated using inhaled pentamidine (300 mg, once a month) and valganciclovir (VGCV). VGCV was appropriately administered as secondary prevention based on the estimated glomerular filtration rate (eGFR) (as an indicator of kidney function) adjusted for Japanese patients (2). The patient received VGCV (450 mg/day) for secondary prevention because his eGFR remained at approximately 50 mL/min/1.73 m2. In addition, azithromycin (1,200 mg, once a week) was started to prevent disseminated nontuberculous mycobacterial disease after the diagnosis of AIDS (CD4 cell count: ≤ 50 cells/mm3).
At four weeks after starting induction therapy for PCP and CMV infection, combination antiretroviral therapy (cART) for HIV infection was started with DTG (50 mg/day), tenofovir (TDF; 300 mg/day), and emtricitabine (FTC; 200 mg/day). The patient's biochemical data at the start of cART are shown in Table. Approximately two months after the start of cART, the patient's platelet count began to gradually decrease. We suspected an adverse reaction to VGCV and discontinued drug; however, the patient's platelet count continued to decrease and reached <50,000 /μL. At four weeks after the discontinuation of VGCV, the reactivation of CMV occurred with no evidence of organ involvement, and three-week induction therapy with VGCV (900 mg/day) was restarted. After the induction therapy, VGCV was continued at a dose of 450 mg/day as secondary prevention. During that period, thrombocytopenia remained. At its lowest, the platelet count decreased to 31,000 /μL without bleeding symptoms. After starting cART, the patient's HIV viral load decreased rapidly, and virological suppression was successfully achieved; however, the patient presented severe thrombocytopenia.
Table.
The Patient’s Biochemical Data at the Start of cART.
| WBC (/µL) | 1,900 | β-D-glucan (pg/mL) | 6.2 |
| RBC (×104 /µL) | 360 | Candida antigen | (-) |
| Hb (g/dL) | 11.3 | Aspergillus antigen | (-) |
| Ht (%) | 33.8 | Cryptococcus antigen | (-) |
| PLT (×103 /µL) | 186 | CMV pp65 AG (positive cell/leukocyte) | 4/50,000 |
| Ret (%) | 2.8 | Anti-Toxoplasma gondii IgG | (-) |
| AST (U/L) | 43 | Helicobacter pylori IgG | (-) |
| ALT (U/L) | 108 | IGRA (T-SPOT®) | (-) |
| LDH (U/L) | 205 | Anti-HAV IgM | (-) |
| T-BIL (mg/dL) | 0.3 | HBs antigen | (-) |
| ALP (U/L) | 402 | Anti-HBs | (+) |
| γ-GTP (U/L) | 205 | Anti-HBc | (+) |
| TP (g/dL) | 7.2 | HBV-DNA | Undetectable |
| ALB (g/dL) | 3.2 | Anti-HCV | (-) |
| CK (U/L) | 28 | HCV-RNA | Undetectable |
| BUN (mEq/L) | 18 | TPHA | ×320 |
| Cr (mg/dL) | 0.89 | RPR | ×1 |
| eGFR (mL/min/1.73m2) | 69 | ||
| TG (mg/dL) | 192 | CD4 (/µL) | 27 |
| HDL-C (mg/dL) | 33 | HIV-RNA (copy/mL) | 3.3×105 |
| LDL-C (mg/dL) | 116 | ||
| HbA1c (%) | 6.0 | ||
| CRP (mg/dL) | 0.85 | ||
| PT-INR | 1.1 | ||
| APTT (sec) | 41.7 | ||
| Fibrinogen (mg/dL) | 830 | ||
| FDP (µg/mL) | 6 | ||
| D-dimer (µg/mL) | 1.6 |
AG: antigemenia, ALB: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, BUN: blood urea nitrogen, cART: combination antiretroviral therapy, CK: creatine kinase, CMV: cytomegalovirus, Cr: creatinine, CRP: C-reactive protein, eGFR: estimated glomerular filtration rate, FDP: fibrinogen degradation product, γ-GTP: gamma-glutamyl transpeptidase, HAV: hepatitis A virus, Hb: hemoglobin, HBV: hepatitis B virus, HCV: hepatitis C virus, HDL-C: high-density lipoprotein cholesterol, HIV: human immunodeficiency virus, Ht: hematocrit, IGRA: interferon-gamma release assay, LDH: lactate dehydrogenase, LDL-C: low-density lipoprotein cholesterol, PLT: platelet, PT-INR: international normalized ratio of prothrombin time, RBC: red blood cell, Ret: reticulocyte, RPR: rapid plasma reagin, T-BIL: total bilirubin, TP: total protein, TPHA: treponema pallidum hemagglutination assay, TG: triglyceride, WBC: white blood cell
Considering the clinical course, we hypothesized that the patient's thrombocytopenia was likely drug-related, and that an antiretroviral drug(s) was most likely responsible. First, TDF and FTC were replaced by abacavir and lamivudine at approximately 18 weeks after the start of cART; however, the patient's platelet count did not improve. At five weeks after the replacement, DTG was switched to ritonavir-boosted darunavir, which is a highly active protease inhibitor with a high genetic barrier. Thereafter, the patient's platelet count promptly improved by >100,000 /μL. At four months after the cessation of DTG, the patient's platelet count remained at >100,000 /μL. The clinical course is summarized in Figure. Inhaled pentamidine, azithromycin, and febuxostat were not changed after the start of cART. In addition, the patient did not take any drugs (other than those listed in Figure), supplements, or Chinese herbs after starting cART.
Figure.
The Clinical course. 3TC: lamivudine, ABC: abacavir, AG: antigemenia, ALT: alanine aminotransferase, AST: aspartate aminotransferase, CK: creatine kinase, CMV: cytomegalovirus, DTG: Dolutegravir, DRV/r: ritonavir-boosted darunavir, FDP: fibrinogen degradation product, Hb: hemoglobin, NE: not evaluated, PT-INR: international normalized ratio of prothrombin time, sCr: serum creatinine, TDF: tenofovir, FTC: emtricitabine, VGCG: valganciclovir, VL: viral load of human immunodeficiency virus, WBC: white blood cell
Discussion
This is the first reported case of severe thrombocytopenia during DTG-containing antiretroviral therapy. DTG is one of the newest antiretroviral drugs, and was approved in the United States and Japan in August 2013 and March 2014, respectively. The efficacy of DTG has been demonstrated in several clinical trials (3-7), and DTG is currently recommended as a first-line agent for the treatment of HIV infection. In a recent safety review of DTG, the most commonly reported ADRs were headache, nausea, and diarrhea; these ADRs were typically mild and did not require discontinuation or a change of treatment (1). The main reported biochemical disturbances have been the elevation of the creatinine, transaminase, and creatine kinase levels. There have been no reports of thrombocytopenia, leukopenia, or anemia. Some older antiretroviral drugs, such as zidovudine, have the potential to cause thrombocytopenia due to bone marrow suppression; however, new-generation agents, including DTG, have rarely been associated with thrombocytopenia.
Thrombocytopenia is a relatively common complication in patients with HIV infection. Many factors, such as HIV-associated immune disorders, co-infection with another virus (e.g. CMV or hepatitis virus) or Helicobacter pylori, malignancy, or drugs can cause thrombocytopenia. Consequently, it is not easy to identify the causative factor. In particular, the diagnosis of drug-induced thrombocytopenia is usually made in complicated situations because patients with HIV infection often take several drugs at the same time. Among the drugs that are frequently used in the treatment of HIV infection and opportunistic diseases, trimethoprim-sulfamethoxazole, ganciclovir (GCV)/VGCV, and rifampicin have been reported to cause secondary thrombocytopenia (8,9). In the present case, with the exception of PCP and CMV infection, the patient did not have active co-infections such as hepatitis virus, Helicobacter pylori, or other opportunistic organisms. The patient required long-term VGCV as induction therapy and secondary prevention for CMV infection. During a period in which DTG and VGCV were concomitantly used, leukopenia and anemia were also observed, along with thrombocytopenia. These blood cell disorders are well known ADRs resulting from bone marrow suppression due to GCV/VGCV (10). Although VGCV could have contributed to the patient's thrombocytopenia, we suspected that DTG was more likely to be the major causative agent because the platelet count recovered after the cessation of DTG, despite the continuous use of VGCV.
The diagnosis of DTG-associated thrombocytopenia is associated with several limitations. We did not evaluate the patient's bone marrow and hence the mechanism of thrombocytopenia remains unclear (e.g. megakaryocytic hypoplasia, or platelet destruction due to immune responses). The possibility of malignancy cannot be completely ruled out for the same reason. In addition, the patient's serum concentration of DTG was not measured; the patient took a normal dose of DTG. Thus, the association between the dose of DTG and the ADR observed in the present case is also unclear. Despite these limitations, we believe that the severe thrombocytopenia was more likely associated with the use of DTG than other potentially influential factors in the present case (e.g. VGCV or viremia due to HIV and CMV) for the following reasons: a) despite the successful suppression of HIV and CMV, the patient's platelet count continued to decrease, and during this period, DTG-containing cART and VGCV (450 mg/day), which can cause bone marrow suppression, were administered; b) after the replacement of DTG, the patient's platelet count rapidly improved; and c) despite the continuous use of VGCV (450 mg/day), the patient's platelet count remained at >100,000 /μL after the replacement of DTG, which suggested that VGCV (450 mg/day) was not responsible for the severe thrombocytopenia. To the best of our knowledge, there are no published reports on this ADR in patients receiving DTG.
In conclusion, we encountered a case of severe thrombocytopenia during DTG-containing antiretroviral therapy. Although several potentially influential factors were present at the same time, we suspected that DTG was the cause of the patient's severe thrombocytopenia, based on the clinical course. DTG has generally been regarded as a well-tolerated drug; however, the information on DTG-associated ADRs remains insufficient. Clinicians should carefully observe patients to detect the signs of ADRs in patients who are treated with DTG. The further accumulation of clinical experience in relation to the administration of DTG is needed.
Author's disclosure of potential Conflicts of Interest (COI).
Seiji Hori: Advisory role, Kyorin Pharmaceutical; Fees for promotional materials, Daiichi-Sankyo.
Acknowledgement
The authors would like to thank the nurses, clerks, laboratory technicians, pharmacists, and resident doctors of our hospital for their vigorous contribution to our practice.
References
- 1. Kandel CE, Walmsley SL. Dolutegravir - a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther 9: 3547-3555, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 369: 1807-1818, 2013. [DOI] [PubMed] [Google Scholar]
- 4. van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. LnID 12: 111-118, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. LnID 13: 927-935, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382: 700-708, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 383: 2222-2231, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Arnold DM, Nazi I, Warkentin TE, et al. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev 27: 137-145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danziger-Isakov L, Mark Baillie G. Hematologic complications of anti-CMV therapy in solid organ transplant recipients. Clin Transplant 23: 295-304, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Jabs DA, Ahuja A, Van Natta M, Dunn JP, Yeh S. Complications of AIDS Research Group: Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 120: 1262-1270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]