Abstract
Objective
In DSM-5, pain-related fear during anticipation of vaginal penetration is a diagnostic criterion of Genito-Pelvic Pain/Penetration Disorder (GPPPD). We aimed to investigate subjective and brain responses during anticipatory fear and subsequent induction of vestibular pain in women with GPPPD.
Methods
Women with GPPPD (n = 18) and age-matched healthy controls (HC) (n = 15) underwent fMRI scanning during vestibular pain induction at individually titrated pain threshold after a cued anticipation period. (Pain-related) fear and anxiety traits were measured with questionnaires prior to scanning, and anticipatory fear and pain intensity were rated during scanning using visual analog scales.
Results
Women with GPPPD reported significantly higher levels of anticipatory fear and pain intensity. During anticipation and pain induction they had stronger and more extensive brain responses in regions involved in cognitive and affective aspects of pain perception, but the group difference did not reach significance for the anticipation condition. Pain-related fear and anxiety traits as well as anticipatory fear ratings were positively associated with pain ratings in GPPPD, but not in HC. Further, in HC, a negative association was found between anticipatory fear ratings and brain responses in regions involved in cognitive and affective aspects of pain perception, but not in women with GPPPD.
Conclusions
Women with GPPPD are characterized by increased subjective and brain responses to vestibular pain and, to a lesser extent, its anticipation, with fear and anxiety associated with responses to pain, supporting the introduction of anticipatory fear as a criterion of GPPPD in DSM-5.
Abbreviations: aMCC, anterior midcingulate cortex; dlPFC, dorsolateral prefrontal cortex; DSM-5, Diagnostic Statistical Manual of Mental Disorders, fifth edition; FM, fibromyalgia; fMRI, functional magnetic resonance imaging; FPQ, Fear of Pain Questionnaire; GPPPD, Genito-Pelvic Pain/Penetration Disorder; HC, healthy controls; IBS, irritable bowel syndrome; n, number; OFC, orbitofrontal cortex; pACC, perigenual anterior cingulate cortex; PASS, Pain Anxiety Symptoms Scale; PVD, provoked vestibulodynia; Q1, Quartile 1; Q3, Quartile 3; SAS, statistical analysis software; SD, standard deviation; SII, secondary somatosensory cortex; SMA, supplementary motor area; SPM8, Statistical Parametric Mapping, SPM8; SPSS, Statistical Package for Social Sciences; STAI, State-Trait Anxiety Inventory; TR/TE, repetition time/echo time; VAS, Visual Analogue Scale; vlPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex
Keywords: Anticipation of pain, Pain-related fear and anxiety, Vestibular pain, fMRI, Genito-pelvic pain/penetration disorder, Provoked vestibulodynia
Highlights
-
•
Both subjective and brain responses during anticipation and induction of vestibular pain are increased in women with GPPPD.
-
•
Between-group differences were found in brain regions involved in cognitive and affective aspects of the pain experience.
-
•
These results support the addition of pain-related fear and anxiety in the diagnostic criteria of GPPPD in DSM-5.
1. Introduction
In DSM-5, Genito-Pelvic Pain/Penetration Disorder (GPPPD) is defined as persistent or recurrent difficulties with vaginal penetration during intercourse (American Psychiatric Association, 2013). GPPPD is a prevalent condition, being present in 11% to 19% of pre-menopausal women (Traeen and Stigum, 2010, Graaf et al., 2012), and is associated with high levels of anxiety and depression, low levels of sexual satisfaction, and sexual dysfunction (Bergeron et al., 2014). Despite the fact that GPPPD is categorized as a sexual dysfunction in DSM-5, it may also be conceptualized as a persistent pain condition or “functional pain syndrome” (van Lankveld et al., 2010, Basson, 2012, Binik, 2005), as it shares critical features with other such conditions including Irritable Bowel Syndrome (IBS) and fibromyalgia (FM), among others (Mayer and Bushnell, 2009) (chapter 4 and 25). More specifically, this may be the case for provoked vestibulodynia (PVD), the most prevalent form of GPPPD in pre-menopausal women, characterized by provoked vulvar pain during sexual and non-sexual activities (Mayer and Bushnell, 2009). In line with such conceptualization, according to DSM-5, one of the criteria for GPPPD is ‘marked fear or anxiety about vulvovaginal or pelvic pain in anticipation of, during or as a result of vaginal penetration’ (American Psychiatric Association, 2013) (p.437), postulating a central role of anticipatory fear or anxiety in this condition. Women with GPPPD report higher levels of pain-related fear and anxiety, hypervigilance to pain, and pain catastrophizing than pain-free controls, and these levels are associated with higher perceived pain intensity in GPPPD patients, but work in this disorder is limited by the use of cross-sectional designs and self-report measures (Payne et al., 2005, Payne et al., 2007, Brauer et al., 2007), with mechanistic experimental studies being almost entirely lacking. Pain perception is characterized by a complex non-linear relationship with its nociceptive input due to a complex interplay between “bottom-up” pain signalling/processing on the one hand and “top-down” pain modulation on the other (Wiech et al., 2008). Through the latter, cognitive-affective processes such as (anticipatory) fear and anxiety can profoundly influence pain perception in healthy humans (Wiech et al., 2008, Wager et al., 2013, Bushnell et al., 2013, Fairhurst et al., 2007, Porro et al., 2004, Atlas and Wager, 2012, Denny et al., 2014, Wager and Atlas, 2013, Wager et al., 2004, Berman et al., 2008). In persistent pain conditions such as irritable bowel syndrome (IBS) and fibromyalgia (FM), these mechanisms may be dysfunctional, with anticipatory fear and chronic (pain-related) anxiety amplifying pain transmission and processing and, hence, perception (Berman et al., 2008, Larsson et al., 2012).
Although still incompletely understood, knowledge on the neural mechanisms underlying pain perception and its modulation by fear/anxiety, and how these may go awry in functional pain syndromes, is growing. The “pain neuromatrix” is a descriptive collective term for pain-responsive brain regions (Wager et al., 2013, Mayer et al., 2006, Apkarian et al., 2005). These regions, however, are by no means specifically or only responsive to pain, and most of them also respond to the anticipation of pain (Bushnell et al., 2013). Indeed, a recent meta-analysis of brain imaging studies on pain anticipation confirmed the partial overlap of brain regions activated during anticipation and induction of pain in healthy subjects (Palermo et al., 2015). Further, the “pain neuromatrix” consists of distinct but highly intertwined networks, each of which have their own function in pain perception. This can be illustrated by the brain networks involved in IBS symptom generation. Afferent pain signals are processed in a sensorimotor network consisting of thalamus, basal ganglia, posterior insula, and primary and secondary sensorimotor cortices, whereas the salience of this afferent input is computed in the salience network, consisting of amygdala, medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior midcingulate cortex (aMCC) and anterior insula. The central executive network (dorsolateral PFC, posterior parietal regions) and emotional-arousal network (locus coeruleus, amygdala, hippocampus, pregenual and subgenual anterior cingulate cortex (pACC, sACC), ventrolateral (vl)PFC and mPFC) are involved in cognitive and emotional responses to and modulation of afferent pain signals. Finally, most of the regions of the emotional-arousal and salience networks overlap with a central autonomic network, including descending modulatory projections to brainstem nuclei (periaqueductal gray (PAG), rostrolateral ventral medulla, which in turn project in a top-down fashion to the dorsal horn of the spinal cord where ongoing pain transmission is being modulated (Mayer et al., 2015). A meta-analysis of brain imaging studies on pain responses in IBS primarily showed altered responses in networks involved in cognitive and affective aspects of pain perception and its modulation (salience, central executive, emotional-arousal, and central autonomic network), rather than in sensory aspects of pain processing (sensorimotor network) (Tillisch et al., 2011). Moreover, a study in healthy subjects demonstrated that interindividual differences in pain-related fear and anxiety (but not general anxiety) correlate with brain responses to experimentally induced heat pain in key regions of the emotional-arousal and salience network, including pACC, mPFC and vlPFC/OFC, thereby confirming the key role of these networks in (anticipatory) pain-related fear and anxiety and its role in pain perception (Ochsner et al., 2006).
However, despite pain-related fear and anxiety being key criteria of a DSM-5 GPPPD diagnosis in women, brain mechanisms underlying pain perception in GPPPD in general, and the putative influence of anticipatory fear and anxiety hereupon in particular, remain poorly understood, as none of the few published brain imaging studies in GPPPD/PVD (Pukall et al., 2005, Schweinhardt et al., 2008, Hampson et al., 2013, Gupta et al., 2015) focused on anticipatory fear and anxiety.
The primary aim of the present study was therefore to investigate subjective and brain responses during anticipation and induction of vestibular pain in women with GPPPD/PVD and in healthy controls (HC). On the behavioural level, we expected in GPPPD versus HC 1) lower vulvar pressure thresholds for moderate pain, 2) higher levels of pain-related fear and anxiety traits (but not general anxiety) as well as higher levels of fear in anticipation of an individually titrated moderate intensity vestibular pain stimulus, and 3) similar pain ratings in response to the pain stimulus (because of the individual titration before scanning). Given the limited amount of previous functional brain imaging research in GPPPD, we refrained from formulating too specific hypotheses on the brain responses, but based on studies in healthy volunteers as well as other persistent pain conditions outlined above, we hypothesized increased brain responses primarily in regions of the central executive, salience, emotional-arousal networks and the overlapping central autonomic network (hence, including prefrontal, cingulate, parietal, medial temporal, and brainstem regions) during both anticipation of and induction of vestibular pain in women with GPPPD compared to HC. As a secondary aim, we explored the association between individual differences in (anticipatory) pain-related fear and anxiety and subsequent pain perception, both at subjective and brain levels. We hypothesized a stronger association between (anticipatory) pain-related fear and anxiety (but not general anxiety) and pain responses, primarily in GPPPD (based on behavioural research in GPPPD), and focused these exploratory analysis on two key regions of the emotional-arousal and salience networks (pACC/mPFC, vlPFC/OFC) (based on the abovementioned study in healthy subjects).
2. Methods and materials
2.1. Participants
Participants included 18 women diagnosed with GPPPD/PVD (23.3 ± 2.1 years) and 15 age-matched, healthy pain-free controls (23.2 ± 1.6 years), all right-handed. In women with GPPPD, gynecological and/or medical conditions potentially causing vulvovaginal pain were excluded by means of a gynecological assessment performed by an experienced gynecologist (LA) in order to include a homogeneous sample of women with PVD. The psychometric assessment included the MINI International Neuropsychiatric Interview (Lecrubier et al., 1997), which was performed by a trained and experienced psychiatrist (LVO) to exclude current psychiatric disorders. Detailed information about the gynecologic and psychometric assessment as well as the in- and exclusion criteria are provided as Supplementary Material. Participants gave written informed consent and received a monetary compensation for their participation. The study was reviewed and approved by the Medical Ethical Committee of the University Hospitals Leuven (Identification Number S52995).
2.2. Course of the study
During the first visit, participants filled out questionnaires to measure pain-related fear and anxiety traits, including the State-Trait Anxiety Inventory (STAI), Trait scale (van der Ploeg, 2000, Spielberger et al., 1983); the Pain Anxiety Symptoms Scale (PASS) (McCracken et al., 1992); and the Fear of Pain Questionnaire (FPQ) (McNeil and Rainwater, 1998). Further, a high resolution structural magnetic resonance imaging (MRI) scan was acquired (details below). The second visit started with the determination of the individual pressure threshold corresponding with moderate pain levels (score of 4 on a visual analogue scale (VAS) ranging from 0 (no pain) to 10 (worst pain imaginable) using a modified vulvalgesiometer (details in Supplementary Methods, device and procedure illustrated in Supplementary Figs. S1 & S2). This individually titrated threshold was used throughout the fMRI session to induce vestibular pain; anticipatory fear was not rated during this individual titration of the pressure threshold for moderate pain prior to scanning. To avoid novelty effects, participants were familiarized with the stimulus and rating procedure during a practice run inside the scanner, prior to the actual fMRI scan session.
2.2.1. fMRI data acquisition and pre-processing
Neuroimaging data were acquired on a Philips Achieva 3.0 Tesla scanner (Philips Medical Systems, Best, the Netherlands) with a 32 channel head-coil. Functional images were collected using an echo-planar imaging sequence with blood oxygen level-dependent contrast (TR/TE = 3000/30 ms, voxel size = 2.50 × 2.50 × 2.50 mm3, flip angle 90°, 48 slices of 2.5 mm thick). The high resolution T1-weighted structural scan of the whole-brain was acquired (TR/TE = 9.6/4.6 ms, voxel size = 0.98 × 0.98 × 1.20 mm3).
We pre-processed and analyzed the fMRI data using SPM8 software (Wellcome Trust Centre for Neuroimaging, UCL) (details in Supplementary Methods).
2.2.2. fMRI session: experimental paradigm
The fMRI session consisted of six runs with 2 min rest between the runs. Each run comprised 12 trials and lasted approximately 10 min. Each trial contained a cued anticipation period of variable duration, a pain induction period and a rating and rest period (Fig. 1 and Supplementary Methods), resulting in a total duration of 45 to 54 s per trial and of approximately 68 min for the entire fMRI session. During the anticipation period, an exclamation point (“!”) indicated a 100% chance that pain would be induced during the subsequent pain period (certain condition); a zero (“0”) indicated that no pain would be induced (safe condition); and a question mark (“?”) indicated a 50% chance that pain would be induced (uncertain condition). Uncertain anticipation conditions were thus part of the paradigm (Fig. 1, Supplementary Table S1), but to keep the current paper sufficiently focused and concise (i.e. within an acceptable word limit), we decided to only report the analyses testing our hypotheses on the certain anticipation and certain pain conditions herein; comparisons with the uncertain conditions will be reported in a separate manuscript on the effect of uncertainty on subjective and brain responses to pain and its anticipation in different conditions including GPPPD.
Fig. 1.
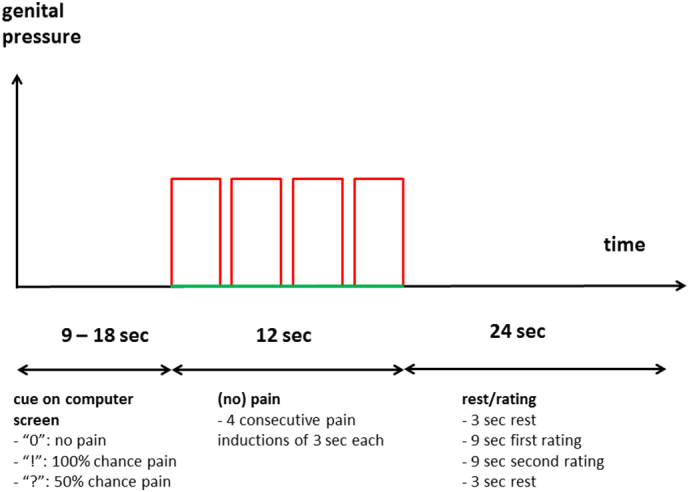
Schematic overview of a single trial of the fMRI experiment. For detailed description see Supplementary Methods.
2.3. Data analysis
2.3.1. Descriptive data
Pain threshold and pain-related fear and anxiety traits were compared between groups using independent samples Student's t-tests in SPSS statistical software (version 22.0; Chicago, Inc., IL). All data are shown as mean ± SD or median (Q1; Q3). Since we had an a priori hypothesis about the direction of the difference (i.e. lower pain threshold and higher fear and anxiety traits in GPPPD), we considered a one-tailed p-value < 0.05 significant. Since we performed 3 bivariate tests on the 3 trait variables, we applied a Bonferroni correction to these p-values.
2.3.2. Subjective responses to vestibular pain and its anticipation: behavioural data
Anticipatory fear and pain ratings obtained during scanning were analyzed on a trial-by-trial basis using linear mixed models in SAS 9.3 software (SAS Institute, Cary, NC, USA). Fear and pain ratings were the dependent variables, independent variables included “repetition” (within-subject continuous variable to test the effect of repeated stimulus administration), “condition” (within-subject categorical variable; safe, certain for anticipatory fear; safeno pain and certainpain for pain) and “group” (between-subject categorical variable; GPPPD, HC). All possible interaction effects were included, but for reasons of parsimony, only significant interaction effects were retained in the final models. Planned contrasts using t-tests (one-tailed for the reason mentioned above) with Bonferroni correction for multiple testing were used to test our specific hypotheses (higher levels of anticipatory fear during anticipation in GPPPD versus HC, similar levels of subsequent pain perception due to the individual titration). Random intercepts and slopes for repetition (per participant as well as per condition within participant) were used to model the variance-covariance structure of the data as this model was shown to fit the data best based on Akaike's Information Criterion.
2.3.3. Brain responses to vestibular pain induction and its anticipation: fMRI data
2.3.3.1. First level analysis
Statistical analyses were conducted with a combined event (anticipation period) and block (pain period) design using the generalized linear model (Friston et al., 1994). To test the hypotheses formulated in this paper, the following contrasts were computed for each individual: 1) anticipation of vestibular pain (certain – safe) and 2) induction of vestibular pain (certainpain – safeno_pain).
2.3.3.2. Second level analysis
Whole-brain voxel-based analysis was conducted in SPM8 at a voxel-level threshold of puncorrected < 0.001 combined with a cluster-level threshold of pFWE-corrected < 0.05 for the within-group analyses (one sample Student's t-test for each contrast). Differences between women with GPPPD and HC were tested using two sample Student's t-tests. Due to lower statistical power, these between-group comparisons were performed at a less stringent threshold of puncorrected < 0.005 at voxel-level combined with a cluster-level threshold of pFWE-corrected < 0.05. For all analyses, a gray matter mask was used.
2.3.4. Relationship between pain-related fear and anxiety and pain responses at subjective and brain level
For the purpose of regression analysis, we calculated the average of the online VAS ratings of anticipatory fear during the certain and safe anticipation conditions over all trials, and subtracted the ratings obtained during the safe condition from the ratings obtained during the certain condition in each subject (Δcertain - safe). In a similar way, we averaged and subtracted online VAS ratings of pain intensity during the certainpain and safeno_pain conditions (Δcertainpain - safeno_pain). The associations between these variables, as well as the association with questionnaire data was tested using robust regression models in SAS 9.3 software. Associations with brain responses were conducted using regression analysis in SPM8. As mentioned in the introduction, these exploratory analyses focused on two key regions of the emotional-arousal and salience networks (pACC/mPFC, vlPFC/OFC), based on earlier work on healthy volunteers (Ochsner et al., 2006). Six mm spheres around the coordinates of the local maxima from this previous study were used as regions of interest for these analyses (pACC/mPFC: − 10, 48, 0; vlPFC/OFC: 30, 40, − 8). Voxel-level threshold was set at pFWE-corrected < 0.05 within a mask consisting of both ROIs combined.
2.3.4.1. Questionnaire measures of (pain-related) fear and anxiety traits
The associations between subjective questionnaire measures of pain-related fear and anxiety (FPQ, STAI, PASS) on the one hand and subjective online ratings (Δcertainpain - safeno_pain) and brain responses to vestibular pain induction (contrast certainpain - safeno_pain) on the other hand were tested using (robust) regression analysis, in the entire sample, and in women with GPPPD and HC separately.
2.3.4.2. Online ratings of anticipatory fear and pain
The associations between online anticipatory fear ratings during certain anticipation compared to safe anticipation (Δcertain - safe) on the one hand and subjective online ratings (Δcertainpain - safeno_pain) and brain responses to vestibular pain induction (contrast certainpain - safeno_pain) on the other hand were tested using (robust) regression analysis, in the entire sample, and in women with GPPPD and HC separately.
3. Results
3.1. Descriptive results: pain threshold and pain-related fear and anxiety traits
Contrary to our hypothesis, pain threshold was not significantly different between HC and women with GPPPD [300 g (range: 250–400 g) versus 289.44 g (range: 200–400 g), Z = 0.34, p = 0.37].
On average, FPQ was significantly higher in GPPPD than in HC [67.50 ± 14.92 vs 55.93 ± 13.77, t(31)=2.296, p = 0.04, Cohen's d = 0.81] (as hypothesized), while no significant differences were found on PASS (not as hypothesized) and STAI (as hypothesized) (Table 1).
Table 1.
Comparison of (pain-related) fear and anxiety traits in women with genito-pelvic pain/penetration disorder (GPPPD; n = 18) and healthy controls (HC; n = 15).
| Measures | GPPPD | HC | t-value | p-Value Bonferroni corrected one-tailed | Cohen d |
|---|---|---|---|---|---|
| State-Trait Anxiety Inventory, Trait Scale | 32.83 ± 7.63 | 30.47 ± 4.07 | 1.078 | 0.44 | 0.40 |
| Pain Anxiety Symptoms Scale | 55.56 ± 18.24 | 52.60 ± 21.45 | 0.428 | 1 | 0.15 |
| Fear of Pain Questionnaire | 67.50 ± 14.92 | 55.93 ± 13.77 | 2.296 | 0.04 | 0.81 |
Data are shown as mean ± SD. Bold p-values < 0.05, are considered as significant.
3.2. Primary aim: comparison of subjective and brain responses to anticipation and induction of vestibular pain between HC and GPPPD
3.2.1. Anticipation of vestibular pain
3.2.1.1. Anticipatory fear ratings (online VAS)
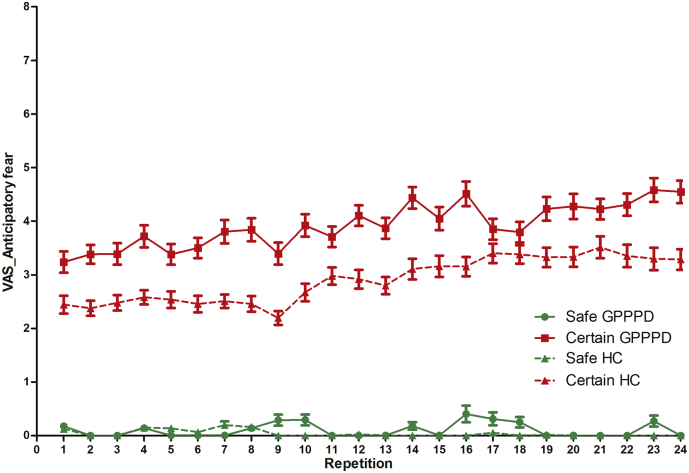
A significant main effect of condition [F(1,31) = 103.5, p < 0.0001] was found, due to significantly higher ratings in certain (3.39 ± 0.23) compared to safe (0.07 ± 0.23) anticipation, indicating that pain induction indeed resulted in significant levels of anticipatory fear (of low to moderate intensity on average). A significant main effect of repetition [β = 0.03 ± 0.01, F(1,32)=13.5, p = 0.0009] (positive slope over both groups and conditions) and a trend for a main effect of group [F(1,1324) = 2.94, p = 0.087] (higher ratings over both conditions and all repetitions in GPPPD) were also found. A significant repetition ∗ condition interaction effect was found [F(1,32) = 18.4, p = 0.0002], due to a significantly higher slope of repetition in certain (βrepetition = 0.06 ± 0.01, p < 0.0001) compared to safe (βrepetition = − 0.003 ± 0.01, p = 0.74) anticipation conditions, indicating a linear increase in anticipatory fear ratings with repeated stimulation in the former condition. The condition ∗ group interaction effect was not significant [F(1,1324) = 2.43, p = 0.12], but planned contrasts demonstrated significantly higher ratings in GPPPD versus HC in the certain condition (3.92 ± 0.31 versus 2.88 ± 0.34, p = 0.021), and no difference in the safe condition (0.10 ± 0.31 versus 0.05 ± 0.34, p = 1.0) (Fig. 2). In summary, our hypothesis that individually titrated moderate levels of vestibular pain would provoke higher levels of anticipatory fear in GPPD versus HC was confirmed in planned contrast analysis directly testing this hypothesis.
Fig. 2.
Trial-by-trial online anticipatory fear ratings in women with genito-pelvic pain/penetration disorder (GPPPD) and healthy control women (HC). For results of the statistical analysis, see text.
3.2.1.2. Brain responses during anticipation of vestibular pain
3.2.1.2.1. GPPD patients
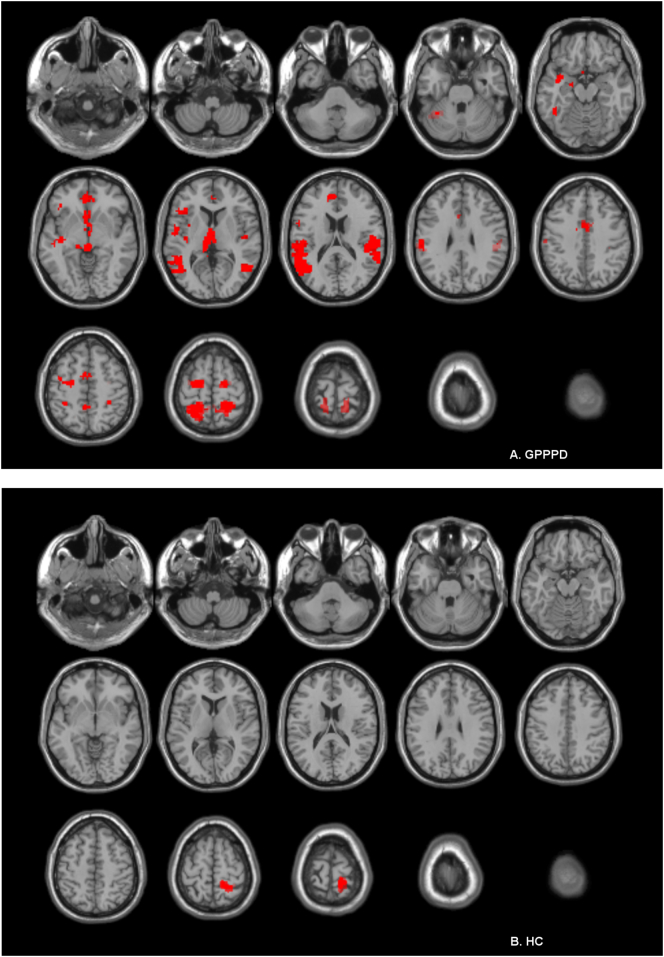
Significant activations (certain - safe) were found in key regions of virtually all networks comprising the “pain neuromatrix” including sACC, pACC, aMCC, vlPFC, OFC, (pre)motor cortex, SI/SII, (posterior) insula, caudate head & pallidum, thalamus, midbrain, parahippocampal gyrus, hypothalamus, precuneus, and inferior parietal cortex. No significant deactivations (safe - certain) were found (Fig. 3A, Table 2A).
Fig. 3.
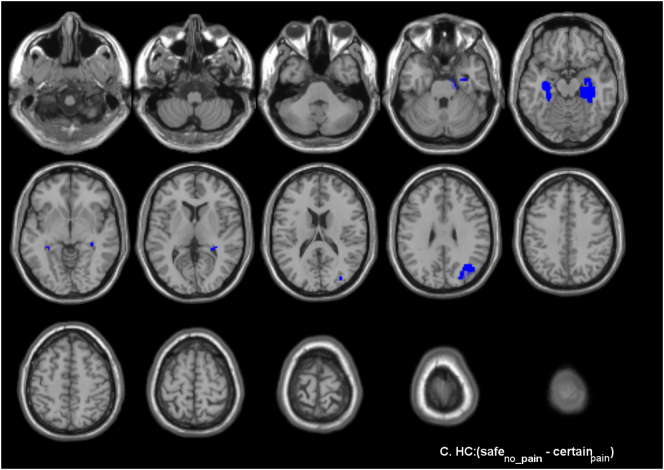
Brain responses during anticipation of vestibular pain (contrast certain - safe) in (A) women with genito-pelvic pain/penetration disorder (GPPPD) and (B) healthy control women (HC). A voxel level threshold of puncorrected < 0.001 combined with a cluster level threshold of pFWE-corrected < 0.05 was used.
Table 2.
Brain responses during anticipation of vestibular pain (contrast: certain – safe), in (A) women with genito-pelvic pain/penetration disorder (GPPPD) and (B) healthy control women (HC).
| Cluster level |
Peak level |
MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Contrast | pFWE-corr | kE | T | x | y | z | Tentative anatomical localization | |
| A | GPPPD | Certain - safe | < 0.001 | 351 | 8.43 | − 3 | 6 | − 11 | Left Caudate Head |
| 6.72 | 3 | − 24 | − 5 | Right Thalamus | |||||
| 6.41 | − 3 | − 9 | 7 | Left Thalamus | |||||
| 6.30 | 0 | 18 | − 8 | sACC/olfactory cortex | |||||
| 6.29 | 6 | − 3 | − 8 | Right Hypothalamus | |||||
| 5.92 | − 9 | − 33 | 4 | Left Parahippocampal gyrus/PCC | |||||
| 5.03 | 3 | 6 | − 2 | Right Caudate Head | |||||
| 4.85 | − 21 | − 12 | − 11 | Left Pallidum | |||||
| 4.51 | − 6 | − 6 | − 8 | Left Hypothalamus | |||||
| 4.21 | − 12 | − 36 | − 8 | Left Parahippocampal gyrus | |||||
| < 0.001 | 523 | 7.60 | 21 | − 42 | 70 | Right Postcentral gyrus (SI) | |||
| 7.03 | − 18 | − 51 | 70 | Left Superior Parietal Lobule | |||||
| 6.74 | − 3 | − 45 | 55 | Left Precuneus | |||||
| 5.87 | − 15 | − 42 | 61 | Left Superior Parietal Lobule | |||||
| 5.82 | − 30 | − 54 | 64 | Left Superior Parietal Lobule | |||||
| 4.57 | − 33 | − 42 | 58 | Left Postcentral Gyrus (SI) | |||||
| 3.97 | 33 | − 30 | 46 | Right Postcentral Gyrus (SI) | |||||
| 3.95 | 6 | − 51 | 64 | Right Precuneus | |||||
| < 0.001 | 75 | 7.49 | 0 | 42 | − 5 | Left pACC | |||
| 5.41 | − 3 | 60 | − 14 | Left Midorbital Gyrus (OFC) | |||||
| 3.92 | − 3 | 30 | − 2 | Right Midorbital Gyrus (OFC) | |||||
| < 0.001 | 627 | 7.19 | − 54 | − 63 | 16 | Left Middle Temporal Gyrus | |||
| 5.99 | − 57 | − 45 | 19 | Left Superior Temporal Gyrus | |||||
| 5.78 | − 42 | − 63 | 7 | Left Middle Temporal Gyrus | |||||
| 5.14 | − 39 | − 66 | 25 | Left Middle Occipital Gyrus | |||||
| 5.00 | − 51 | − 36 | 22 | Left Inferior Parietal Lobule | |||||
| 4.90 | − 57 | − 21 | 31 | Left Inferior Parietal Lobule | |||||
| 4.82 | − 60 | − 27 | 22 | Left Supramarginal Gyrus | |||||
| 4.11 | − 57 | − 39 | 10 | Left Superior Temporal Gyrus | |||||
| < 0.001 | 167 | 6.96 | − 15 | − 12 | 67 | Left Paracentral Lobule | |||
| 6.21 | − 27 | − 12 | 58 | Left Precentral Gyrus | |||||
| 4.50 | − 39 | − 3 | 55 | Left Precentral Gyrus | |||||
| < 0.001 | 84 | 6.61 | − 39 | − 51 | − 23 | Left Fusiform Gyrus/Cerebellum | |||
| < 0.001 | 60 | 6.18 | 21 | − 12 | 64 | Right Superior Frontal Gyrus (premotor cortex) | |||
| 4.52 | 30 | − 9 | 58 | Right Superior Frontal Gyrus (premotor cortex) | |||||
| < 0.001 | 84 | 5.95 | − 36 | − 21 | − 5 | Left Posterior Insula | |||
| 5.43 | − 36 | − 15 | 7 | Left Posterior Insula | |||||
| 5.36 | − 36 | 0 | − 14 | Left Posterior Insula | |||||
| < 0.001 | 341 | 5.91 | 57 | − 24 | 19 | Right SII | |||
| 5.32 | 48 | − 60 | 7 | Right Middle Temporal Gyrus | |||||
| 5.20 | 60 | − 48 | 16 | Right Inferior Parietal Lobule | |||||
| 4.81 | 57 | − 39 | 22 | Right Inferior Parietal Lobule | |||||
| 4.74 | 45 | − 33 | 16 | Right Inferior Parietal Lobule/SII | |||||
| 4.64 | 48 | − 12 | 4 | Right Heschl's Gyrus | |||||
| 3.70 | 60 | − 18 | 31 | Right Inferior Parietal Lobule/SI | |||||
| < 0.001 | 62 | 5.86 | − 45 | 24 | 7 | Left Inferior Frontal Gyrus | |||
| 5.62 | − 39 | 33 | − 5 | Left Inferior Frontal Gyrus (vlPFC) | |||||
| < 0.001 | 71 | 5.53 | − 9 | 42 | 22 | Left Superior Medial Gyrus (dmPFC) | |||
| 5.02 | − 3 | 42 | 13 | Left pACC | |||||
| < 0.001 | 76 | 5.07 | − 54 | 0 | 7 | Left Rolandic Operculum | |||
| 4.54 | − 42 | − 3 | 16 | Left Rolandic Operculum | |||||
| 4.28 | − 33 | 0 | 10 | Left Midinsula | |||||
| < 0.001 | 106 | 4.61 | 12 | 6 | 37 | Right aMCC | |||
| 4.50 | − 3 | 3 | 49 | Left Posterior Medial Frontal Gyrus | |||||
| 4.34 | − 12 | − 3 | 40 | Left MCC | |||||
| 4.09 | 6 | 0 | 55 | Right Posterior Medial Frontal Gyrus | |||||
| 3.76 | − 3 | 15 | 31 | Left aMCC | |||||
| B | HC | Certain - safe | < 0.001 | 171 | 7.62 | 21 | − 39 | 73 | Right postcentral gyrus (SI) |
| 6.29 | 30 | − 45 | 61 | Right postcentral gyrus (SI) | |||||
A voxel level threshold of puncorr < 0.001 combined with a cluster level threshold of pFWE-corr < 0.05 was used. Local maxima > 10 mm apart are shown. sACC, subgenual anterior cingulate cortex; PCC, posterior cingulate cortex; SI, primary somatosensory cortex; pACC, pregenual anterior cingulate cortex; OFC, orbitofrontal cortex; SII, secondary somatosensory cortex; vlPFC, ventrolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; aMCC, anterior midcingulate cortex; FWE, family-wise error; kE, cluster size extent; MNI, Montreal Neurological Institute.
3.2.1.2.2. Healthy controls
In HC, significant activations (certain - safe) were found in right SI only. No deactivations (safe - certain) were found (Fig. 3B, Table 2B).
3.2.1.2.3. Between-group comparison
No significant differences were found at the pre-defined combined voxel- and cluster-level threshold. However, stronger activation was found in women with GPPPD compared to HC [contrast (certain - safe)GPPPD > (certain - safe)HC] in a key region of the emotional-arousal network (pACC/vmPFC; local maximum − 6, 42, 16), as hypothesized, reaching significance at the voxel-level threshold (T = 3.41, puncorr = 0.001), but not at the additional cluster-level threshold (k = 44, puncorr = 0.072, pFWE-corr = 0.77). No regions showed stronger activation in HC compared to GPPPD [contrast (certain - safe)HC > (certain - safe)GPPPD], even when the additional cluster-level threshold was omitted.
3.2.2. Induction of vestibular pain
3.2.2.1. Pain intensity ratings (online VAS)
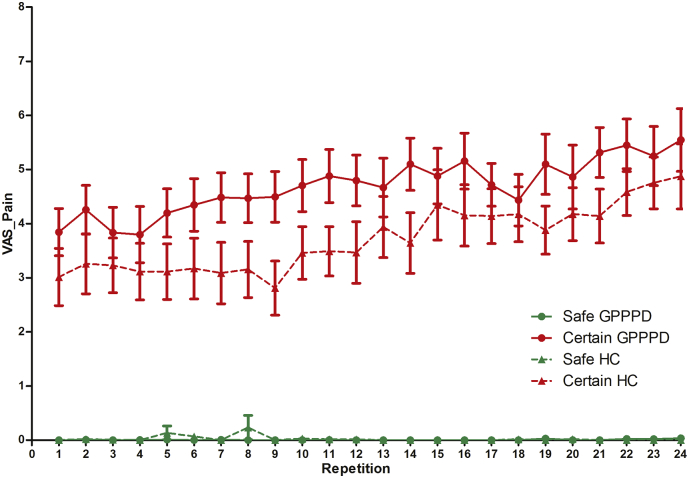
A significant main effect of condition [F(1,31) = 184.1, p < 0.0001] was found, due to significantly higher ratings in certainpain (4.17 ± 0.22) compared to safeno_pain (0.01 ± 0.22), indicating that pain induction indeed resulted in moderate levels of pain as intended. A significant main effect of repetition [β = 0.04 ± 0.01, F(1,32) = 25.0, p < 0.0001] was found (positive slope over both groups and conditions), the main effect of group was not significant [F(1,1324) = 2.72, p = 0.10]. Further, a significant repetition ∗ condition interaction effect was found [F(1,32) = 26.4, p < 0.0001], due to a significantly higher slope of repetition in certainpain (βrepetition = 0.07 ± 0.01, p < 0.0001) compared to safeno_pain (βrepetition = − 0.001 ± 0.01, p = 0.94), indicating a linear increase in pain intensity ratings with repeated stimulation in the former condition. The condition ∗ group interaction effect [F(1,1324) = 2.43, p = 0.08] revealed a trend, but planned contrasts demonstrated significantly higher ratings in GPPPD versus HC in the certainpain condition (4.69 ± 0.29 versus 3.66 ± 0.32, p = 0.017) (contrary to what we expected because of the individual titration of the stimulus intensity prior to scanning), and no difference in the safeno_pain condition (0.002 ± 0.29 versus 0.03 ± 0.32, p = 1.0) (Fig. 4).
Fig. 4.
Trial-by-trial online pain intensity ratings in women with genito-pelvic pain/penetration disorder (GPPPD) and healthy control women (HC). For results of the statistical analysis, see text.
3.2.2.2. Brain responses during induction of vestibular pain
3.2.2.2.1. GPPPD patients
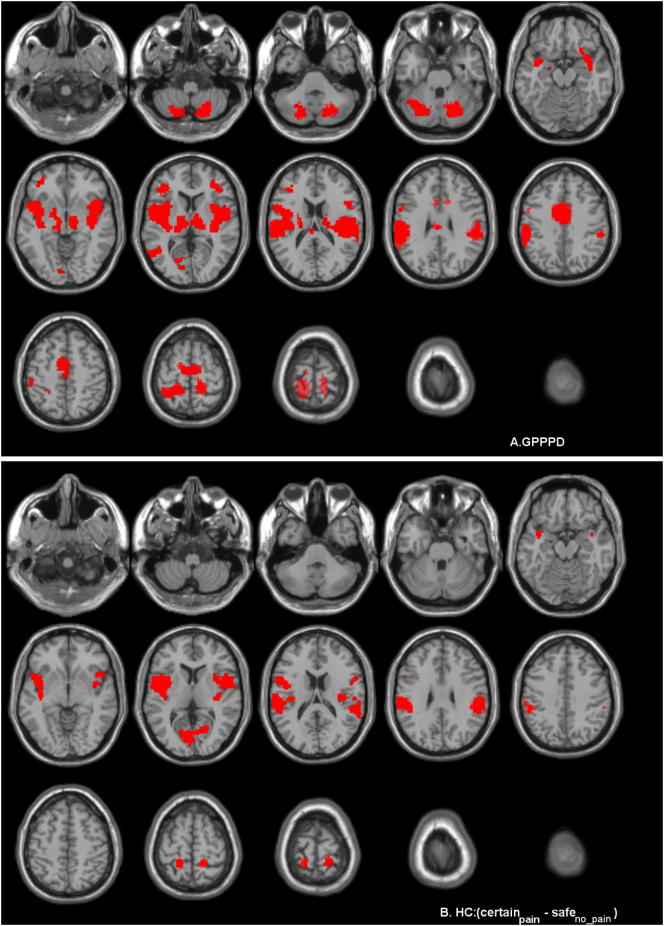
Activations (certainpain - safeno_pain) were found in key regions of virtually all networks comprising the “pain neuromatrix” including vlPFC, dlPFC, insula, aMCC, thalamus, caudate, SI, SII, supramarginal gyrus, inferior parietal lobule, precentral gyrus, and premotor cortex, as well as some regions that are not generally considered part of the “pain neuromatrix”, including middle temporal gyrus, cerebellum, and left calcarine and lingual gyrus. No significant deactivations (safeno_pain - certainpain) were found (Table 3A and Fig. 5A).
Table 3.
Brain responses during induction of vestibular pain (contrast certainpain – safeno_pain) in (A) women with genito-pelvic pain/penetration disorder (GPPPD) and (B) healthy controls (HC).
| Cluster level |
Peak level |
MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Contrast | pFWE-corr | kE | T | x | y | z | Tentative anatomical localization | |
| A | GPPPD | Certainpain - safeno_pain | < 0.001 | 1262 | 16.24 | 60 | − 24 | 16 | Right SII |
| 14.42 | 39 | − 3 | − 5 | Right Midinsula | |||||
| 10.04 | 36 | 3 | 10 | Right Midinsula | |||||
| 10.00 | 48 | − 27 | 22 | Right SII | |||||
| 9.13 | 36 | − 18 | 16 | Right SII/posterior insula | |||||
| 8.55 | 54 | 3 | 4 | Right Rolandic Operculum | |||||
| 7.12 | 36 | − 21 | − 2 | Right Posterior Insula | |||||
| 7.01 | 57 | − 36 | 31 | Right Supramarginal Gyrus | |||||
| 5.02 | 51 | 15 | 22 | Right Inferior Frontal Gyrus | |||||
| 4.87 | 57 | 9 | 16 | Right Inferior Frontal Gyrus | |||||
| 4.52 | 27 | 18 | − 17 | Right Anterior Insula | |||||
| < 0.001 | 1791 | 12.44 | − 60 | − 33 | 40 | Left Interior Parietal Lobule | |||
| 11.15 | − 21 | − 42 | 67 | Left SI | |||||
| 11.04 | − 36 | 0 | 10 | Left Midinsula | |||||
| 10.87 | − 51 | 0 | 4 | Left Rolandic Operculum | |||||
| 9.94 | − 36 | 6 | − 5 | Left Midinsula | |||||
| 9.03 | − 60 | − 21 | 28 | Left SI | |||||
| 8.59 | − 60 | − 24 | 37 | Left SI | |||||
| 7.98 | − 39 | − 18 | − 5 | Left Posterior Insula | |||||
| 7.38 | − 39 | − 15 | 13 | Left Posterior Insula | |||||
| 7.07 | − 54 | 6 | 19 | Left Inferior Frontal Gyrus | |||||
| < 0.001 | 481 | 12.43 | 15 | − 75 | − 50 | Right Cerebellum (lobule VII) | |||
| 8.74 | 21 | − 73 | − 18 | Right Cerebellum (lobule VI) | |||||
| 0.046 | 59 | 10.74 | 42 | 39 | 7 | Right Inferior Frontal Gyrus (dlPFC) | |||
| < 0.001 | 643 | 8.37 | − 6 | 3 | 40 | Left aMCC | |||
| 7.08 | − 3 | − 18 | 61 | Left Medial Frontal Gyrus (premotor cortex) | |||||
| 6.53 | − 12 | − 15 | 73 | Left Paracentral Lobule (premotor cortex) | |||||
| 5.89 | 6 | − 12 | 61 | Right Medial Frontal Gyrus (premotor cortex) | |||||
| 5.83 | 12 | − 12 | 70 | Right Medial Frontal Gyrus (premotor cortex) | |||||
| 5.71 | − 12 | − 3 | 67 | Left Medial Frontal Gyrus (premotor cortex) | |||||
| 5.64 | 9 | 6 | 37 | Right aMCC | |||||
| 5.19 | 12 | 15 | 31 | Right aMCC | |||||
| 4.44 | − 3 | − 21 | 31 | Left pMCC | |||||
| < 0.001 | 404 | 8.15 | − 18 | − 72 | − 47 | Left Cerebellum (Lobule VII) | |||
| 7.88 | − 24 | − 69 | − 23 | Left Cerebellum (Lobule VI) | |||||
| 5.28 | − 39 | − 54 | − 26 | Left Cerebellum (Lobule VI) | |||||
| 0.002 | 118 | 8.03 | 18 | − 39 | 70 | Right pre/postcentral gyrus | |||
| 6.05 | 12 | − 33 | 76 | Right pre/postcentral gyrus | |||||
| < 0.001 | 164 | 6.40 | 9 | − 15 | 4 | Right Thalamus | |||
| 5.42 | − 12 | − 24 | 1 | Left Thalamus | |||||
| 5.37 | − 6 | − 18 | 4 | Left Thalamus | |||||
| 4.31 | 18 | − 15 | 19 | Right Caudate Head | |||||
| 0.004 | 105 | 6.10 | − 39 | 36 | 10 | Left Inferior Frontal Gyrus (dlPFC) | |||
| 4.66 | − 42 | 36 | 1 | Left Inferior Frontal Gyrus | |||||
| 4.39 | − 36 | 45 | − 5 | Left Middle Frontal/Orbital Gyrus (vlPFC) | |||||
| 0.039 | 62 | 5.10 | − 45 | − 57 | 7 | Left Middle Temporal Gyrus | |||
| 0.008 | 91 | 4.32 | − 21 | − 72 | 7 | Left Calcarine Gyrus | |||
| 4.04 | − 12 | − 87 | − 2 | Left Lingual Gyrus | |||||
| Safeno_pain - certainpain | No suprathreshold clusters | ||||||||
| B | HC | Certainpain - safeno_pain | < 0.001 | 952 | 9.49 | − 63 | − 21 | 19 | Left SII |
| 9.13 | − 39 | − 21 | 16 | Left SII | |||||
| 8.57 | − 39 | − 3 | 1 | Left Posterior Insula | |||||
| 7.58 | − 60 | − 21 | 34 | Left Supramarginal Gyrus | |||||
| 7.36 | − 51 | 9 | 16 | Left Inferior Frontal Gyrusq | |||||
| 7.31 | − 45 | − 3 | 13 | Left Rolandic Operculum | |||||
| 6.26 | − 57 | 9 | 4 | Left Rolandic Operculum | |||||
| 5.53 | − 54 | − 30 | 19 | Left Inferior Parietal Lobule/SII | |||||
| 5.37 | − 51 | − 36 | 40 | Left Inferior Parietal Lobule | |||||
| 5.27 | − 39 | − 15 | − 2 | Left Posterior Insula | |||||
| 5.12 | − 42 | 0 | − 11 | Left Midinsula | |||||
| 0.010 | 83 | 8.66 | 21 | − 42 | 73 | Right SI | |||
| 6.44 | 15 | − 42 | 64 | Right Superior Parietal Lobule/SI | |||||
| < 0.001 | 286 | 7.43 | 54 | − 33 | 25 | Right Inferior Parietal Lobule/SI | |||
| 6.08 | 54 | − 21 | 31 | Right Inferior Parietal Lobule/SI | |||||
| 4.99 | 57 | − 42 | 10 | Right Middle Temporal Gyrus | |||||
| < 0.001 | 340 | 7.42 | 60 | 9 | 13 | Right Rolandic Operculum | |||
| 5.74 | 45 | 18 | 1 | Right Anterior Insula/Inferior Frontal Gyrus | |||||
| 4.87 | 36 | 6 | 7 | Right Midinsula | |||||
| 4.74 | 42 | 0 | − 2 | Right Midinsula | |||||
| 0.008 | 87 | 7.41 | − 18 | − 45 | 67 | Left Superior Parietal Lobule | |||
| 0.014 | 77 | 6.46 | 39 | − 18 | 16 | Right SII | |||
| < 0.001 | 236 | 6.10 | − 6 | − 78 | 10 | Left Calcarine Gyrus | |||
| 5.79 | 18 | − 63 | 4 | Right Calcarine Gyrus | |||||
| 4.87 | − 18 | − 66 | 4 | Left Calcarine Gyrus | |||||
| Safeno_pain - certainpain | < 0.001 | 229 | 7.44 | 36 | − 30 | − 14 | Right Hippocampus | ||
| 5.15 | 30 | − 12 | − 17 | Right Hippocampus | |||||
| 4.96 | 21 | − 36 | − 11 | Right Parahippocampal Gyrus | |||||
| 0.013 | 78 | 6.80 | − 33 | − 21 | − 17 | Left Hippocampus | |||
| 5.48 | − 27 | − 36 | − 14 | Left Parahippocampal Gyrus | |||||
| 5.00 | − 33 | − 36 | − 5 | Left Hippocampus | |||||
| 0.003 | 113 | 5.45 | 39 | − 66 | 28 | Right Angular Gyrus | |||
| 4.81 | 27 | − 81 | 28 | Right Superior Occipital Gyrus | |||||
A voxel level threshold of puncorr < 0.001 combined with a cluster level threshold of pFWE-corr < 0.05 was used. SII, secondary somatosensory cortex; SI, primary somatosensory cortex; dlPFC, dorsolateral prefrontal cortex; a/pMCC, anterior/posterior midcingulate cortex; vlPFC, ventrolateral prefrontal cortex; FWE, family-wise error; kE, cluster size extent; MNI, Montreal Neurological Institute.
Fig. 5.
Brain responses during induction of vestibular pain (contrast certainpain – safeno pain) in (A) women with genito-pelvic pain/penetration disorder (GPPPD) and (B) healthy control women (HC). A voxel level threshold of puncorrected < 0.001 combined with a cluster level threshold of pFWE-corrected < 0.05 was used.
3.2.2.2.2. Healthy controls
Significant activations (certainpain - safeno_pain) were primarily found in sensorimotor network regions including (posterior-mid) insula, SI, and SII as well as in one central executive regions (inferior parietal lobule). Activations were also found in regions outside the “pain neuromatrix” including inferior frontal gyrus, middle temporal gyrus, calcarine and lingual gyrus. The hippocampus and parahippocampal gyrus, key emotional-arousal/central autonomic regions were deactivated (safeno_pain - certainpain), as well as central executive region (angular gyrus), and middle occipital gyrus outside the “pain neuromatrix” (Table 3B and Fig. 5B & C).
3.2.2.2.3. Between-group comparison
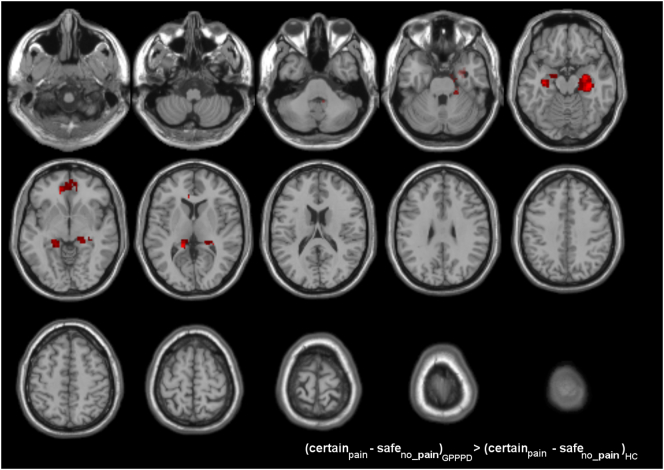
In comparison to HC, women with GPPPD showed significantly stronger activations [contrast (certainpain - safeno_pain)GPPPD > (certainpain - safeno_pain)HC] in key emotional-arousal/central autonomic network regions: amygdala, hippocampus, and parahippocampal gyrus (significant at cluster level in the right hemisphere, trend at cluster level in the right hemisphere) (Table 4, Fig. 6). Further, stronger activation in GPPPD compared to HC was found in bilater pACC/vmPFC, but this difference did not reach significance at cluster level. All these differences were driven by deactivation in HC and activation in GPPPD, as evident from the within-group analyses reported above (Fig. 4, Table 3). No regions showed more activation in HC compared to GPPPD [contrast (certainpain - safeno_pain)HC > (certainpain - safeno_pain)GPPPD].
Table 4.
Differences in brain responses between women with GPPPD and healthy controls during induction of vestibular pain.
| Cluster level |
Peak level |
MNI coordinates |
||||
|---|---|---|---|---|---|---|
| pFWE-corr | kE | T | x | y | z | Tentative anatomical localization |
| 0.007 | 255 | 4.64 | 36 | − 21 | − 17 | Right Hippocampus |
| 4.18 | 33 | − 3 | − 23 | Right Amygdala | ||
| 4.10 | 30 | − 12 | − 17 | Right Hippocampus | ||
| 3.75 | 21 | − 33 | − 32 | Right Cerebellum | ||
| 3.75 | 30 | − 36 | 4 | Right Hippocampus | ||
| 3.66 | 18 | − 30 | − 11 | Right Parahippocampal Gyrus | ||
| 3.48 | 24 | − 27 | − 23 | Right Parahippocampal Gyrus | ||
| 3.04 | 18 | − 33 | 7 | Right Thalamus (Pulvinar) | ||
| 0.089 | 141 | 4.28 | − 33 | − 18 | − 17 | Left Hippocampus |
| 3.62 | − 18 | − 36 | − 2 | Left Parahippocampal Gyrus | ||
| 3.49 | − 24 | − 9 | − 11 | Left Amygdala | ||
| 3.39 | − 21 | − 30 | − 5 | Left Parahippocampal Gyrus | ||
| 3.10 | − 18 | − 6 | − 17 | Left Amygdala | ||
| 0.488 | 70 | 3.85 | − 12 | 39 | − 5 | Left pACC |
| 3.65 | − 12 | 45 | − 5 | Left Medial Frontal Gyrus (vmPFC) | ||
| 3.20 | 12 | 48 | − 5 | Right Medial Frontal Gyrus (vmPFC) | ||
| 3.05 | 3 | 51 | − 5 | Right Medial Frontal Gyrus (vmPFC) | ||
| 2.82 | 3 | 42 | − 2 | Right pACC | ||
A voxel level threshold of puncorr < 0.005 was used, clusters surviving an additional cluster level threshold of pFWE-corr < 0.05 are shown in bold, and a trend at cluster level is shown in italic. pACC, perigenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; FWE, family-wise error; kE, cluster size extent; MNI, Montreal Neurological Institute.
Fig. 6.
Brain responses during induction of vestibular pain (contrast certainpain - safeno pain): comparisons between genito-pelvic pain/penetration disorder (GPPPD) and healthy control women (HC). A voxel level threshold of puncorrected < 0.005 combined with an extent threshold of 50 voxels was used for visualization purposes.
3.3. Secondary aim: relationship between fear, anxiety and pain responses
3.3.1. (Pain-related) fear & anxiety traits (questionnaires)
3.3.1.1. Association with subjective pain ratings (online VAS)
As hypothesized, in the entire sample (HC + GPPPD), scores on the FPQ (βrobust = 0.035 ± 0.017, p = 0.043) and PASS (βrobust = 0.030 ± 0.013, p = 0.026), but not STAI-trait anxiety (βrobust = − 0.036 ± 0.044, p = 0.42) were significantly and positively associated with online pain ratings (Δcertainpain - safeno_pain). Again in line with our hypothesis, the association with the PASS score is driven by GPPPD (βrobust = 0.044 ± 0.018, p = 0.013) and not HC (βrobust = 0.012 ± 0.018, p = 0.51). This was also the case for the FPQ score, although the association in GPPPD only did not reach significance here (GPPPD: βrobust = 0.035 ± 0.024, p = 0.15; HC: βrobust = 0.010 ± 0.043, p = 0.81).
3.3.1.2. Association with brain responses to pain
In HC, ROI-based regression analysis showed a significant negative association of FPQ score with brain responses to pain (contrast certainpain - safeno_pain) in left pACC (local max − 9, 45, − 5, T = 3.21, pFWE-corr = 0.045). No significant positive associations were found in HC. In GPPPD, no significant positive nor negative associations were found (Supplementary Fig. S3A). There were no significant between-group differences.
No significant within-group associations were found with STAI and PASS scores, nor were any between-group differences found.
3.3.2. Anticipatory fear ratings (online VAS)
3.3.2.1. Association with pain ratings (online VAS)
In the entire sample (HC + GPPPD), anticipatory fear ratings (Δcertain - safe) were significantly and positively associated (βrobust = 0.74 ± 0.14, p < 0.0001) with pain ratings (Δcertainpain - safeno_pain). This was driven by a significant association in GPPPD (βrobust = 0.070 ± 0.017, p < 0.0001) which was absent in HC (βrobust = 0.57 ± 0.47, p = 0.23). These findings confirm our hypothesis.
3.3.2.2. Association with brain responses to pain
Compared to women with GPPPD, HC showed a significantly stronger negative association between anticipatory fear ratings (Δcertain - safe) and brain responses to pain (contrast certainpain – safeno_pain) in left pACC (ROI analysis, interaction effect: local max − 6, 45, − 2, T = 3.87, pFWE-corr = 0.0.011). Within-group analysis showed that the interaction effect was driven by a significant negative association in HC (ROI analysis: local max − 6, 45, − 2, T = 4.65, pFWE-corr = 0.0018) (Supplementary Fig. S3B).
4. Discussion
The primary aim of this study was to examine subjective and brain responses to experimentally induced vestibular pain and its anticipation in women with GPPPD/PVD and HC. Contrary to our hypothesis, we found similar vestibular pain thresholds between both groups., Hence, intensity of the individually titrated pain stimuli used during scanning was on average similar between both groups, yet perceived pain intensity was higher in GPPPD (contrary to what we expected). However, as hypothesized anticipatory fear ratings toward the pain stimuli were higher in women with GPPPD compared to HC, although it should be noted that significance was only found in planned contrast analysis testing our hypotheses directly, with only trends being found for the main effect of group and group-by-condition interaction effects. In parallel, brain responses during anticipation and induction of vestibular pain were more extensive and stronger in women with GPPPD compared to HC, although it should be acknowledged that between-group differences did not reach statistical significance for the anticipation contrast (except for one cluster in the pACC if the additional cluster-level threshold was omitted). As hypothesized, these differences were found primarily in networks of the “pain neuromatrix” that are primarily involved in cognitive and affective aspects of pain perception, including descending pain modulation, such as the salience, emotional-arousal, central executive and central autonomic networks (Bushnell et al., 2013, Mayer et al., 2006, Mayer et al., 2015). Further, exploratory correlation analyses demonstrated that pain-related fear and anxiety traits as well as anticipatory fear ratings (online VAS) were positively associated with online pain ratings in GPPPD but not in HC. Also, in HC, a negative association between pain-related fear and anxiety traits and online anticipatory fear ratings on the one hand and brain responses to pain on the other was found in the pACC (Atlas and Wager, 2012, Wager and Atlas, 2013, Vogt, 2005).
Brain responses during anticipation of vestibular pain were found in women with GPPPD in regions involved in salience detection (aMCC) (Vogt, 2005, Bushnell et al., 2013, Spielberger et al., 1983) as well as cognitive and affective aspects of and responses to pain perception (pACC, parahippocampal gyrus, vlPFC, posterior parietal cortex) (Bushnell et al., 2013, Mayer et al., 2006, Bishop et al., 2004, Bishop, 2009, Coghill et al., 1999, Kano et al., 2013). In addition, we found increased activity in sensorimotor network regions such as basal ganglia, thalamus, posterior insula, SI and SII, and premotor cortex, which may be interpreted as an increased preparatory response of the body to react to a threat such as pain (Cunnington et al., 2005). In controls, only activation in SI was found during anticipation. As between-group differences for the anticipation contrast did not reach significance (except for a pACC cluster which did not survive the additional corrected cluster-level significance threshold), potentially due to a lack of power to detect between-group differences, we need to be cautious in interpreting these results. We nevertheless believe they are in line with our hypothesis, although replicating in a larger sample would be needed to confirm significance of the between-group results. Generally, these findings are in line with studies (in HC) showing that key regions in various networks of the “pain neuromatrix” including aMCC, vlPFC, vmPFC and dlPFC, SII, thalamus, and insula (Wager et al., 2013, Bushnell et al., 2013, Apkarian et al., 2005, Yoshida et al., 2013, Sawamoto et al., 2000, Song et al., 2006) are not only activated during actual pain induction, but also during its anticipation, suggesting that these activations have a preparatory function. Although there is some overlap in brain regions activated during anticipation of pain and during pain induction, recently, it has been demonstrated that anticipation of pain activates a specific neural network in healthy participants, distinct from the one involved in pain perception (Palermo et al., 2015). The brain can either up-regulate or down-regulate sensory and cognitive/affective and autonomic brain regions during anticipation based on expected intensity, previous experience and familiarity with the stimulus (Atlas and Wager, 2012, Denny et al., 2014, Mayer et al., 2006). More specifically, in healthy participants, brain activity in key emotional-arousal and autonomic brain regions (dorsal brainstem, (anterior) insula, amygdala and sACC) decreases during certain expectation of a painful stimulus, while this is not the case in patients with other persistent pain conditions such as IBS and FM (Berman et al., 2006, Burgmer et al., 2011). Indeed, compared to HC, IBS and FM patients showed increased anticipatory activation in regions involved in salience detection and emotional-arousal responses (“dorsal anterior cingulate cortex”, corresponding to aMCC/pACC, vlPFC, dorsal brainstem) (Berman et al., 2008, Lowen et al., 2015), cognitive control and attention (dlPFC, posterior parietal cortex) (Burgmer et al., 2011, Lee et al., 2012, Icenhour et al., 2015), and descending pain modulation (midbrain/PAG, dorsal brainstem) (Burgmer et al., 2011, Lowen et al., 2015). As such, the present findings in GPPPD corroborate findings in other persistent pain conditions, and we may speculate that the activations of brain regions involved in salience detection, emotion/arousal, executive functioning, and autonomic and pain modulatory responses during the anticipation of pain may underlie the hypervigilance toward painful stimuli and the increased levels of pain-related fear and anxiety found in previous non-brain imaging studies in women with GPPPD (Payne et al., 2005). To the best of our knowledge, no studies on brain responses to pain anticipation in GPPPD have been performed before. However, Gupta et al. found that the decreased connectivity between the posterior cingulate cortex and the other regions of the default mode resting state network between GPPPD and HC could be accounted for by increased levels of (general) anxiety in GPPPD patients. Due to the differences in design (task-based versus resting state) and measurement of anxiety (pain-specific fear and anxiety traits versus levels of general anxiety over the past two weeks), comparing the results of both studies is difficult.
As hypothesized, and in line with the brain imaging literature in other persistent pain conditions (Bushnell et al., 2013, Apkarian et al., 2005), brain responses during pain induction were significantly stronger in emotional-arousal regions (parahippocampal gyrus, amygdala, hippocampus), and, to a lesser extent, pACC/vmPFC (as the latter cluster did not survive the additional corrected cluster-level threshold) (Bushnell et al., 2013, Atlas and Wager, 2012, Mayer et al., 2006, Vogt, 2005, Kano et al., 2013) in women with GPPPD. Results diverge from the only two previous fMRI studies on vestibular pain induction in women with GPPPD. Pukall et al. found significantly stronger responses in insular cortex, precentral gyrus and left supramarginal gyrus (Pukall et al., 2005). The fact that, in the latter study, no cued anticipation condition was included and that controls perceived the vestibular touch as non-painful due to the use of stimuli of fixed intensity rather than individually titrated stimuli may explain the divergent findings. In a more recent study using stimuli at individually titrated pain threshold, no differences in brain activation were found during vestibular pain induction when comparing women with GPPPD and controls (Hampson et al., 2013). These findings may be due to the heterogeneous group of GPPPD participants, consisting of provoked and unprovoked as well as primary and secondary vestibulodynia. In our study, increased brain responses in women with GPPPD were mainly found in regions involved in cognitive and affective-motivational aspects of the pain experience (Mayer et al., 2006, Vogt, 2005, Ploghaus et al., 2003, Ploghaus et al., 1999). These findings may indicate that in GPPPD, pain induction activates more negative emotions, cognitions and memories and may reflect an inability to reduce emotional arousal during repeated painful stimulation. Alternatively, the higher perceived pain intensity in GPPPD may have accounted for these results. However, this interpretation is unlikely as adding the average online VAS ratings of pain intensity during the certainpain and safeno_pain conditions (Δcertainpain - safeno_pain) as a covariate to the comparison of brain responses between GPPPD and HC had virtually no impact on the results (details not shown).
At the subjective level, pain-related fear and anxiety traits as well as online anticipatory fear ratings were positively associated with online pain ratings in women with GPPPD, but not in HC. Further, scores on the FPQ (reflecting the trait of pain-related anxiety), but not on the PASS or the STAI (the latter reflecting non-specific trait anxiety) were higher in women with GPPPD, as expected. At the brain level, in HC, an inverse association between online momentary anticipatory fear ratings and brain responses to pain was found in the pACC (Atlas and Wager, 2012, Wager and Atlas, 2013, Vogt, 2005). This negative association was not found in GPPPD, yielding a significant between-group difference. A similar pattern was observed for FPQ scores (i.e. negative association in HC, but not in GPPPD), but the between-group difference did not reach significance here. Our findings reveal that chronic, trait-like pain-related anxiety may upregulate pain perception in GPPPD, while trait anxiety (that is not specific to pain) does not, indicating the central role of pain-related anxiety in vestibular pain perception. In HC, acute anticipatory fear for the upcoming vestibular pain stimulus down-regulates the subsequent pACC response to this pain stimulus, which may be adaptive and in line with acute anti-nociceptive effects of fear (Rhudy and Meagher, 2000). This mechanism was not found in GPPPD, which may be reflected in the positive association between anticipatory fear and pain ratings in GPPPD. Findings support the modulatory role of pain-related fear and anxiety on pain perception, and the differential brain response pattern previously found in patients with persistent pain conditions (Bushnell et al., 2013, Apkarian et al., 2005). Our results therefore provide empirical evidence validating the addition of pain-related fear and anxiety in the diagnostic criteria of GPPPD in DSM-5 (American Psychiatric Association, 2013).
Contrary to previous findings in GPPPD, pressure thresholds for moderate pain were similar in both groups (Pukall et al., 2002, Pukall et al., 2004, Granot et al., 2002). The need for individually titrated pain thresholds and repeated painful stimulation without causing injury in the present study may have led to the exclusion of both GPPPD women with lower pain thresholds, and HC with higher pain thresholds, which may account for these unexpected results. Despite the resulting similar (average) intensity of the pain stimuli applied during scanning (due to the individual titration and lack of difference in pain thresholds), in both groups, pain intensity ratings (and online ratings of anticipatory fear, as expected) were significantly higher in women with GPPPD compared to HC, which is in line with studies showing that the pain experience is amplified in patients with chronic pain conditions (Bushnell et al., 2013, Apkarian et al., 2005). Finally, stress/fear/anxiety induced by the scanner environment may have amplified pain perception (as well as the underlying brain responses) more strongly in GPPPD patients than controls, which may provide an additional explanation for the higher pain ratings during scanning in GPPPD, despite similar intensity stimuli being used in both groups. Such an interpretations would provide further arguments for an important role of fear and anxiety in upregulating pain perception and, hence, symptom generation, in GPPPD. Partly in line with this interpretation, we recently demonstrated that the MRI scanner environment amplifies visceral (but not somatic) pain perception in IBS, albeit a similar phenomenon was found in healthy volunteers (in whom the difference in pain ratings correlated with levels of psychological distress over the past weeks) (Wong et al., 2016).
Several limitations of this study need to be mentioned. Although common in neuroimaging studies using pain induction in patients with persistent pain conditions, the sample size is relatively small, resulting in low statistical power (Button et al., 2013), which may explain the lack of between-group differences surviving the additional corrected cluster-level threshold in the anticipation condition. Also, the clinical sample was limited to women with PVD without any involuntary pelvic floor muscle contraction (‘vaginistic reflex’) during gynecological examination. Although PVD mostly co-occurs with a certain degree of pelvic floor muscle tension (Reissing et al., 2004), participants with involuntary pelvic floor muscle tension were excluded in order to limit movement during the scanning session. Finding may thus not generalize to all women with GPPPD and to pain-free women.
The results of this study have implications for our understanding of the pathophysiology of GPPPD, which may in turn have implications for improving treatment. Since pain-related fear and anxiety are important targets of current treatment modalities such as cognitive-behavioural therapy (ter Kuile and Weijenborg, 2006, Bergeron et al., 2008) and body-mind therapies (Brotto et al., 2010), increased knowledge of brain mechanisms in these patients can be a crucial first step toward a better understanding of the mechanisms of action and efficacy of these treatments, and may in turn contribute to the development, and/or improvement of future treatments.
In conclusion, this study is the first to demonstrate that, despite similar intensity of pain stimuli, not only subjective, but also brain responses during induction and, to a lesser extent, anticipation of vestibular pain are increased in GPPPD, mostly in regions involved in cognitive and affective aspects of the pain experience. At the subjective level, pain-related fear and anxiety traits as well as momentary anticipatory fear ratings were positively associated to pain perception in GPPPD, while at the brain level, questionnaire-based trait measures of pain-related fear and anxiety, and acute, momentary measures of anticipatory fear for the vestibular pain stimulus were differentially associated with pACC responses during pain induction. Although confirmation in a larger sample is needed, we believe our findings support the addition of pain-related fear and anxiety in the diagnostic criteria of GPPPD in DSM-5, emphasize the importance of (anticipatory) pain-related fear and anxiety in the modulation of pain perception in GPPPD, as well as a start to unravel its biological basis.
Conflict of interest and source of funding
Dr. Pazmany, Dr. Ly, and Prof. Van Oudenhove are supported by the KU Leuven Special Research Fund.
Preliminary results of this study were presented at the annual meeting of the International Academy of Sex Research, Dubrovnik, Croatia, June 2014, and the Congress Emotions 2015, Tilburg, The Netherlands, October 2015.
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgement
We would like to thank Prof. Dr. Annemie Devreese, affiliated to the Department of Physical Revalidation (KU Leuven), for giving us advice about the best possible position of the participants during scan sessions, as well as Mr. Jos Van Bael, affiliated to Medical Instrumentation (University Hospitals Leuven) for the construction of the knee-support and the modified vulvalgesiometers.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.07.017.
Appendix A. Supplementary data
Supplementary material
References
- American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. Aug. (PubMed PMID: 15979027. Epub 2005/06/28. eng) [DOI] [PubMed] [Google Scholar]
- Atlas L.Y., Wager T.D. How expectations shape pain. Neurosci. Lett. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039. Jun 29. (PubMed PMID: 22465136. Epub 2012/04/03. eng) [DOI] [PubMed] [Google Scholar]
- Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J. Sex. Med. 2012;9(8):2077–2092. doi: 10.1111/j.1743-6109.2012.02803.x. Aug. (PubMed PMID: 22672388. Epub 2012/06/08. eng) [DOI] [PubMed] [Google Scholar]
- Bergeron S., Khalife S., Glazer H.I., Binik Y.M. Surgical and behavioral treatments for vestibulodynia: two-and-one-half year follow-up and predictors of outcome. Obstet. Gynecol. 2008;111(1):159–166. doi: 10.1097/01.AOG.0000295864.76032.a7. Jan. (PubMed PMID: 18165405. Epub 2008/01/01. eng) [DOI] [PubMed] [Google Scholar]
- Bergeron S., Likes W.M., Steben M. Psychosexual aspects of vulvovaginal pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28(7):991–999. doi: 10.1016/j.bpobgyn.2014.07.007. Oct. (PubMed PMID: 25104563. Epub 2014/08/12. eng) [DOI] [PubMed] [Google Scholar]
- Berman S.M., Naliboff B.D., Suyenobu B., Labus J.S., Stains J., Bueller J.A. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am. J. Phys. Regul. Integr. Comp. Phys. 2006;291(2):R268–76. doi: 10.1152/ajpregu.00065.2006. Aug. (PubMed PMID: 16614061. Epub 2006/04/15. eng) [DOI] [PubMed] [Google Scholar]
- Berman S.M., Naliboff B.D., Suyenobu B., Labus J.S., Stains J., Ohning G. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. Jan 9. (PubMed PMID: 18184777. Epub 2008/01/11. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binik Y.M. Should dyspareunia be retained as a sexual dysfunction in DSM-V? A painful classification decision. Arch. Sex. Behav. 2005;34(1):11–21. doi: 10.1007/s10508-005-0998-4. Feb. (PubMed PMID: 15772767. Epub 2005/03/18. eng) [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009;12(1):92–98. doi: 10.1038/nn.2242. Jan. (PubMed PMID: 19079249. Epub 2008/12/17. eng) [DOI] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A.D. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat. Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. Feb. (PubMed PMID: 14703573. Epub 2004/01/03. eng) [DOI] [PubMed] [Google Scholar]
- Brauer M., ter Kuile M.M., Janssen S.A., Laan E. The effect of pain-related fear on sexual arousal in women with superficial dyspareunia. Eur. J. Pain. 2007;11(7):788–798. doi: 10.1016/j.ejpain.2006.12.006. Oct. (PubMed PMID: 17303453. Epub 2007/02/17. eng) [DOI] [PubMed] [Google Scholar]
- Brotto L.A., Sadownik L., Thomson S. Impact of educational seminars on women with provoked vestibulodynia. J. Obstet. Gynaecol. Can. 2010;32(2):132–138. doi: 10.1016/S1701-2163(16)34427-9. Feb. (PubMed PMID: 20181314. Epub 2010/02/26. eng) [DOI] [PubMed] [Google Scholar]
- Burgmer M., Petzke F., Giesecke T., Gaubitz M., Heuft G., Pfleiderer B. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom. Med. 2011;73(9):751–759. doi: 10.1097/PSY.0b013e318236588a. Nov–Dec. (PubMed PMID: 22048836. Epub 2011/11/04. eng) [DOI] [PubMed] [Google Scholar]
- Bushnell M.C., Ceko M., Low L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. Jul. (PubMed PMID: 23719569. Epub 2013/05/31. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. May. (PubMed PMID: 23571845. Epub 2013/04/11. eng) [DOI] [PubMed] [Google Scholar]
- Coghill R.C., Sang C.N., Maisog J.M., Iadarola M.J. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J. Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. Oct. (PubMed PMID: 10515983. Epub 1999/10/09. eng) [DOI] [PubMed] [Google Scholar]
- Cunnington R., Windischberger C., Moser E. Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum. Mov. Sci. 2005;24(5–6):644–656. doi: 10.1016/j.humov.2005.10.001. Oct–Dec. (PubMed PMID: 16337295. Epub 2005/12/13. eng) [DOI] [PubMed] [Google Scholar]
- Denny B.T., Ochsner K.N., Weber J., Wager T.D. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Soc. Cogn. Affect. Neurosci. 2014;9(4):403–411. doi: 10.1093/scan/nss148. Apr. (PubMed PMID: 23202664. Pubmed Central PMCID: PMC3989121. Epub 2012/12/04. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst M., Wiech K., Dunckley P., Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1–2):101–110. doi: 10.1016/j.pain.2006.09.001. Mar. (PubMed PMID: 17070996. Epub 2006/10/31. eng) [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.B., Frith C.D., Frackowiak R.S. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Graaf H.D., Kruijer H., Acker J.V., Meijer S. Eburon; Delft: 2012. Seks onder je 25ste 2: Seksuele gezondheid van jongeren in Nederland anno 2012. [Google Scholar]
- Granot M., Friedman M., Yarnitsky D., Zimmer E.Z. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. BJOG. 2002;109(8):863–866. doi: 10.1111/j.1471-0528.2002.01416.x. Aug. (PubMed PMID: 12197364. Epub 2002/08/29. eng) [DOI] [PubMed] [Google Scholar]
- Gupta A., Rapkin A.J., Gill Z., Kilpatrick L., Fling C., Stains J. Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain. 2015;156(5):809–819. doi: 10.1097/01.j.pain.0000461289.65571.54. May. (PubMed PMID: 25735001. Pubmed Central PMCID: PMC4402252. Epub 2015/03/04. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson J.P., Reed B.D., Clauw D.J., Bhavsar R., Gracely R.H., Haefner H.K. Augmented central pain processing in vulvodynia. J. Pain. 2013;14(6):579–589. doi: 10.1016/j.jpain.2013.01.767. Jun. (PubMed PMID: 23578957. Pubmed Central PMCID: PMC3672331. Epub 2013/04/13. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenhour A., Langhorst J., Benson S., Schlamann M., Hampel S., Engler H. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol. Motil. 2015;27(1):114–127. doi: 10.1111/nmo.12489. Jan. (PubMed PMID: 25557224. Epub 2015/01/06. eng) [DOI] [PubMed] [Google Scholar]
- Kano M., Farmer A.D., Aziz Q., Giampietro V.P., Brammer M.J., Williams S.C. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304(8):G687–99. doi: 10.1152/ajpgi.00385.2012. Apr 15. (PubMed PMID: 23392235. Pubmed Central PMCID: PMC3625873. Epub 2013/02/09. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Kuile M.M., Weijenborg P.T. A cognitive-behavioral group program for women with vulvar vestibulitis syndrome (VVS): factors associated with treatment success. J Sex Marital Ther. 2006;32(3):199–213. doi: 10.1080/00926230600575306. May–Jun. (PubMed PMID: 16809249. Epub 2006/07/01. eng) [DOI] [PubMed] [Google Scholar]
- van Lankveld J.J., Granot M., Weijmar Schultz W.C., Binik Y.M., Wesselmann U., Pukall C.F. Women's sexual pain disorders. J. Sex. Med. 2010;7(1 Pt 2):615–631. doi: 10.1111/j.1743-6109.2009.01631.x. Jan. (PubMed PMID: 20092455. Epub 2010/01/23. eng) [DOI] [PubMed] [Google Scholar]
- Larsson M.B., Tillisch K., Craig A.D., Engstrom M., Labus J., Naliboff B. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142(3):463–472. doi: 10.1053/j.gastro.2011.11.022. Mar. e3. (PubMed PMID: 22108191. Pubmed Central PMCID: PMC3288538. Epub 2011/11/24. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D.B., Weiller E., Amorim P., Bonora I., Harnett-Sheehan K. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured Interview: reliability and validity according to the CIDI. Eur. Psychiatry. 1997;12:224–231. [Google Scholar]
- Lee H.F., Hsieh J.C., Lu C.L., Yeh T.C., Tu C.H., Cheng C.M. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153(6):1301–1310. doi: 10.1016/j.pain.2012.03.018. Jun. (PubMed PMID: 22541443. Epub 2012/05/01. eng) [DOI] [PubMed] [Google Scholar]
- Lowen M.B., Mayer E., Tillisch K., Labus J., Naliboff B., Lundberg P. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterol. Motil. 2015;27(5):646–655. doi: 10.1111/nmo.12537. May. (PubMed PMID: 25777251. Epub 2015/03/18. eng) [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Bushnell M.C. IASP Press; 2009. Pain IAftSo. Functional Pain Syndromes: Presentation and Pathophysiology. [Google Scholar]
- Mayer E.A., Naliboff B.D., Craig A.D. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–1942. doi: 10.1053/j.gastro.2006.10.026. Dec. PubMed PMID: 17188960. Epub 2006/12/26. eng. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Labus J.S., Tillisch K., Cole S.W., Baldi P. Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015;12(10):592–605. doi: 10.1038/nrgastro.2015.121. 08/25. (PubMed PMID: PMC5001844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken L.M., Zayfert C., Gross R.T. The Pain Anxiety Symptoms Scale: development and validation a scale to measure fear of pain. Pain. 1992;50:67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- McNeil D.W., Rainwater A.J., 3rd. Development of the Fear of Pain Questionnaire—III. J. Behav. Med. 1998;21(4):389–410. doi: 10.1023/a:1018782831217. Aug. (PubMed PMID: 9789168. Epub 1998/10/28. eng) [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ludlow D.H., Knierim K., Hanelin J., Ramachandran T., Glover G.C. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1–2):69–77. doi: 10.1016/j.pain.2005.10.014. Jan. (PubMed PMID: 16364548. Pubmed Central PMCID: PMC2914607. Epub 2005/12/21. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo S., Benedetti F., Costa T., Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum. Brain Mapp. 2015;36(5):1648–1661. doi: 10.1002/hbm.22727. May. (PubMed PMID: 25529840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne K.A., Binik Y.M., Amsel R., Khalife S. When sex hurts, anxiety and fear orient attention towards pain. Eur. J. Pain. 2005;9(4):427–436. doi: 10.1016/j.ejpain.2004.10.003. Aug. (PubMed PMID: 15979023. Epub 2005/06/28. eng) [DOI] [PubMed] [Google Scholar]
- Payne K.A., Binik Y.M., Pukall C.F., Thaler L., Amsel R., Khalife S. Effects of sexual arousal on genital and non-genital sensation: a comparison of women with vulvar vestibulitis syndrome and healthy controls. Arch. Sex. Behav. 2007;36(2):289–300. doi: 10.1007/s10508-006-9089-4. Apr. (PubMed PMID: 17136588. Epub 2006/12/01. eng) [DOI] [PubMed] [Google Scholar]
- van der Ploeg H.M. Swets & Zeitlinger; Lisse, The Netherlands: 2000. Handleiding bij de Zelf Beoordelings Vragenlijst (ZBV) [Test Manual Dutch State Trait Anxiety Inventory] [Google Scholar]
- Ploghaus A., Tracey I., Gati J.S., Clare S., Menon R.S., Matthews P.M. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. doi: 10.1126/science.284.5422.1979. Jun 18. (PubMed PMID: 10373114. Epub 1999/06/18. eng) [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Becerra L., Borras C., Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn. Sci. 2003;7(5):197–200. doi: 10.1016/s1364-6613(03)00061-5. May. (PubMed PMID: 12757820. Epub 2003/05/22. Eng) [DOI] [PubMed] [Google Scholar]
- Porro C.A., Lui F., Facchin P., Maieron M., Baraldi P. Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies. Magn. Reson. Imaging. 2004;22(10):1539–1548. doi: 10.1016/j.mri.2004.10.003. Dec. (PubMed PMID: 15707803. Epub 2005/02/15. eng) [DOI] [PubMed] [Google Scholar]
- Pukall C.F., Binik Y.M., Khalife S., Amsel R., Abbott F.V. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96(1–2):163–175. doi: 10.1016/s0304-3959(01)00442-0. Mar. (PubMed PMID: 11932072. Epub 2002/04/05. eng) [DOI] [PubMed] [Google Scholar]
- Pukall C.F., Binik Y.M., Khalife S. A new instrument for pain assessment in vulvar vestibulitis syndrome. J Sex Marital Ther. 2004;30(2):69–78. doi: 10.1080/00926230490275065. Mar–Apr. (PubMed PMID: 15043051. Epub 2004/03/27. eng) [DOI] [PubMed] [Google Scholar]
- Pukall C.F., Strigo I.A., Binik Y.M., Amsel R., Khalife S., Bushnell M.C. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115(1–2):118–127. doi: 10.1016/j.pain.2005.02.020. May. (PubMed PMID: 15836975. Epub 2005/04/20. eng) [DOI] [PubMed] [Google Scholar]
- Reissing E.D., Binik Y.M., Khalife S., Cohen D., Amsel R. Vaginal spasm, pain, and behavior: an empirical investigation of the diagnosis of vaginismus. Arch. Sex. Behav. 2004;33(1):5–17. doi: 10.1023/B:ASEB.0000007458.32852.c8. Feb. (PubMed PMID: 14739686. Epub 2004/01/24. eng) [DOI] [PubMed] [Google Scholar]
- Rhudy J.L., Meagher M.W. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84(1):65–75. doi: 10.1016/S0304-3959(99)00183-9. Jan. (PubMed PMID: 10601674. Epub 1999/12/22. eng) [DOI] [PubMed] [Google Scholar]
- Sawamoto N., Honda M., Okada T., Hanakawa T., Kanda M., Fukuyama H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J. Neurosci. Off. J. Soc. Neurosci. 2000;20(19):7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. Oct 1. (PubMed PMID: 11007903. Epub 2000/09/29. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P., Kuchinad A., Pukall C.F., Bushnell M.C. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–419. doi: 10.1016/j.pain.2008.09.014. Dec. (PubMed PMID: 18930351. Epub 2008/10/22. eng) [DOI] [PubMed] [Google Scholar]
- Song G.H., Venkatraman V., Ho K.Y., Chee M.W., Yeoh K.G., Wilder-Smith C.H. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126(1–3):79–90. doi: 10.1016/j.pain.2006.06.017. Dec 15. (PubMed PMID: 16846694. Epub 2006/07/19. eng) [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R. Consulting Psychologists Press; Palo Alto: 1983. Manual for the State-Trait Anxiety Inventory STAI (Form Y) [Google Scholar]
- Tillisch K., Mayer E.A., Labus J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140(1):91–100. doi: 10.1053/j.gastro.2010.07.053. Jan. (PubMed PMID: 20696168. Pubmed Central PMCID: PMC3253553. Epub 2010/08/11. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeen B., Stigum H. Sexual problems in 18–67-year-old Norwegians. Scand. J. Publ. Health. 2010 Jul;38(5):445–456. doi: 10.1177/1403494810371245. (PubMed PMID: 20494944. Epub 2010/05/25. eng) [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. Jul. (PubMed PMID: 15995724. Pubmed Central PMCID: PMC2659949. Epub 2005/07/05. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y. How is pain influenced by cognition? Neuroimaging weighs in. Perspect. Psychol. Sci. 2013;8(1):91–97. doi: 10.1177/1745691612469631. Jan 1. (PubMed PMID: 24761154. Pubmed Central PMCID: PMC3994173. Epub 2013/01/01. Eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Rilling J.K., Smith E.E., Sokolik A., Casey K.L., Davidson R.J. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. Feb 20. (PubMed PMID: 14976306. Epub 2004/02/21. eng) [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. Apr 11. (PubMed PMID: 23574118. Pubmed Central PMCID: PMC3691100. Epub 2013/04/12. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K., Ploner M., Tracey I. Neurocognitive aspects of pain perception. Trends Cogn. Sci. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. Aug. (PubMed PMID: 18606561. Epub 2008/07/09. eng) [DOI] [PubMed] [Google Scholar]
- Wong R.K., Van Oudenhove L., Li X., Cao Y., Ho K.Y., Wilder-Smith C.H. Visceral pain perception in patients with irritable bowel syndrome and healthy volunteers is affected by the MRI scanner environment. United European Gastroenterol J. 2016;4(1):132–141. doi: 10.1177/2050640615580888. Feb. (PubMed PMID: 26966533. Pubmed Central PMCID: PMC4766539. Epub 2016/03/12. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W., Seymour B., Koltzenburg M., Dolan R.J. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal gray. J. Neurosci. Off. J. Soc. Neurosci. 2013;33(13):5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. Mar 27. (PubMed PMID: 23536078. Pubmed Central PMCID: PMC3701089. Epub 2013/03/29. eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material