Abstract
Objective
We examined the relationship between alcohol use trajectories and HIV disease severity among men and women participating in the Veterans Aging Cohort Study (VACS).
Design
Prospective cohort of HIV-infected persons in care at eight US Veterans Health Administration sites.
Methods
Between 2002 and 2010, we assessed alcohol consumption annually using the alcohol use disorders identification test-consumption (AUDIT-C). HIV disease severity was ascertained using the VACS index, a validated measure of morbidity and all-cause mortality. We examined the relationship between alcohol use and HIV disease severity patterns using joint trajectory modeling. Alcohol use trajectories were validated using phosphatidylethanol – a biomarker of alcohol consumption – measured between 2005 and 2006 among a subset of participants. We examined associations between membership in alcohol use and VACS index trajectories using multinomial regression.
Results
Among eligible participants, we identified four alcohol consumption trajectories: abstainers (24% of the sample), lower risk (44%), moderate risk (24%), and higher risk drinkers (8%). Alcohol use trajectories were highly correlated with phosphatidylethanol (Cramér’s V = 0.465, P < 0.001): mean concentrations were 4.4, 17.8, 57.7, and 167.6 ng/ml in the abstainer, lower risk, moderate risk, and higher risk groups, respectively. Four VACS index trajectories were identified: low (2%), moderate (46%), high (36%), and extreme (16%). Higher risk drinkers were most common in the extreme VACS index group, and were absent in the low index group. In multivariable analysis, the association between alcohol use and VACS index trajectory membership remained significant (P = 0.002).
Conclusion
Alcohol use trajectories characterized by persistent unhealthy drinking are associated with more advanced HIV disease severity among HIV-infected veterans in the United States.
Keywords: alcohol, HIV infection, morbidity, trajectories, trends, veterans
Introduction
Unhealthy alcohol use – the spectrum of alcohol consumption characterized by at-risk and heavy episodic drinking, along with alcohol use disorder [1] – is prevalent among people living with HIV [2]. The effects of unhealthy alcohol use on HIV disease progression have been studied extensively [3]. Studies have demonstrated a significant detrimental impact of unhealthy alcohol use on viral load suppression, primarily mediated through antiretroviral therapy adherence [4–7], as well as retention in care [8]. In addition, unhealthy alcohol use has direct effects on the immune system [9], and has been associated with CD4+ cell count decline in individuals on antiretroviral therapy [10,11]. Compared with uninfected individuals, those living with HIV experience increased mortality at lower levels of alcohol consumption [12]. This body of research provides strong evidence that unhealthy alcohol use negatively influences HIV disease progression through a combination of behavioral (i.e. adherence) and biological processes.
Nonetheless, elucidating the relationships between long-term patterns of alcohol use and HIV disease severity has been limited by several factors. For example, many studies only measure alcohol use at a single time point. Furthermore, in longitudinal studies of alcohol use among HIV-infected populations, traditional analytic methods are often unable to differentiate reductions in alcohol consumption because of declining health (i.e. ‘sick quitters’) from the potentially deleterious effects of unhealthy alcohol use on HIV disease progression [3]. Alcohol use trajectories have begun to be explored, but their associations with HIV disease progression have yet to be fully elucidated [13]. The classification of alcohol use phenotypes may lead to more precise efforts to identify patients with distinct patterns of alcohol consumption that place that them at increased risk of long-term HIV disease morbidity and mortality.
In this cohort study of HIV-infected patients in care, we sought to characterize alcohol use trajectories and their relationship with HIV disease severity over time. To achieve these objectives, we employed a joint trajectory modeling approach in which two longitudinal outcomes are analyzed contemporaneously [14]. We hypothesized that, independent of other relevant behavioral factors, persistent unhealthy patterns would be associated with more advanced HIV disease severity over time.
Methods
Study design and eligibility criteria
The study utilized data collected from the Veterans Aging Cohort Study (VACS) survey sample, which enrolled HIV-infected participants receiving medical care at Veterans Health Administration (VHA) facilities in Atlanta, Baltimore, Houston, Los Angeles, Manhattan and The Bronx, Pittsburgh, and Washington, DC. Design of the VACS has been described in detail elsewhere [15,16]. In brief, the open cohort began enrolment in 2002 with follow-up assessments scheduled approximately annually. For this analysis, we used data from six waves of data collection, representing an 8-year (2002–2010) study period.
At each assessment participants completed a detailed questionnaire that elicited information regarding socio-demographics, general health status, health conditions, and behavioral factors including alcohol and other substance use. Participant survey data were then linked with VHA electronic medical records, which include information on all clinical encounters, laboratory data, and diagnoses recorded using the International Classification of Diseases, 9th Revision codes [17]. The VACS was approved by the institutional review boards at each participating VHA Medical Center and affiliated academic institutions.
Measures
We assessed two outcome measures. First, we examined each participant’s set of scores on the alcohol use disorders identification test-consumption (AUDIT-C) questionnaire [18]. Scores range from 0 to 12; values at least 3 and 4 are considered positive for unhealthy alcohol use in women and men, respectively [19]. The AUDIT-C is a reliable and valid measure to assess risk of unhealthy alcohol use among HIV-infected individuals, and has been used in previous VACS studies [13,20].
The second outcome was the VACS index, a composite measure that predicts all-cause mortality, cause-specific mortality, and other clinical outcomes in those with HIV [21]. Validation studies show the VACS index has reproducible accuracy and validity in HIV-infected populations [22–24]. As shown in Table S1, http://links.lww.com/QAD/B70 the score is created by summing points for age, indicators of HIV disease (i.e. CD4+ cell count and HIV-1 RNA), general indicators of organ system injury, as well as history of hepatitis C virus (HCV) coinfection [21]. Each five-point increment indicates an approximately 20% increase in 5-year mortality risk [21,22]. Thus, the VACS index is a clinically relevant measure of overall HIV disease severity. At each survey wave, the VACS index was calculated as previously reported [12], using laboratory values closest to the participant’s AUDIT-C assessment. The score is recalculated each time a lab is updated, with components of the index carried forward up to a year to calculate the index.
In addition to exploring associations between the two outcome measures, we also considered the following independent covariates. These included: age at baseline, sex, race (white, African American, vs. other), Hispanic/Latino ethnicity (yes vs. no) educational attainment (high school or less education, yes vs. no), marital status, and lifetime homelessness. We also examined current smoking status at baseline (defined as any smoking in the past week), and self-reported history of injection drug use. We assessed depressive symptomatology using the Patient Health Questionnaire, where a score at least 10 is considered positive for moderate or greater depressive severity [25]. Finally, we assessed HCV coinfection status (positive vs. negative, based on a combination of International Classification of Diseases, 9th Revision codes for this diagnosis and laboratory data indicating positive HCV antibody or HCV–RNA positivity), CD4+ cell count (cells/μl), and viral suppression, defined as less than 400 copies/ml. Finally, any participant who filled a prescription for an antiretroviral on the Veterans Affairs (VA) formulary and within the VA between 90 days prior and 7 days following the date of the baseline interview were considered to be on highly active antiretroviral therapy at baseline.
As conducted previously [13], we identified participants who died during the study period (between 2002 and up to September 2010) from the VA vital status file. Finally, we defined loss to follow-up (for reasons other than death) as failing to complete a study assessment within 1 year prior to the end of the study period (i.e. their last assessment occurred before September 2009).
Statistical analyses
We used a semiparametric, group-based mixture modeling approach (Proc TRAJ) to identify joint AUDIT-C and VACS index score trajectories [26]. In brief, the modeling procedure sorts each participant’s set of AUDIT-C values and VACS index scores into ‘clusters’ and estimates distinct trajectories. The time scale was follow-up time (in years) from study enrolment. The procedure calculates each individual’s probability of belonging in each trajectory and assigns the one for which they have the highest probability of membership [27]. We used a zero-inflated Poisson model for AUDIT-C scores and a censored normal model for VACS index scores (minimum = 0, maximum = 164, with higher scores indicating greater HIV disease severity).
After each outcome had been separately modelled, we used the joint modeling procedure in Proc TRAJ [14]. This model permits analysis of two outcomes that evolve contemporaneously over time. First, the model estimates the trajectories for each outcome and the conditional probabilities of membership in each combination of trajectories. Second, the model assigns each individual to the combination with the highest probability of membership. Additional details regarding the determination of the optimal number of trajectory groups and each trajectory’s shape are provided in the supplemental file.
Once trajectory groups were assigned, we used the χ2 test and the Wilcoxon rank sum test to compare the baseline characteristics of individuals in each VACS index trajectory group. Then, we used multinomial logistic regression to estimate odds of membership in each VACS index trajectory, conditional on membership in each AUDIT-C group, and other covariates. As there were no persons assigned to the higher risk AUDIT-C/low VACS index subgroup, we were unable to use either of these classes as the referent level for the multivariable model. As such, the moderate AUDIT-C and moderate VACS index classes were chosen as the referent categories. The final multivariable model was chosen using an iterative, manual backwards selection procedure. First, variables significant at P < 0.05 in bivariable analyses were included in a preliminary model. Baseline HCV status, HIV treatment status, CD4+ cell count, and viral load were not considered for inclusion in the multivariable model as they are components of the VACS index. Next, variables with the highest P value were removed sequentially, with the final model based on that with the lowest Akaike information criterion. Finally, we calculated and applied inverse probability of censoring weights to account for potential biases arising from differential loss to follow-up (see Supplemental File, http://links.lww.com/QAD/B70). All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA) and all P values are two-sided.
Validation and sensitivity analyses
To validate AUDIT-C trajectories, we conducted a subanalysis of participants enrolled in the VACS tissue repository. As described previously [28], blood and DNA specimens were collected from a subset of 1532 VACS participants between 2005 and 2006. All samples were tested for phosphatidylethanol (PEth), a direct biomarker of alcohol exposure with high specificity for alcohol abstinence that modestly correlates with dose of alcohol consumed in the past 21 days [29,30]. Additional details are provided in the supplemental file. We examined mean PEth concentrations and the proportion with PEth above the limit of detection (≥ 4 ng/ml), stratified by AUDIT-C trajectory membership.
In post hoc analyses, we compared the characteristics of persons classified in specific subgroups of interest (i.e. ‘healthy abstainers’, ‘sick quitters’). In addition we examined the proportion in each subgroup who reported sexual activity in the past year. Next, in a series of sensitivity analyses, we used last observation carried forward (LOCF) to account for potential biases arising from individuals who were lost to follow-up. The LOCF approach carries forward the last observation to the last time point for each participant who dropped out, treating these carried forward data as observed data [31]. Then, we restricted the trajectory analyses to persons who completed at least two study assessments during the study period (87% of the analytic sample). Finally, to determine whether mortality affected the shape of each VACS index and AUDIT-C score pattern, we re-estimated the trajectories excluding those participants who died during the study period.
Results
Patient characteristics
Of 3631 HIV-infected participants, 3539 (97%) had at least once AUDIT-C and one VACS index score and were thus included in this analysis. The 3539 eligible participants contributed 13 090 AUDIT-C observations and 12 958 VAC index score values for a total of 15 354 person-years over the 8-year study period. A total of 3072 (86.8%) participants completed at least two assessments. The median age was 49 [interquartile range (IQR) = 44–55], 98% were men, and 68% were African American. At baseline, median CD4+ cell count was 367 cells/μl (IQR = 213–555) and median viral load was 413 copies/ml (IQR = 75–14500). The median AUDIT-C score was 1 (IQR = 0–4), and the median VACS index score was 29 (IQR = 17–46).
Alcohol use disorders identification test-consumption score trajectories
In the joint model, we identified four distinct AUDIT-C score trajectories (Fig. 1, Table S2, http://links.lww.com/QAD/B70). We labelled the AUDIT-C trajectories as abstainers, lower risk, moderate risk, and higher risk. The abstainer group (24% of the sample) had consistently low mean AUDIT-C scores. The lower risk group (44%) had an AUDIT-C trajectory indicative of stable, lower risk drinking. The moderate risk group (24%) had an average AUDIT-C score between 3.2 and 3.9, just below the standard cutoff of 4 for men. Finally, we identified a higher risk group (8%), who had a consistently elevated AUDIT-C score, indicative of persistent unhealthy alcohol use. The baseline characteristics of persons in each of the four AUDIT-C groups are shown in Table S3, http://links.lww.com/QAD/B70.
Fig. 1. AUDIT-C score trajectories among 3539 HIV-infected participants in the VACS, 2002–2010. AUDIT-C; alcohol use disorders identification test-consumption; VACS, Veterans Aging Cohort Study.
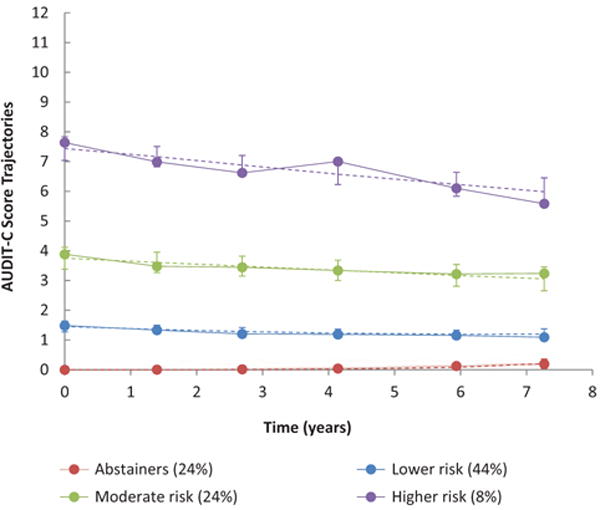
Dashed lines represent predicted values and error bars represent 95% confidence intervals for each wave’s estimate; solid lines represent empirical averages. Time points are based on the average time since baseline for each of the six waves of data collection during the 8-year study period.
Validation of alcohol use disorders identification test-consumption score trajectories
Among 1525 persons included in the VACS tissue repository, 1499 (98.3%) were included in this analysis. The distribution of trajectories and characteristics of persons included in the subsample were similar to those of the overall analytic sample (Table S4, http://links.lww.-com/QAD/B70). We observed significant correlations between measures of PEth concentration and membership in the AUDIT-C trajectories (Figure S1, http://links.lww.com/QAD/B70). For example, the mean PEth concentrations were 4.4, 17.8, 58.8, and 167.6 ng/ml among persons classified in the abstainer, lower risk, moderate risk, and higher risk groups, respectively (P < 0.001). Over 90% of participants in the higher risk group were above the PEth limit of detection, compared with only 18% in the abstainer group (P < 0.001).
Veterans Aging Cohort Study index trajectories
We identified four distinct VACS index trajectories (Table S2 http://links.lww.com/QAD/B70 and Fig. 2). We characterized these groups as low, moderate, high, and extreme. The low group (2%) had an average VACS index score of 0 at baseline, increasing to 4 at the last follow-up. Almost half of participants (46%) belonged to the moderate group, which was characterized by a slightly decreasing VACS index trajectory from an average of 19 to 16. One-third of participants (36%) were classified as belonging to the high group, with a stable VACS index score. Finally, we identified an extreme group (16%), who had consistently elevated VACS index scores.
Fig. 2. VACS index score trajectories among 3539 HIV-infected participants in the VACS, 2002–2010. AUDIT-C; alcohol use disorders identification test-consumption; VACS, Veterans Aging Cohort Study.
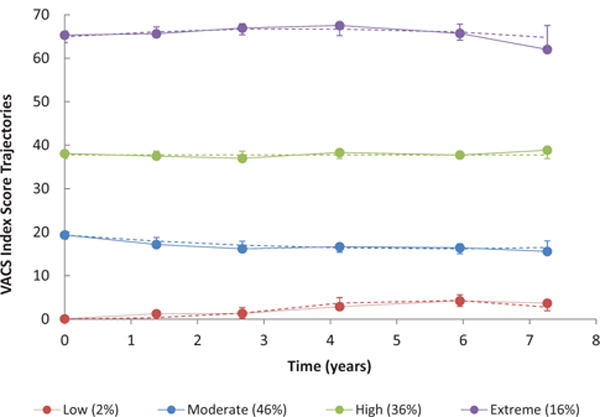
Dashed lines represent predicted values and error bars represent 95% confidence intervals for each wave’s estimate; solid lines represent empirical averages. Time points are based on the average time since baseline for each of the six waves of data collection during the 8-year study period.
Bivariable analyses
We observed a significant association between membership in AUDIT-C and VACS index trajectories (χ2 = 73, P < 0.001, see Fig. 3). Persons in the abstainer and higher risk AUDIT-C trajectories were most likely to belong to the extreme VACS index trajectory. Notably, there were no individuals classified as higher risk drinkers in the Low (i.e. healthiest) VACS index trajectory.
Fig. 3. Relationship between membership in trajectories by VACS index and AUDIT-C among 3359 HIV-infected participants in the VACS. AUDIT-C; alcohol use disorders identification test-consumption; VACS, Veterans Aging Cohort Study.
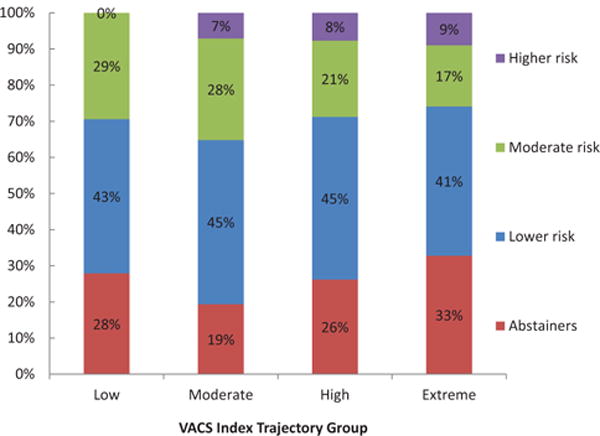
Association between membership in VACS index score trajectory and AUDIT-C score trajectory was statistically significant (χ2 = 73, P < 0.001).
As expected (as age, CD4+ cell count, viral load, and HCV status are components of the VACS index), we observed significant associations between age, baseline CD4+ cell count, viral suppression, and HCV coinfection with VACS index trajectory membership (Table 1). Providing further validation of the VACS index trajectories, the proportion of participants who died over the study period was only 6% in the healthiest group increasing to over 70% in the Extreme group (P < 0.001). We also observed significant associations between race, educational attainment, marital status, history injection drug use, lifetime homelessness, and smoking status by membership in the VACS index trajectory groups (P < 0.001).
Table 1.
Baseline characteristics, alcohol use disorders identification test-consumption trajectory group, death, and loss to follow-up among eligible participants, overall and by Veterans Aging Cohort Study index score trajectory group (n, % except as noted).
| Characteristic | VACS index trajectory group
|
P value | ||||
|---|---|---|---|---|---|---|
| Overall | Low | Moderate | High | Extreme | ||
|
| ||||||
| N = 3,539 | N = 68 (2%) | N = 1629 (46%) | N = 1290 (36%) | N = 552 (16%) | ||
| Age (median, IQR) | 49 (44–55) | 42 (38–44) | 46 (41–51) | 52 (47–56) | 53 (48–58) | < 0.001 |
| Baseline VACS index (median, IQR) | 17 (29–46) | 0 (0–0) | 12 (18–24) | 30 (38–46) | 65 (56–76) | < 0.001 |
| Female | 90 (3) | 2 (3) | 45 (3) | 31 (2) | 12 (2) | 0.858 |
| Race | < 0.001 | |||||
| White | 827 (23) | 26 (39) | 447 (28) | 271 (22) | 83 (16) | |
| African American | 2395 (68) | 37 (55) | 1042 (66) | 909 (73) | 407 (78) | |
| Other | 182 (5) | 4 (6) | 91 (6) | 58 (5) | 29 (6) | |
| Hispanic/Latino | 332 (9.4) | 9 (13) | 162 (10) | 110 (9) | 51 (9) | 0.405 |
| On HAART at baseline | 2615 (73.9) | 56 (82.4) | 1177 (72.3) | 963 (74.7) | 419 (75.9) | 0.075 |
| CD4+ cell count (median, IQR cells/μl) | 367 (213–555) | 719 (638–904) | 450 (310–620) | 309 (187–483) | 170 (6–331) | < 0.001 |
| Virally suppressed (< 400 copies/ml) | 1482 (42) | 48 (71) | 782 (48) | 484 (38) | 169 (30) | < 0.001 |
| HCV positive | 1790 (51) | 0 (0) | 599 (37) | 799 (62) | 394 (71) | < 0.001 |
| Current smoker | 1880 (53) | 27 (40) | 838 (52) | 709 (55) | 306 (56) | < 0.001 |
| Injection drug use, ever | 1170 (33) | 10 (15) | 400 (25) | 517 (41) | 243 (52) | < 0.001 |
| Depressive symptomatologya | 790 (22) | 9 (13) | 357 (22) | 289 (23) | 135 (25) | 0.147 |
| Education, high school or less | 1476 (42) | 18 (27) | 585 (36) | 590 (46) | 283 (51) | < 0.001 |
| Marital status | < 0.001 | |||||
| Married/living with partner | 806 (23) | 13 (19) | 397 (25) | 300 (24) | 96 (18) | |
| Divorced/separated/widowed | 1432 (40) | 17 (25) | 563 (35) | 567 (45) | 285 (53) | |
| Never married | 1249 (35) | 38 (56) | 649 (40) | 407 (32) | 155 (29) | |
| Homeless, ever | 1388 (39) | 15 (22) | 606 (37) | 542 (42) | 225 (41) | < 0.001 |
| Baseline AUDIT-C (median, IQR) | 1 (0–4) | 1 (0–3) | 2 (0–4) | 1 (0–3) | 1 (0–4) | < 0.001 |
| AUDIT-C trajectory group | < 0.001 | |||||
| Abstainers | 854 (24) | 19 (28) | 316 (19) | 338 (26) | 181 (33) | |
| Lower risk | 1576 (45) | 29 (43) | 739 (45) | 580 (45) | 228 (41) | |
| Moderate risk | 843 (24) | 20 (29) | 458 (28) | 272 (21) | 93 (17) | |
| Higher risk | 266 (7) | 0 (0) | 116 (7) | 100 (8) | 50 (9) | |
| Died during study period | 1105 (31) | 4 (6) | 241 (15) | 469 (36) | 391 (71) | < 0.001 |
| Loss to follow-up | 772 (22) | 20 (29) | 461 (29) | 244 (19) | 47 (9) | < 0.001 |
Not all cells add to 100% because of missing values. AUDIT-C; alcohol use disorders identification test-consumption; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; IQR, interquartile range; VACS, Veterans Aging Cohort Study.
Patient Health Questionnaire-9 score at least 10 indicates moderate depressive severity [25].
Multivariable results
As shown in Table 2, AUDIT-C trajectory group membership was independently associated with VACS index trajectory groups (P = 0.002). Specifically, compared with the moderate risk AUDIT-C group, Abstainers were more likely to be assigned to the low and extreme groups compared with the moderate VACS index group. Furthermore, membership in the lower risk AUDIT-C class was marginally associated with membership in the extreme VACS index group (adjusted odds ratio = 1.35, 95% confidence interval: 1.02–1.79), compared with the moderate group. Finally, the association between membership in the higher-risk AUDIT-C group and the extreme VACS index trajectory group was significant (adjusted odds ratio = 1.83, 95% confidence interval: 1.21–2.78).
Table 2.
Weighted multinomial logistic regression of factors associated with membership in Veterans Aging Cohort Study index score trajectories among HIV-positive participants.
| Characteristic | VACS index trajectory group, adjusted odds ratio (95% CI)
|
P value | |||
|---|---|---|---|---|---|
| Low | Moderate | High | Extreme | ||
| Age (per unit increase) | 0.94 (0.91–0.96) | REF | 1.12 (1.10–1.12) | 1.13 (1.12–1.15) | < 0.001 |
| Race (ref: White) | < 0.001 | ||||
| African American | 0.42 (0.26–0.66) | REF | 1.80 (1.52–2.14) | 2.58 (1.98–3.38) | |
| Other | 0.41 (0.16–1.05) | REF | 1.44 (1.01–2.04) | 2.41 (1.47–3.95) | |
| High school education | 1.30 (0.80–2.13) | REF | 0.77 (0.66–0.90) | 0.69 (0.56–0.84) | < 0.001 |
| Marital status (ref: Married) | 0.006 | ||||
| Divorced/separated/widowed | 1.35 (0.71–2.58) | REF | 1.03 (0.85–1.24) | 1.56 (1.18–2.07) | |
| Never married | 2.06 (1.16–3.64) | REF | 1.00 (0.82–1.21) | 1.28 (0.96–1.73) | |
| IDU, ever | 0.57 (0.31–1.08) | REF | 1.77 (1.52–2.07) | 2.03 (1.64–2.52) | < 0.001 |
| AUDIT-C group | REF | 0.002 | |||
| Abstainers | 1.92 (1.07–3.44) | REF | 1.18 (0.96–1.47) | 1.90 (1.40–2.57) | |
| Lower risk | 1.07 (0.64–1.79) | REF | 1.14 (0.95–1.36) | 1.35 (1.02–1.79) | |
| Moderate risk | REF | REF | REF | REF | |
| Higher risk | a | REF | 1.21 (0.89–1.63) | 1.83 (1.21–2.78) | |
Final model chosen using a manual backwards selection procedure based on the AIC, starting with all variables significant at P < 0.05 in Table 1. Inverse probability of censoring weights were used to account for loss to follow-up for reasons other than death. Final model also adjusted for year of recruitment. AIC, Akaike information criterion; AUDIT-C; alcohol use disorders identification test-consumption; CI, confidence interval; REF, reference; VACS, Veterans Aging Cohort Study.
Effect estimate could not be calculated because of zero cell counts.
Post hoc and sensitivity analyses
In post hoc analyses, we compared the characteristics of persons in the abstainer category who were also classified in the low and extreme VACS index groups, respectively. Persons in the abstainer/extreme subgroup were older (mean = 56 years) compared with those in the abstainer/low subgroup (mean = 41). Furthermore, persons in the abstainer/extreme subgroup were significantly less likely than those in the abstainer/low subgroup to report any sexual activity in the past year at baseline (45.3 vs. 79.0%, P < 0.001). Notably, over 74.0% of persons in the abstainer/extreme subgroup died over the study period, compared with only 10.5% of the abstainer/low VACS index subgroup. In the entire abstainer group, 809 (94.7%) reported abstinence from alcohol throughout the entire study period, a proportion that did not vary significantly between the abstainer/low and abstainer/extreme subgroups (89.5 vs. 97.8%, P = 0.114).
In several sensitivity analyses, we found no substantive difference in our findings. Applying LOCF values did not meaningfully alter the shape or composition of the AUDIT-C and VACS index trajectories (Figures S2, S3, http://links.lww.com/QAD/B70). Restricting the analysis to the 3072 participants who completed at least two assessments during the study period did not substantially alter our results (Figures S4, S5, http://links.lww.com/QAD/B70), nor did excluding those who died (Figures S6, S7, http://links.lww.com/QAD/B70).
Discussion
In this 8-year prospective cohort study of HIV-infected patients engaged in care, long-term alcohol use patterns were associated with HIV disease severity trajectories. Notably, no individuals belonging to the healthiest trajectory (indicative of normal immune system functioning and well controlled HIV disease) were classified as consistently higher risk drinkers. Moreover, persons with long-term unhealthy alcohol use were over-represented in the extreme VACS index trajectory (in which over 70% of participants died). Finally, abstainers were overrepresented in both the low and extreme VACS index trajectories, suggesting distinct groups of ‘sick quitters’ and ‘healthy abstainers’. The observed alcohol use trajectories were highly correlated with PEth – a biomarker of alcohol consumption assessed in a subsample of VACS participants – demonstrating that trajectory-based approaches are a valid method for discriminating between different patterns of long-term alcohol exposure.
These results extend previous work demonstrating that unhealthy alcohol use is associated with increased physiologic injury among HIV-infected persons [12]. One possible explanation for this finding is that alcohol consumption may adversely affect medication adherence and thus promote greater HIV disease severity. Prior studies have shown that alcohol use, including moderate consumption, has been associated with poorer adherence to therapy among HIV-infected individuals [4,5]. Alcohol exposure may also influence HIV disease severity through biological effects not mediated by adherence. For example, animal and human studies have shown that alcohol-mediated alterations in immune function can result in chronic inflammation and T-cell activation that may accelerate HIV disease progression [32]. Finally, previous studies have shown fewer drinks to get a ‘buzz’ among HIV-infected individuals [33], suggesting greater exposure to alcohol at lower levels of consumptions.
A key strength of this study was the use of PEth to validate the observed alcohol use trajectories. This finding demonstrates that trajectory-based approaches based on self-reported alcohol use offer promise as an empirical method to identify distinct behavioral phenotypes of long-term alcohol consumption. Future work should seek to identify genetic, social, and other risk factors for persistent unhealthy drinking in HIV-infected populations. Another strength was the use of the VACS index to measure HIV disease severity. The index is a more comprehensive assessment than either CD4+ or viral suppression and accounts for both HIV-specific measures and general organ system injury.
The ‘sick quitter’ phenomenon, in which individuals consume less alcohol as they become too sick to drink [34], has been hypothesized to explain the apparent protective effect of alcohol use on physical functioning [35] and mortality [36] among people living with HIV. To our knowledge, this study, the first to provide empirical support for a distinct subgroup of ‘sick quitters’ among HIV-infected individuals. Specifically, in post hoc analyses, we found that almost three in four ‘sick quitters’ died over the study period, compared with only 10% of the ‘health abstainers’. Future studies assessing the relationship between alcohol use and HIV disease outcomes should account for ‘sick quitters’ by either excluding nondrinkers or by identifying individuals who abstain from alcohol as a result of poor health.
The observed association between membership in the lower risk AUDIT-C class and the extreme VACS index class was unexpected and requires further investigation. It is possible that unmeasured confounding may explain the association. Alternatively, this finding may reflect a distinct subgroup of persons who continue to consume alcohol at relatively healthy levels despite the prevalence of other comorbidities. Finally, it is possible that this marginally significant association (P = 0.035) is because of random chance.
The study has a number of important limitations. First, although a validated measure was used to assess alcohol consumption, alcohol use patterns were self-reported. However, in a subanalysis of persons involved in the VACS biomarker cohort, AUDIT-C group membership was highly correlated with PEth concentrations. These findings increase confidence in the observed AUDIT-C patterns. Second, there may be unmeasured confounding affecting the observed associations between membership in the alcohol use and VACS index score trajectories. For example, we were not able to assess family history of alcohol problems, which may affect both long-term drinking patterns and overall health. Third, the trajectory-based approach did not permit analysis of whether specific changes in drinking behavior result in subsequent changes in VACS index scores. Fourth, differential loss to follow-up may have affected the shape and classification of alcohol and VACS index trajectories, particularly as the proportion who dropped out varied across the groups. We evaluated the extent to which these biases influenced our results by conducting a series of sensitivity analyses, and found that the differences in trajectory membership, shape, and effect estimates were minimal. Fifth, the small number of persons assigned to the low VACS index class is in part a function of the fact that persons 50 years and older are assigned at least 12 points on the VACS index. Research is underway to refine how age is accounted for in the scoring algorithm. Finally, our study was restricted to HIV-infected veterans receiving care in the Veterans Healthcare System and who were primarily men. Thus, the results may not necessarily be generalizable to all veterans or populations living with HIV.
In conclusion, long-term alcohol consumption and VACS index trajectories were linked and interrelated among HIV-infected patients in care. A pattern of persistent unhealthy alcohol use was most common among persons with greater HIV disease severity, whereas abstainers were overrepresented in the healthiest and unhealthiest groups (suggesting distinct groups of ‘healthy abstainers’ and ‘sick quitters’). Future research should explore motivations and reasons for alcohol abstinence among HIV-infected populations to more fully understand this relationship. Second, studies should seek to determine the extent to which changes in alcohol consumption may impact long-term HIV disease progression. Finally, further research is needed to identify whether interventions that successfully reduce alcohol consumption improve HIV-related morbidity and mortality.
Supplementary Material
Acknowledgments
We would like to acknowledge the Veterans who participate in the Veterans Aging Cohort Study (VACS) and the study coordinators and staff at each VACS site and at the West Haven Coordinating Center. We would also like to thank Melissa Skanderson for her assistance and support during data acquisition. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, grant numbers U24-AA022000, U10-AA013566, U01-AA020795, U01-AA020790, U24-AA020794, and P01-AA019072), the National Institute of Allergy and Infectious Diseases (grant number P30-AI042853), the National Institute on Drug Abuse (NIDA, grant number R01-DA035616) and in kind by the US Department of Veterans Affairs. R.L.C. is supported by the NIAAA (grant number U24-AA022002). J.R.G. is supported by the NIDA (grant number F31-DA035567). E.J.E. is a Yale-Drug Abuse, Addiction, and HIV Research Scholar (grant number K12-DA033312). B.D.L.M. is supported a Henry Merrit Wriston Fellowship from Brown University.
Footnotes
All authors made significant contributions to the study’s design and conduct. Author B.D.L.M. designed the analytic plan and conducted the statistical analysis, with scientific input from J.P.T. and A.C.J. Author B.D.L.M. wrote the first draft of the manuscript, and all authors participated in its preparation and critical revision for important intellectual content. All authors contributed to and have approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) HIV surveillance special report 16. Atlanta, GA: CDC; Published; Jan, 2016. Behavioral and clinical characteristics of persons receiving medical care for HIV infection–—medical monitoring project, United States, 2013 Cycle (June 2013–May 2014) [Google Scholar]
- 3.Hahn JA, Samet JH. Alcohol HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7:226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 5.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 6.Ferro EG, Weikum D, Vagenas P, Copenhaver MM, Gonzales P, Peinado J, et al. Alcohol use disorders negatively influence antiretroviral medication adherence among men who have sex with men in Peru. AIDS Care. 2015;27:93–104. doi: 10.1080/09540121.2014.963013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahler CW, Liu T, Cioe PA, Bryant V, Pinkston MM, Kojic EM, et al. Direct and indirect effects of heavy alcohol use on clinical outcomes in a longitudinal study of HIV patients on ART. AIDS Behav. 2016 doi: 10.1007/s10461-016-1474-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy alcohol use is associated with worse retention in HIV care. J Acquir Immune Defic Syndr. 2016;73:419–425. doi: 10.1097/QAI.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagby GJ, Amedee AM, Siggins RW, Molina PE, Nelson S, Veazey RS. Alcohol and HIV effects on the immune system. Alcohol Res. 2015;37:287–297. doi: 10.35946/arcr.v37.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall BD, Operario D, Bryant KJ, Cook RL, Edelman EJ, Gaither JR, et al. Drinking trajectories among HIV-infected men who have sex with men: a cohort study of United States veterans. Drug Alcohol Depend. 2015;148:69–76. doi: 10.1016/j.drugalcdep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Method Res. 2007;35:542–571. [Google Scholar]
- 15.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice AC, Landefeld CS, Asch SM, Gifford AL, Whalen CC, Covinsky KE. Justification for a new cohort study of people aging with and without HIV infection. J Clin Epidemiol. 2001;54:S3–S8. doi: 10.1016/s0895-4356(01)00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. International classification of diseases, ninth revision, clinical modification (ICD-9-CM) Atlanta, GA: National Center for Health Statistics (NCHS); 2013. [Google Scholar]
- 18.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 19.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, Fiellin DA. Comparing alcohol screening measures among HIV-infected and uninfected men. Alcohol Clin Exp Res. 2013;37:435–442. doi: 10.1111/j.1530-0277.2012.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, et al. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:226–230. doi: 10.1097/QAI.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown ST, Tate JP, Kyriakides TC, Kirkwood KA, Holodniy M, Goulet JL, et al. The VACS index accurately predicts mortality and treatment response among multidrug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PLoS One. 2014;9:e92606. doi: 10.1371/journal.pone.0092606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 27.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29:374–393. [Google Scholar]
- 28.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn JA, Dobkin LM, Mayanja B, Emenyonu NI, Kigozi IM, Shiboski S, et al. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012;36:854–862. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 31.Shao J, Zhong B. Last observation carry-forward and last observation analysis. Stat Med. 2003;22:2429–2441. doi: 10.1002/sim.1519. [DOI] [PubMed] [Google Scholar]
- 32.Molina PE, Bagby GJ, Nelson S. Biomedical consequences of alcohol use disorders in the HIV-infected host. Curr HIV Res. 2014;12:265–275. doi: 10.2174/1570162x12666140721121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGinnis KA, Fiellin DA, Tate JP, Cook RL, Braithwaite RS, Bryant KJ, et al. Number of drinks to ‘Feel a Buzz’ by HIV status and viral load in men. AIDS Behav. 2016;20:504–511. doi: 10.1007/s10461-015-1053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2:1267–1273. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 35.Samet JH, Pace CA, Cheng DM, Coleman S, Bridden C, Pardesi M, et al. Alcohol use and sex risk behaviors among HIV-infected female sex workers (FSWs) and HIV-infected male clients of FSWs in India. AIDS Behav. 2010;14(Suppl 1):S74–S83. doi: 10.1007/s10461-010-9723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walley AY, Cheng DM, Libman H, Nunes D, Horsburgh CR, Jr, Saitz R, et al. Recent drug use, homelessness and increased short-term mortality in HIV-infected persons with alcohol problems. AIDS. 2008;22:415–420. doi: 10.1097/QAD.0b013e3282f423f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


