Abstract
The molecular chaperones, α–crystallins, belong to the small heat shock protein (sHSP) family and prevent the aggregation and insolubilization of client proteins. Studies in vivo have shown that the chaperone activity of the α–crystallins is raised or lowered in various disease states. Therefore, the development of tools to control chaperone activity may provide avenues for therapeutic intervention, as well as enable a molecular understanding of chaperone function. The major human lens α–crystallins, αA- (HAA) and αB- (HAB), share 57% sequence identity and show similar activity towards some clients, but differing activities towards others. Notably, both crystallins contain the “α–crystallin domain” (ACD, the primary client binding site), like all other members of the sHSP family. Here we show that RNA aptamers selected for HAA, in vitro, exhibit specific affinity to HAA but do not bind HAB. Significantly, these aptamers also exclude the ACD. This study thus demonstrates that RNA aptamers against sHSPs can be designed that show high affinity and specificity – yet exclude the primary client binding region – thereby facilitating the development of RNA-aptamer based therapeutic intervention strategies
Keywords: aptamer, crystallin, cataract, heat shock proteins, SELEX, RNA, chaperone, melittin
1. Introduction
α–crystallins are the most prominent members of the small heat shock (sHSP) family of proteins. They are molecular chaperones that bind to client or target proteins as these targets unfold, keeping them in solution. The α–crystallin concentration in the eye lens is very high (≥ 100 mg/ml [1, 2]), and they keep the other lens proteins in a stable and soluble state – thereby minimizing light scattering and maintaining lens transparency. The human sHSP family members, Hsp27 (or HspB1), HAA (or HspB4) and HAB (or HspB5) also show anti-apoptotic properties [3, 4], which are apparently associated with their chaperone property [5, 6]. Because of their role in cellular protection as well as in abnormal cellular proliferation in some cases, sHSPs have been viewed as therapeutic targets for modulating their biological function. For HspB1, peptide aptamers have been designed as therapeutic compounds to modulate its expression to higher or lower levels, depending on the beneficial effects the expression levels produce in a particular disease [7]. Such therapeutic modulation has also been suggested for HSPs B4 (HAA) and B5 (HAB) [8].
Besides peptide aptamers, DNA and RNA aptamers have also been developed for a variety of experimental and therapeutic applications, including the RNA aptamers we isolated to modify the Hsp70 chaperone system [9, 10] and Heat Shock Factor 1 [11–13]. Here we describe the selection of three distinct RNA aptamers against HAA using the Systematic evolution of ligands by exponential enrichment (SELEX) methodology [14]. α–crystallins are not known to bind RNA although they do bind ATP [15]. It appears that ATP binding is close to the putative substrate binding region [15–17] on the chaperone, which may or may not be true for aptamer binding. Interestingly however, in a kinase, GRK2, which also binds ATP but does not normally recognize nucleic acid polymers, the RNA aptamer exploits the ATP binding site [18] and inserts an adenine from an RNA loop into this site – which suggests an overlap between the two sites. Thus, it is conceivable that RNA aptamers selected for the α–crystallins could bind at or near the ATP binding site on the chaperone, thereby affecting not only substrate binding but also the anti-apoptotic function of the protein.
Aptamers are believed to be the most competitive class of therapeutics for the eye [19]. Our motivation for this study is not only to modulate the chaperone activity of the α–crystallins, but also to understand the mechanistic basis of their chaperone function. Several key issues remain unresolved in understanding the molecular bases of the α–crystallin chaperone function [21]. For example, we do not know why the α–crystallins — promiscuous “molecular sponges” [22] for destabilized proteins — hardly bind to partially-unfolded mutants of another major eye-lens protein, γD-crystallin [23, 24]. Also puzzling is how a 19 or 20 amino-acid fragment of the α-crystallins (mini α–crystallin) shows chaperone activity despite being only 1/10th the size of the parent protein’s monomer [25–27]. RNA aptamers could serve as valuable tools to examine the underlying molecular mechanisms in such cases, by revealing how aptamer-binding alters chaperone function, and whether the chaperone-complexes with natural substrates/clients, also bind these aptamers.
Aptamer binding to a protein not only affects the properties of the binding region, but possibly, the properties of other domains [28–30] as well. Thus, unexpected gains or losses of chaperone function towards different clients could result from aptamer binding, which would help to distinguish between their binding preferences for different clients. Since α-crystallins typically unfold around 60–65° C [31], it is desirable to enhance their stability by means of RNA aptamer binding, because both HAA and HAB protect client proteins under harsh unfolding conditions. These are our motivating factors for generating RNA aptamers for the α–crystallins.
2. Materials and Methods
2.1 Preparation of recombinant HAA and HAB
Expression and purification of HAA has been described earlier [32]. The HAB clone (generous gift from Prof. J. Horwitz, Jules Stein Eye Institute, UCLA), was expressed and purified according to Horwitz et al [33]. Melittin was obtained from Genscript (Piscataway, NJ), and used without further purification.
2.2 Oligonucleotides
All oligonucleotides were purchased from Integrated DNA Technologies. The initial RNA pool contained ~1.8 × 1015 different sequences, each having a 50-nt randomized region in the middle flanked by 25-nt constant regions on either side. The sequence of the constant regions in the context of full-length RNA pool sequences is given below:
5’GGGAGAAUUCAACUGCCAUCUA-(N55)-
GACUACAAGCUUCUGGACUCGGU3’
2.3 In vitro selection of aptamer by SELEX
2-Fluoropyrimidine (2F-Py)-RNA pools were prepared by in vitro transcription using DuraScribe® T7 Transcription Kit (Epicenter Biotechnologies), according to the manufacturer’s recommendations. Selection and amplification steps were carried out as previously described [34] with minor modifications. In the first cycle 15 nmole 2’F-Py-RNA pool was dissolved in 1mL binding buffer (150 mM NaCl, 5 mM MgCl2, 20 mM HEPES, pH 7.2), and heated at 65°C for 10 minutes, then incubated at 37°C for another 10 minutes. 100 µg of purified HAA was added and the mixture was incubated for 2hrs at 37°C. The mixture was partitioned using a 0.22 µm nitrocellulose membrane filter paper (Millipore, Cat No. GSPWP025000), and the filter was washed with 25 mL binding buffer. Except for the first cycle, negative selection was performed against the nitrocellulose filter to enrich aptamers in the pool. Through the course of selection, the following parameters were changed: amount of RNA, amount of protein, total volume of reaction, incubation time and amount of competitor, yeast genomic RNA. A total of 22 cycles were carried out to obtain the desired aptamers. The 12th cycle pool of DNA was cloned and sequenced to monitor the progression of the selection process. In the first 12 cycles the nitrocellulose filter paper was washed with binding buffer. For the last 10 cycles the washing buffer was supplemented with 0.01% v/v TritonX-100 (Sigma-Aldrich).
2.4 Cloning and sequencing
Cloning was done as per Sambrook & Russell [35]. Selected DNA pools were cloned at the EcoRI- HindII site in the pGEM-3Z vector (Promega). Sequencing was performed in the Life Sciences Core Facility at the University at Albany.
2.5 Aptamer-Protein binding assay
Aptamer-protein binding assays were performed by the electrophoretic mobility shift assay (EMSA) technique, following our basic protocol [34] with some modifications. We used 4% polyacrylamide gel with an acrylamide to bis-acrylamide ratio of 70:1, and the material was electrophoresed for 5 hr. at 4°C. Radioactively-labeled RNA was prepared using the DuraScribe® T7 Transcription Kit. In all binding reactions 10 µg yeast genomic RNA (Ambion) was mixed to decrease non-specific interaction between the aptamer and protein.
3. Results
3.1 Isolation of RNA aptamers for HAA
The HAA-chaperone exists in solution as a large assembly with an average mol. wt. of 540 kDa [36] and may be subject to a higher level of non-specific binding that could decrease the efficiency of selection. After 12 cycles, we checked the binding ability of a few, later generations (10th, 11th & 12th selected pools) with the target protein using EMSA (data not shown) and observed a αA-crystallin-dependent band-shift for these pools, and cloned the 12th cycle DNA. We analyzed 103 sequences and found them all to be unique (Figure 1A). This indicated that the 12th pool was still very diverse, which supported our expectation based on the special properties of αA-crystallin noted above. We also noticed that the amount of retrieved RNA from each round of selection was significantly higher compared to other protein targets.
Figure 1. Isolation of RNA aptamers for HAA.
A. Schematic diagram of the SELEX process: “Gx” represents an aptamer candidate pool at the x-th generation of selection and amplification. Number of sequences in the two samples are indicated.
B. Modification of washing conditions: The PCR products of the G13 DNA pool are shown. Three different conditions were used to wash the binding mixture on the filter, as indicated. Markers are the 100-bp ladder standards.
C. Sequence of aptamers for HAA: Lowercase letters, constant region sequences; uppercase letters, variable region sequences. The two 25-base constant regions are each covered by a 22-base primer in PCR amplification. The number of identical sequences isolated in the G22 pool is indicated.
To increase the efficiency of selection, we tested a battery of washing buffers for the RNA-protein complex immobilized on nitrocellulose. These washing buffers were essentially the binding buffer (150 mM NaCl, 5 mM MgCl2, 20 mM HEPES pH 7.2) with a single variation: We used different concentrations of NaCl (250 mM, 500 mM, 750 mM and 1 M), eliminated MgCl2, or added TritonX-100 (0.1%, 0.01% and 0.005%). We varied the NaCl concentration and eliminated MgCl2 because of the well-known, general and specific salt-effects on RNA-binding; but the inclusion of Triton X-100 is not common. Using these variations, we observed a significant decrease of RNA retrieval only when TritonX-100 was included (Figure-1B).
The prevention of non-specific binding in the presence of TritonX-100 is not surprising: sHSPs are essentially “molecular sponges” [22] – and hence susceptible to non-specific binding which is minimized in the presence of a non-ionic detergent such as Triton. We therefore continued the selection process with 0.01% TritonX-100 (v/v) included in the wash-buffer in subsequent cycles. After ten more rounds of selection and amplification, we cloned the 22nd cycle DNA pool and analyzed 177 sequences (Figure 1A). There were three sequences with multiple isolates occurring 6, 5, and 2 times respectively. We synthesized the templates for these three candidates and tested each of them using EMSA. They were confirmed to be true aptamers and named AptAC-1, 2, and 3, with sequences shown in Figure 1C.
3.2 Characterization of aptamers to HAA
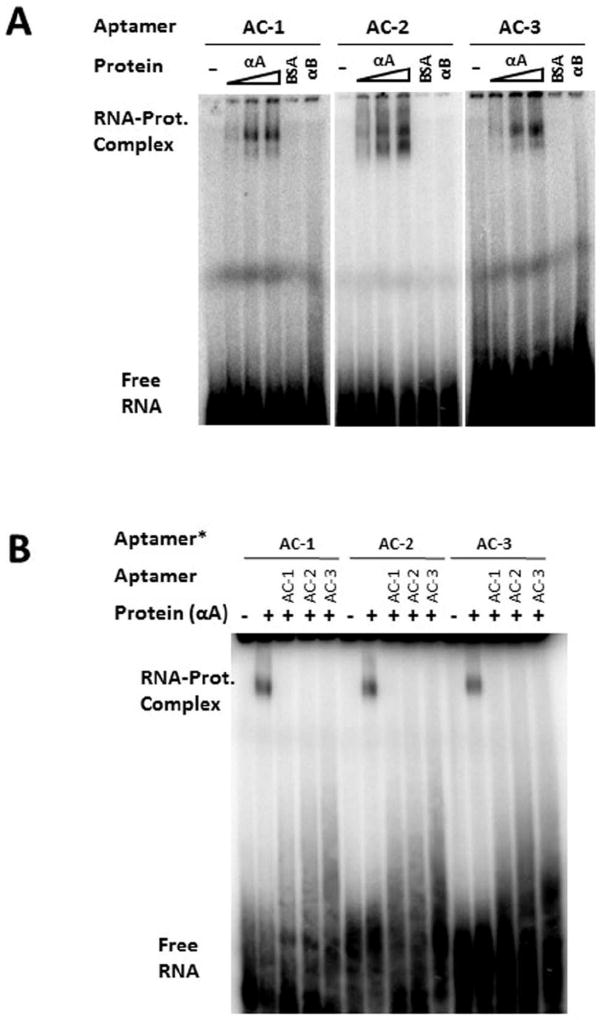
To confirm the affinity and investigate the specificity of the isolated aptamers, we used radio-labeled aptamers in EMSA. This independent binding assay is different from the filter binding conditions used during selection. Here a small amount of the labeled RNA is incubated with the proteins in excess. In addition to the intended target, HAA, we used BSA as a negative control. The binding reaction was set up in the presence of at least a 10,000-fold excess of non-specific yeast genomic RNA. In Figure 2A, an RNA-protein-retarded migratory complex is visible in EMSA in the presence of HAA, but more importantly, the amount of RNA-protein complex increases with increasing amounts of HAA. Such dose-dependency indicated that binding was specific to a defined site on the surface of the target protein. However, since HAA exists as an assembly in solution and the stoichiometry of binding is unknown, no attempt was made to assign a Kd (dissociation constant) value based on band intensity. We also examined whether these aptamers recognized HAB, the other member of the α–crystallin chaperone family, with a ~57% sequence identity with [21]. As shown in Figure 2A, HAB did not produce a shifted band with any of the three aptamers. Therefore, these aptamers clearly distinguish between the two proteins. We sometimes observed – especially when the EMSA gel image was overexposed – multiple shifted bands, which may correspond to the aptamers binding to different populations of the protein target. In Figure 2A at least three such distinct, shifted bands can be discerned. The three aptamers seem to exhibit different relative intensities of these bands, which suggests different modes of binding.
Figure 2. Characterization of Aptamers to HAA.
A. Specific, concentration-dependent binding of aptamers to HAA. Each aptamer was radiolabeled (~1 nM, asterisk). Three different concentrations of HAA were used in binding reactions: 1.5 µg, 3 µg, and 4.5 µg. HAB (4.5 µg) was used to demonstrate specificity. BSA (4.5µg) was used as a control. In all binding reactions 10 µg yeast RNA (~100 nt in length) was used as a competitor (~30 mM or 60,000-fold in excess compared to target aptamer).
B. Competition between aptamers for binding to HAA: Each radiolabeled aptamer (~1 nM, asterisk) was mixed with 1500× unlabeled aptamer in the binding reactions in the presence of 4µg HAA. Labeled and unlabeled aptamers were mixed together before adding protein. In all binding reactions, 10 µg yeast RNA was included.
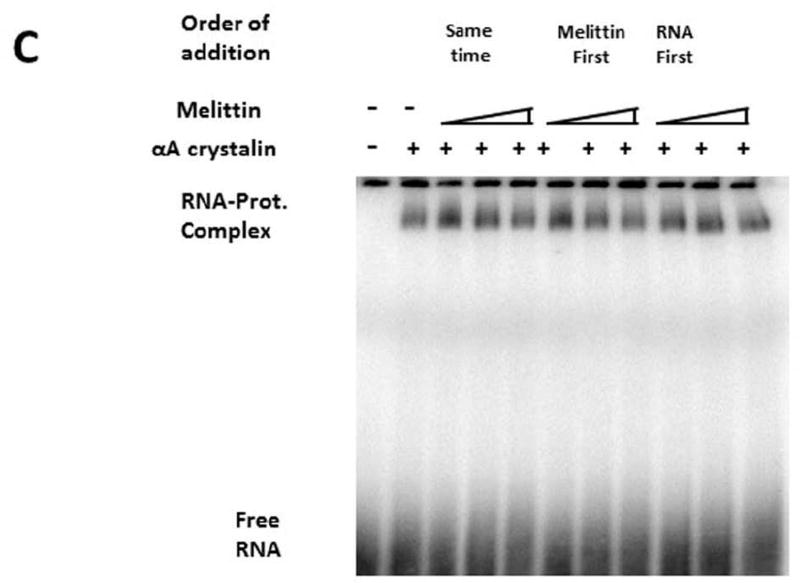
C. Effect of melittin on the binding of aptamers: Radiolabeled AptAC-2 was used in all binding reactions (concentration ~0.5 nM). HAA concentration (all lanes except the left most one, 4 µg, ~18 µM). Melittin used at three different concentrations: 1.5 µM, 3 µM, and 4.5 µM. Three different orders of addition were tested as indicated. In all lanes 10 µg yeast RNA (~100 nt) was included as competitor.
In view of the potentially high level of non-specific binding, we took a three-pronged approach to confirm the specificity of each isolated aptamer. In addition to the dose dependent binding to HAA, and the inability to bind HAB (Figure 2A), we also performed a competition assay in which each radiolabeled aptamer is co-incubated with at least 1,000-fold excess of unlabeled “cold” aptamer. All three aptamers were capable of self-competition, which confirmed the existence of a defined binding site for each (Figure 2B). Moreover, despite their different binding modes (Figure 2A), each aptamer competed with the other two. This suggests that all these aptamers are interacting with HAA around the same locus.
In order to locate the binding site on HAA we employed bee venom melittin, which is known to bind to the α-crystallins at or adjacent to the ACD [25]. We tested whether aptamer binding is affected by melittin binding to HAA or vice versa. Three different orders of addition were tested (Figure 2C): simultaneous incubation (lanes 3, 4 & 5), melittin first (lanes 6, 7 & 8) and RNA first (lanes 9, 10 &11). Each segment of incubation was for 30 minutes except for lanes 3, 4 & 5, for which it was 1hr. With about 9,000-fold excess melittin (in lane 5, 8 & 11), the radioactivity of the aptamer was not reduced by half (compared to lanes 3, 6 & 9). We note that with increasing concentration of melittin, the intensity of the band located at the top of the gel also qualitatively increases, which indicates that the aptamers bind to HAA irrespective of melittin binding – and even in its presence. We note that melittin (Mol. Wt. 2600 Da), and aptamer (approximate Mol. Wt. 3300 Da) being small in comparison with the target protein (Average Mol. Wt. 540 kDa), the migration rates of all complexes are nearly indistinguishable in Figure 2C.
4. Discussion
We set out to find aptamers specific for the α-crystallins and to determine whether they bind to ACD, which consists of 90 to 100 amino acid residues, and is common to all sHSPs [20]. If indeed the aptamers bind to ACD, they are likely to affect the chaperone activity – thus revealing the mechanism of chaperone action, similar to enzyme-inhibitors that reveal the molecular basis of enzyme activity. If however, the aptamers bind to other regions besides ACD, they might modulate chaperone function; thereby also revealing the structural basis of chaperone function.
It is important to determine the binding locus on the target before other considerations (such as controlling target protein function) can be taken into account. We have described three RNA aptamers that specifically bind HAA at a locus that does not include the ACD, the critical, client-binding region around which bee venom melittin is known to bind [25]. Because the aptamers do not affect melittin binding, we can presume that they spare the substrate/client binding site. This would allow us to examine the effect of aptamer-binding on various chaperone complexes. Thus, the generation of these aptamers provides an avenue for controlling the functional properties of HAA.
Among the sHSP family, αA- and αB-crystallins are probably the best characterized members, both structurally and functionally [21]. sHSPs have generally been described as natural molecular sponges [22], since they are promiscuous in sequestering non-native proteins. An analysis of the αA-interactome using human protein microarrays, identified hundreds of interacting proteins [37]. Similarly, a proteomics study found hundreds of proteins in heat-stressed HeLa cell lysate protected from aggregation by HAB [38]. These crystallins also play an active role in apoptosis, which seems related to their chaperone activity [5]. Furthermore – based on their apparent role in disease-related processes – sHSPs in general, and α–crystallins in particular, have been associated with exacerbating some diseases while alleviating others [39, 40]. Thus in considering their potential therapeutic role, it is desirable to regulate the functional activity of the α–crystallins positively, as well as negatively.
Cataract-associated α–crystallin mutants fall into both categories; namely, those leading to an increase and others leading to a decrease in chaperone activity [21], suggesting that the chaperone activity of the α–crystallins is optimized in the lens. For therapeutic purposes, modulation of this activity is desirable, and one mechanism for such regulation in vivo may be the phosphorylation of the Ser residues of the chaperones [21]. For therapeutically controlling the functional activity of the α–crystallins and other sHSPs, peptide aptamers have been proposed and designed [7]. However, as stated in the introduction, RNA aptamers have some distinct, positive attributes [41].
In conclusion, we have described the isolation and characterization of three aptamers for HAA, using SELEX, with a stringent surfactant-purge (using Triton X-100). None of the three aptamers bind to the ACD. Previous studies on melittin-binding to α-crystallin qualifies it as a good surrogate for client proteins [25, 27]. However, melittin-binding is known to be weak [42], and our results are in agreement with earlier studies. We note that the binding of aptamers to non-ACD sites is of paramount importance because they do not directly affect substrate\client binding, but may exert an indirect regulatory effect, in an allosteric manner. While HAA and HAB are both molecular chaperones, there are clear differences in their interactions with other proteins with respect to their functional activity and specificity [43, 44]. Thus it is not surprising that the aptamers against HAA do not bind HAB. In fact, this specificity in RNA-aptamer binding is significant, if such aptamers are to be used to regulate the activity of individual HSPs.
This work shows for the first time, the successful design of RNA aptamers against HAA, which do not bind to HAB. More importantly, such binding to HAA spares the ACD region that is common to all sHSPs and is intimately involved in the binding of substrates and clients during the course of chaperone function.
Supplementary Material
Highlights.
Selection of RNA aptamers against the chaperone, human αA-crystallin is described.
The aptamers do not appear to bind to the client-binding, “α-crystallin domain”.
αA-crystallin-aptamer binding is independent of melittin (substrate mimic) binding.
These aptamers do not bind to the closely related αB-crystallin.
Acknowledgments
We thank Dr. Ajay Pande for his contributions to planning the study, writing the manuscript, and providing pure alpha crystallin preparations.
Supported by NIH Grant No. EY010535 to J.P.
Abbreviations
- sHSP
Small heat shock protein
- ACD
α–crystallin domain
- EMSA
Electrophoretic mobility shift assay
- BSA
Bovine serum albumin
- SELEX
Systematic evolution of ligands by exponential enrichment
- HAA
Human recombinant αA-crystallin
- HAB
Human recombinant αB-crystallin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no competing financial interests.
References
- 1.Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens - the distribution of protein. Experimental eye research. 1981;33:615–620. doi: 10.1016/s0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz J. Alpha-crystallin. Experimental eye research. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 3.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 4.Andley UP. Effects of alpha-crystallin on lens cell function and cataract pathology. Curr Mol Med. 2009;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- 5.Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D, Nagaraj RH. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santhoshkumar P, Karmakar S, Sharma KK. Structural and functional consequences of chaperone site deletion in alphaA-crystallin. Biochimica et biophysica acta. 2016;1864:1529–1538. doi: 10.1016/j.bbapap.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibert B, Simon S, Dimitrova V, Diaz-Latoud C, Arrigo AP. Peptide aptamers: tools to negatively or positively modulate HSPB1(27) function. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368:20120075. doi: 10.1098/rstb.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrigo AP, Gibert B. HspB1, HspB5 and HspB4 in Human Cancers: Potent Oncogenic Role of Some of Their Client Proteins. Cancers (Basel) 2014;6:333–365. doi: 10.3390/cancers6010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirunavukarasu D, Shi H. An RNA aptamer specific to Hsp70-ATP conformation inhibits its ATPase activity independent of Hsp40. Nucleic Acid Ther. 2015;25:103–112. doi: 10.1089/nat.2014.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thirunavukarasu D, Shi H. Aptamer-Enabled Manipulation of the Hsp70 Chaperone System Suggests a Novel Strategy for Targeted Ubiquitination. Nucleic Acid Ther. 2016;26:20–28. doi: 10.1089/nat.2015.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salamanca HH, Fuda N, Shi H, Lis JT. An RNA aptamer perturbs heat shock transcription factor activity in Drosophila melanogaster. Nucleic Acids Res. 2011;39:6729–6740. doi: 10.1093/nar/gkr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PloS one. 2014;9:e96330. doi: 10.1371/journal.pone.0096330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Shi H, Sevilimedu A, Liachko N, Nelson HC, Lis JT. An RNA aptamer that interferes with the DNA binding of the HSF transcription activator. Nucleic Acids Res. 2006;34:3755–3761. doi: 10.1093/nar/gkl470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold L. SELEX: How It Happened and Where It will Go. J Mol Evol. 2015;81:140–143. doi: 10.1007/s00239-015-9705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasan A, Smith DL, Smith JB. Alpha-crystallin regions affected by adenosine 5'-triphosphate identified by hydrogen-deuterium exchange. Biochemistry. 2002;41:15876–15882. doi: 10.1021/bi026568x. [DOI] [PubMed] [Google Scholar]
- 16.Muchowski PJ, Hays LG, Yates JR, 3rd, Clark JI. ATP and the core "alpha-Crystallin" domain of the small heat-shock protein alphaB-crystallin. The Journal of biological chemistry. 1999;274:30190–30195. doi: 10.1074/jbc.274.42.30190. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh JG, Houck SA, Doneanu CE, Clark JI. The beta4–beta8 groove is an ATP-interactive site in the alpha crystallin core domain of the small heat shock protein, human alphaB crystallin. Journal of molecular biology. 2006;364:364–375. doi: 10.1016/j.jmb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Tesmer VM, Lennarz S, Mayer G, Tesmer JJ. Molecular mechanism for inhibition of g protein-coupled receptor kinase 2 by a selective RNA aptamer. Structure. 2012;20:1300–1309. doi: 10.1016/j.str.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drolet DW, Green LS, Gold L, Janjic N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016;26:127–146. doi: 10.1089/nat.2015.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 21.Haslbeck M, Peschek J, Buchner J, Weinkauf S. Structure and function of alpha-crystallins: Traversing from in vitro to in vivo. Biochimica et biophysica acta. 2016;1860:149–166. doi: 10.1016/j.bbagen.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Eyles SJ, Gierasch LM. Nature's molecular sponges: small heat shock proteins grow into their chaperone roles. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2727–2728. doi: 10.1073/pnas.0915160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau KL, King JA. Cataract-causing defect of a mutant gamma-crystallin proceeds through an aggregation pathway which bypasses recognition by the alpha-crystallin chaperone. PloS one. 2012;7:e37256. doi: 10.1371/journal.pone.0037256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra S, Stein RA, McHaourab HS. Cataract-linked gammaD-crystallin mutants have weak affinity to lens chaperones alpha-crystallins. FEBS Lett. 2012;586:330–336. doi: 10.1016/j.febslet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. The Journal of biological chemistry. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 26.Kumar RS, Sharma KK. Chaperone-like activity of a synthetic peptide toward oxidized gamma-crystallin. J Pept Res. 2000;56:157–164. doi: 10.1034/j.1399-3011.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padlan CS, Malashkevich VN, Almo SC, Levy M, Brenowitz M, Girvin ME. An RNA aptamer possessing a novel monovalent cation-mediated fold inhibits lysozyme catalysis by inhibiting the binding of long natural substrates. RNA. 2014;20:447–461. doi: 10.1261/rna.043034.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont DM, Thuesen CK, Botkjaer KA, Behrens MA, Dam K, Sorensen HP, Pedersen JS, Ploug M, Jensen JK, Andreasen PA. Protein-binding RNA aptamers affect molecular interactions distantly from their binding sites. PloS one. 2015;10:e0119207. doi: 10.1371/journal.pone.0119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Canny MD, De Erkenez A, Krilleke D, Ng YS, Shima DT, Pardi A, Jucker F. A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18902–18907. doi: 10.1073/pnas.0509069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivas P, Narahari A, Petrash JM, Swamy MJ, Reddy GB. Importance of eye lens alpha-crystallin heteropolymer with 3:1 alphaA to alphaB ratio: stability, aggregation, and modifications. IUBMB Life. 2010;62:693–702. doi: 10.1002/iub.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh KS, Pande A, Pande J. Binding of gamma-crystallin substrate prevents the binding of copper and zinc ions to the molecular chaperone alpha-crystallin. Biochemistry. 2011;50:3279–3281. doi: 10.1021/bi200091q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz J, Huang QL, Ding L, Bova MP. Lens alpha-crystallin: chaperone-like properties. Methods in enzymology. 1998;290:365–383. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- 34.Mallik PK, Nishikawa K, Millis AJ, Shi H. Commandeering a biological pathway using aptamer-derived molecular adaptors. Nucleic Acids Res. 2010;38:e93. doi: 10.1093/nar/gkp1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Russell DW. Molecular cloning : a laboratory manual. 3. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2001. [Google Scholar]
- 36.Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Subunit exchange of polydisperse proteins: mass spectrometry reveals consequences of alphaA-crystallin truncation. The Journal of biological chemistry. 2005;280:14485–14491. doi: 10.1074/jbc.M500135200. [DOI] [PubMed] [Google Scholar]
- 37.Fan Q, Huang LZ, Zhu XJ, Zhang KK, Ye HF, Luo Y, Sun XH, Zhou P, Lu Y. Identification of proteins that interact with alpha A-crystallin using a human proteome microarray. Molecular vision. 2014;20:117–124. [PMC free article] [PubMed] [Google Scholar]
- 38.Peschek J, Braun N, Rohrberg J, Back KC, Kriehuber T, Kastenmuller A, Weinkauf S, Buchner J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3780–3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Nagaraj RH, Nahomi RB, Mueller NH, Raghavan CT, Ammar DA, Petrash JM. Therapeutic potential of alpha-crystallin. Biochimica et biophysica acta. 2016;1860:252–257. doi: 10.1016/j.bbagen.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelinas AD, Davies DR, Janjic N. Embracing proteins: structural themes in aptamer-protein complexes. Curr Opin Struct Biol. 2016;36:122–132. doi: 10.1016/j.sbi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Farahbakhsh ZT, Huang QL, Ding LL, Altenbach C, Steinhoff HJ, Horwitz J, Hubbell WL. Interaction of alpha-crystallin with spin-labeled peptides. Biochemistry. 1995;34:509–516. doi: 10.1021/bi00002a015. [DOI] [PubMed] [Google Scholar]
- 43.Andley UP, Malone JP, Townsend RR. In Vivo Substrates of the Lens Molecular Chaperones alphaA-Crystallin and alphaB-Crystallin. PloS one. 2014;9:e95507. doi: 10.1371/journal.pone.0095507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. The Journal of biological chemistry. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.