Abstract
CLR1404 is a cancer-selective alkyl phosphocholine (APC) analog that can be radiolabeled with 124I for PET imaging, 131I for targeted radiotherapy and/or SPECT imaging, or 125I for targeted radiotherapy. Studies have demonstrated avid CLR1404 uptake and prolonged retention in a broad spectrum of preclinical tumor models. The purpose of this pilot trial was to demonstrate avidity of 124I-CLR1404 in human brain tumors and develop a framework to evaluate this uptake for use in larger studies. 12 patients (8 men and 4 women; mean age of 43.9 ± 15.1 y; range 23-66 y) with 13 tumors were enrolled. Eleven patients had suspected tumor recurrence and 1 patient had a new diagnosis of high grade tumor. Patients were injected with 185 MBq ± 10% of 124I-CLR1404 followed by PET/CT imaging at 6-, 24-, and 48-hour. 124I-CLR1404 PET uptake was assessed qualitatively and compared with MRI. After PET image segmentation SUV values and tumor to background ratios were calculated. There was no significant uptake of 124I-CLR1404 in normal brain. In tumors, uptake tended to increase to 48 hours. Positive uptake was detected in 9 of 13 lesions: 5/5 high grade tumors, 1/2 low grade tumors, 1/1 meningioma, and 2/4 patients with treatment related changes. 124I-CLR1404 uptake was not detected in 1/2 low grade tumors, 2/4 lesions from treatment related changes, and 1/1 indeterminate lesion. For 6 malignant tumors, the average tumor to background ratios (TBR) were 9.32 ± 4.33 (range 3.46 to 15.42) at 24 hours and 10.04 ± 3.15 (range 5.17 to 13.17) at 48 hours. For 2 lesions from treatment related change, the average TBR were 5.05 ± 0.4 (range 4.76 to 5.33) at 24 hours and 4.88 ± 1.19 (range 4.04 to 5.72) at 48 hours. PET uptake had areas of both concordance and discordance compared with MRI. 124I-CLR1404 PET demonstrated avid tumor uptake in a variety of brain tumors with high tumor-to-background ratios. There were regions of concordance and discordance compared with MRI, which has potential clinical relevance. Expansion of these studies is required to determine the clinical significance of the 124I-CLR1404 PET findings.
Keywords: CLR1404, alkyl phosphocholine analog, brain tumor, high grade brain tumor, low grade brain tumor, PET, molecular imaging
Introduction
Despite recent diagnostic advances in oncology, the diagnosis and treatment of primary and metastatic brain tumors still face many challenges. Magnetic resonance imaging (MRI) is considered the diagnostic imaging standard for brain tumors and provides excellent structural information of normal brain anatomy and many pathological processes. However, MRI has notable limitations in demarcating the extent of tumor infiltration, discriminating between tumor necrosis and viable tumor, and correctly diagnosing pseudoprogression/treatment related changes from true tumor recurrence in the post-treatment setting [1-4]. These limitations can adversely influence biopsy and surgical planning, radiation therapy planning, and diagnosis in the post-treatment setting which can ultimately have an influence on patient survival [2-5].
In order to address the diagnostic limitations of MRI, numerous positron emission tomography (PET) radiopharmaceuticals have been studied in brain tumors. Some of the more commonly studied PET radiopharmaceuticals include (2-deoxy-2-18F-fluoro-D-glucose) 18F-FDG for tumor glucose metabolism, (L-[methyl-11C]methionine) 11C-MET, (18F-fluoro-ethyl-tyrosine) 18F-FET, and (18F-L-dihydroxyphenylalanine) 18F-DOPA amino acids and analogs, and (3-deoxy-3-18F-fluorothymidine) 18F-FLT proliferation agent [2-14]. Many PET studies have provided complimentary information and improvements to MRI in the delineation of tumor borders and diagnosis of pseudoprogression/treatment related changes from true tumor recurrence; some studies have even demonstrated that improved patient survival can be attained when all metabolically active tumor is resected during surgery [2-14]. Despite such improvements, these radiopharmaceuticals have not been widely adopted clinically due to a variety of limitations such as short physical half-life, lack of Food and Drug Administration (FDA) approval and/or insurance reimbursement, manufacturing limitations, and low tumor to background ratios.
A cancer targeting alkyl phosphocholine (APC) analog, (18-(p-[127I] iodophenyl) octadecyl phosphocholine) CLR1404 (previously known as “NM404”) (Cellectar Biosciences, Inc, Madison, WI), has been developed and optimized by Weichert and colleagues [15-17]. This agent enters cells predominately via lipid rafts, specialized microdomains of plasma membranes enriched in cholesterol and glycosphingolipids, which are found to be overexpressed 6-10 fold in malignant cells compared to normal cells [16]. Additionally, tumor cells typically have deficient phospholipid catabolizing enzymes relative to normal tissue cells, which contributes to prolonged tumor retention of CLR1404 [16,18]. Tumor avidity of CLR1404 has been demonstrated in over 70 cancer types in xenograft, orthotopic, and transgenic rodent models, including brain cancer models [16]. Tumor uptake is relatively slow following injection whereby the tumor will start showing visual uptake at 6 hours and continue to show high uptake beyond 48 hours. Although blood pool uptake in major vascular structures demonstrates high uptake initially, there is no significant CLR1404 uptake in normal brain tissue. Therefore, avid brain tumor uptake results in high tumor to background signal and thus clear identification of viable tumor cells on PET scans [16,19].
The APC molecular platform serves as the tumor targeting moiety for both DIA gnostic and thera PEUTIC, “diapeutic”, radiopharmaceuticals via isosteric iodine substitution with 124I (diagnostic PET), 125I (targeted radiotherapy), 127I (non-radioactive form therapy via AKT inhibition), and 131I (both diagnostic SPECT and targeted radiotherapy) [16,18]. This diapeutic agent can be administered at equivalent mass doses with any iodine isotope which ensures identical in vivo pharmacokinetic properties. Therefore the diagnostic CLR1404 agent acts as the perfect imaging companion for a therapeutic CLR1404 agent and also allows a priori determination of tumor avidity and dosimetry in an individual patient undergoing evaluation for targeted radiotherapy. Furthermore, the tumor targeting specificity of these APC analogs is maintained even after replacing the terminal iodine moiety with a fluorophore of larger size. Accordingly, a near-infrared (NIR) fluorescent analog, CLR1502, is under development to aid in in vivo identification of tumor margins during surgical resection [16-18].
Based on extensive preclinical data and preliminary human data, it is believed that malignant human brain tumors will demonstrate avid 124I-CLR1404 uptake that can be qualitatively and objectively measured. Once a straightforward and reproducible framework to evaluate brain tumor uptake is developed, this information can be used in the evaluation of larger diagnostic and therapeutic trials. Thus, the purpose of this prospective pilot clinical study was to assess for uptake of 124I-CLR1404 in brain tumors above background brain tissue and to develop a methodology to quantify this uptake for use in larger studies.
Materials and methods
Inclusion and exclusion criteria
Adult patients with suspected primary or metastatic brain tumors were eligible for this prospective clinical trial (ClinicalTrials.gov Identifier: NCT01540513). Patients must have a lesion of at least 1 cm on either T1-, T1 gadolinium enhanced-, or T2-MRI within one month of study inclusion. Additional inclusion criteria included a minimum Karnofsky score of 60, baseline platelet count ≥ 75,000/µL, hematocrit ≥ 22%, leukocyte count ≥ 3,000/µL, creatinine ≤ 2.5 mg/dL, alanine aminotransferase ≤ 130 U/L, aspartate aminotransferase ≤ 100 U/L, and urine or serum pregnancy test negative for pregnancy for women of child-bearing potential. Exclusion criteria included age younger than 18 y, radiotherapy less than one month prior to PET imaging, concurrent participation in another clinical trial, and inability or unwillingness to provide consent. Risks and benefits of participating in the study were explained and all patients had to understand the study procedures and provide written informed consent. This study has been approved by the University of Wisconsin Health Sciences Institutional Review Board, and all patients signed an informed consent form.
Patients
From March 2012 to September 2014, 12 patients (8 men and 4 women; mean age of 43.9 ± 15.1 y; range 23-66 y) were enrolled. A total of 13 tumor types were present in 12 patients, one patient had both a high grade glioma and a meningioma. Of the 12 patients, 11 had previously diagnosed and treated primary or metastatic brain tumors and presented with suspected tumor recurrence versus pseudoprogression/treatment related changes on MRI. One patient presented with newly diagnosed suspected high grade glioma, 1 patient had a meningioma, 5 patients had previously treated low grade glioma, 5 patients had previously treated high grade glioma, 1 patient had previously treated medulloblastoma, and 1 patient had a previously treated cerebral metastasis (Table 1). All patients had identifiable lesions of at least 1 cm on either T1, T1 gadolinium-enhanced, and/or T2 MRI within 1 month of obtaining the 124I-CLR1404 PET/CT.
Table 1.
Patient demographics at enrollment
| Patient no. | Age (y) | Sex | Initial histology before PET | Previous therapies |
|---|---|---|---|---|
| 1 | 58 | F | N/A (new diagnosis of suspected high grade glioma by MRI) | None |
| 2 | 35 | M | WHO II glioma | Surgery |
| 3 | 31 | M | WHO II glioma | Surgery |
| 4 | 51 | F | WHO II glioma AND meningioma | Surgery, radiation, chemotherapy |
| 5 | 32 | M | WHO II glioma | Surgery, radiation |
| 6 | 59 | M | WHO II glioma | Surgery, radiation |
| 7 | 60 | F | Metastatic melanoma | Surgery, radiation |
| 8 | 23 | M | WHO II glioma | Biopsy, radiation |
| 9 | 52 | M | WHO IV glioma | Surgery, radiation, chemotherapy |
| 10 | 29 | M | Medulloblastoma | Surgery, radiation, chemotherapy |
| 11 | 66 | M | WHO IV glioma | Surgery, radiation, chemotherapy |
| 12 | 31 | F | WHO III glioma | Surgery, radiation, chemotherapy |
Eleven of 12 patients had either surgical resection (10/11) or biopsy (1/11) prior to enrollment and 1/12 patients had newly diagnosed presumed high grade tumor and did not receive surgery prior to enrollment. Ten of 11 patients with suspected tumor recurrence with 12 distinct tumor types (5/5 high grade tumors, 4/5 low grade tumors, 1/1 metastatic tumor, and 1/1 meningioma) had undergone previous surgical resection and 1/11 patients (1/5 low grade tumors) had undergone previous biopsy. Five of 12 patients received prior chemotherapy (5/6 high grade tumors, 0/5 low grade tumors, 0/1 metastatic tumor, and 0/1 meningioma). Nine of 12 patients received radiation therapy prior to enrollment (5/6 high grade tumors, 3/5 low grade tumors, 1/1 metastatic tumors, and 0/1 meningioma). The time from completion of radiation therapy to 124I-CLR1404 PET/CT scan was at least one month in all patients. Six of 12 patients had pathological confirmation of diagnosis by surgical resection of tumor or biopsy after completion of the PET/CT scan, 1 patient had surgical resection after PET/CT at an outside facility and pathological results were unavailable. All patients had serial clinical and radiographic (MRI) follow-up after the PET/CT scan (Table 2).
Table 2.
Final diagnoses after 124I-CLR1404 PET and method for determining final diagnoses
| Patient no. | Final Diagnosis after PET | Change from initial diagnosis | Method of diagnosis | PET uptake | MRI enhancement | PET/Gad+MRI concordant/discordant |
|---|---|---|---|---|---|---|
| 1 | WHO IV glioma | n/a | Surgery | Y | Y | Both |
| 2 | WHO III glioma | Y | Surgery | Y | Y | Both |
| 3 | WHO II glioma | N | Surgery | N | N | n/a |
| 4 | WHO IV glioma AND meningioma | Y (glioma) | Surgery AND MRI f/u | Y AND Y | Y AND Y | Concordant AND concordant |
| N (meningioma) | ||||||
| 5 | Benign tx related change | Y | Clinical and MRI f/u | N | N | n/a |
| 6 | WHO II glioma | N | Surgery | Y | N | n/a |
| 7 | Benign tx related change | Y | Clinical and MRI f/u | Y | Y | Concordant |
| 8 | Indeterminate* | n/a | Clinical and MRI f/u | N | N | n/a |
| 9 | WHO IV glioma | N | Surgery | Y | Y | Both |
| 10 | Benign tx related change | Y | Clinical and MRI f/u | Y | Y | Concordant |
| 11 | WHO IV glioma | N | Clinical and MRI f/u† | Y | Y | Both |
| 12 | Benign tx related change | Y | Clinical and MRI f/u | N | Y | n/a |
Pathology determined final diagnoses for surgically treated patients and non-surgical patients had a final diagnosis based on 6 months of clinical and MRI follow-up. If MRI showed progression within 6 months, then diagnosis was confirmed as tumor recurrence. If MRI was stable or improving for 6 months, then diagnosis would be benign treatment related change.
Patient 8 had presumed tumor recurrence clinically and was treated with chemotherapy while MRI showed improvement. No pathology was available to confirm clinical findings.
Patient 11 had surgery at an outside hospital after PET and operative and pathological results were not available. By MRI, the patient had obvious progression of disease in the immediate post-operative period.
WHO is World Health Organization grading of brain tumors.
124I-CLR1404 production
Non-radioactive CLR1404 was subjected to isotope exchange conditions as described previously [15]. The 124I PET radioisotope used in the labeling reaction was obtained from IBA (Richmond, VA). CLR1404 (10 µg/37 MBq) was labeled with 124I via isotope exchange, purified by HPLC (>95% radiochemical purity), formulated in aqueous 0.4% polysorbate-20 and sterile filtered through a 0.22 micron filter prior to intravenous injection for PET/CT scans. Pyrogenicity and sterility were also assessed.
Patient preparation and PET/CT imaging
All patients received 3 drops of potassium iodide by mouth (1 gm/ml) 1 to 24 hours prior to injection of 124I-CLR1404 and continued additional daily doses prior to each PET/CT scan for 3 consecutive days, in order to minimize potential free radioiodine uptake by the thyroid gland. All patients were injected with 185 MBq (5 mCi) ± 10% of 124I-CLR1404 intravenously over a period of 1 to 2 minutes followed by a flush of 10 mL of normal saline. The subjects were observed for 30 minutes after injection and at each subsequent imaging visit for possible adverse reactions. They returned for PET/CT scans of the brain at approximately 6-, 24-, and 48-hours post-injection. Each brain imaging session started with a low dose CT which was performed for attenuation correction; this was followed by a 90 minutes dynamic PET acquisition for one bed position on a GE Discovery VCT (10 patients) or GE 710 PET/CT (2 patients) scanner (GE Healthcare, Waukesha, WI). The PET acquisition was acquired in 2D mode and reconstruction was performed using an OS-EM method, 128 × 128 matrix and 30 cm field of view. Attenuation, dead time, and scatter corrections were performed on all images.
Image analysis
For qualitative visual assessment, reconstructed PET/CT images were viewed on a Mirada workstation (Mirada Medical Advanced XD3, v3.6.2.3) by an experienced Nuclear Medicine physician (LH) and fused with clinically obtained MRI images. Visual analysis of PET images included identifying sites of 124I-CLR1404 uptake above background normal brain tissue and correlating with anatomic MRI images. Areas of concordant and discordant PET uptake and MRI signal were identified by comparing areas of T1, T1 contrast enhancement, and T2 signal hyper-intensity with 124I-CLR1404 uptake. Patterns of concordance and discordance are described qualitatively.
For quantitative image analysis, PET/CT and MRI images were next analyzed using Matlab (Matlab R2012b, Mathworks) and Amira (Amira v 5.6, FEI, SAS 2014). Raw, decay- and attenuation-corrected PET image data sets were imported into Matlab and converted into a standardized uptake value (SUV) format. PET, CT, and MRI data sets were then imported into Amira and co-registered (allowing for rigid transformations only) using the normalized mutual information module (Extensive Direction optimizer, followed by a Quasi Newton optimizer for the finest resolution steps). Contrast enhanced tumor margins were contoured on the post-Gad T1 MRI data set. 124I-CLR1404 PET tumor contours were defined based on SUV threshold values of 1.2, 1.6, and 2.0, as well as tumor-to-background ratios (TBR) of 2 and 5, on the 48-hour post-injection PET/CT data set. In addition, the SUV 1.2 contour was “mirrored” and applied to the contralateral brain in order to derive a normal brain contour. An MRI-based contour of the superior sagittal sinus was applied to the PET image data sets to derive imaging-based blood kinetics. All PET contours were then used to extract the mean and maximum SUVs and TBR values of the volumes of interest (VOI) at all imaging time points.
Statistical analysis
Descriptive statistics were used for patient demographics and tumor types. The continuous variables of tumor to background ratio (TBR) and tumor to blood pool ratio (T:Blood Pool) were calculated at each time point and expressed as means with SDs.
Results
124I-CLR1404 PET/CT and MRI images were obtained on 12 patients (8 men and 4 women) with 13 previously diagnosed tumor types (5 high grade tumors, 6 low grade tumors, 1 metastatic tumor, and 1 meningioma). There were no adverse or clinically detectable pharmacological effects in any of the 12 patients. No adverse changes in vital signs or the baseline laboratory studies were observed.
Preliminary diagnosis prior to PET/CT imaging was based on available pathology data in 11/12 patients (12/13 tumors) and clinical and imaging data on 1/12 patients (1/1 tumors). Final diagnosis after PET/CT included 5 high grade tumors (including 2 tumors that progressed from a low grade to a high grade tumor), 2 low grade tumors, 1 meningioma, 4 pseudoprogression/treatment related changes (2 suspected high grade tumors, 1 suspected metastatic tumor, and 1 suspected low grade tumor versus treatment related change), and 1 indeterminate lesion (low grade tumor clinically presumed tumor recurrence with improved MRI findings coinciding with subsequent chemotherapy treatment). Final diagnosis was based on available clinical and imaging follow-up for 6 months (6 patients with 7 tumors) and histology results from surgery (6 patients with 6 tumors) (Table 2).
In normal brain, there was no significant uptake of 124I-CLR1404 at any time point. There was significant blood pool uptake above normal tissue background within the major venous sinuses which was highest at the 6 hour time point and slowly decreased on later time points. In tumors with 124I-CLR1404 uptake above background, uptake was lowest at the 6 hour time point and increased to the 24 hours time point. After 24 hours, tumor uptake tended to increase to 48 hours but at a slower rate. Visually, the 24- and 48-hour time points allowed easier identification of tumor uptake compared to 6 hour images.
124I-CLR1404 PET/CT images were visually assessed for tumor uptake above background, with attention to areas with enhancement and/or increased T2 signal on MRI and the adjacent MRI-negative borders. Nine of 13 tumors had visual uptake of 124I-CLR1404 over background including 1/1 newly diagnosed high grade tumor, 7/11 tumor recurrence versus treatment related changes (4/5 suspected high grade tumors, 2/5 suspected low grade tumors, and 1/1 suspected cerebral metastasis), and 1/1 meningioma (Figures 1, 2, 3, 4 and 5). When compared to final diagnoses, there was positive PET uptake in 5/5 high grade tumors, 1/2 low grade tumors, 1/1 meningioma, and 2/4 patients with treatment related changes (1 metastatic lesion (melanoma) and 1 high grade tumor (medulloblastoma)). There was no PET uptake in 1/2 low grade tumors, 1/1 indeterminate tumor (low grade tumor), and 2/4 treatment related changes (1 high grade tumor and 1 low grade tumor) (Table 2).
Figure 1.
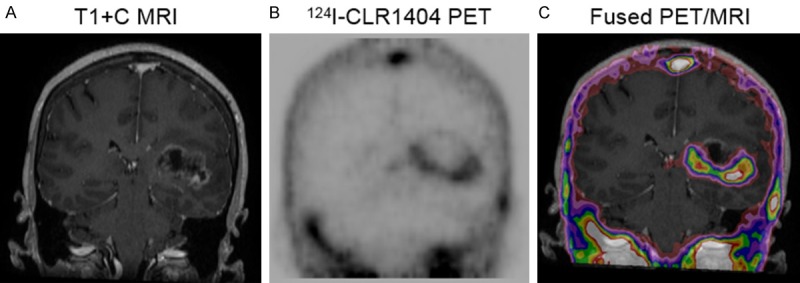
Patient with newly diagnosed high grade tumor (WHO Grade 4 glioma). A: T1 contrast-enhanced coronal MRI demonstrating heterogeneous tumor enhancement. B: Corresponding 124I-CLR1404 coronal PET with intense uptake laterally, inferiorly and medially, minimal uptake superiorly and no uptake centrally. C: Corresponding fused PET/MRI, with PET uptake extending slightly beyond MRI enhancement, except superiorly.
Figure 2.
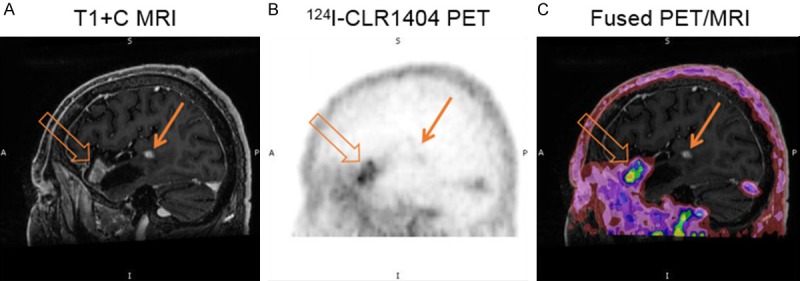
Patient with recurrence of a high grade tumor (WHO Grade 4 glioma) after prior treatments. A: Sagittal T1 contrast MRI demonstrates tumor enhancement adjacent to the anterior (open arrow) and posterior (closed arrow) margins of a resection cavity. B: 124I-CLR1404 sagittal PET demonstrates concordant uptake in the anterior enhancing tumor but no uptake in the posterior enhancing tumor. C: Fused PET/MRI image.
Figure 3.
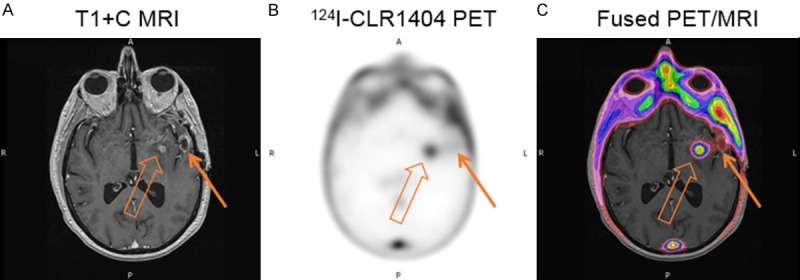
Patient with recurrence of a high grade tumor (WHO Grade 4 glioma). A: Axial T1 contrast MRI demonstrates 2 separate foci of tumor enhancement. B: 124I-CLR1404 axial PET demonstrates concordant uptake in the medial enhancing tumor (open arrow) but no significant uptake in the lateral enhancing tumor (closed arrow). C: Fused PET/MRI image further depicts these findings.
Figure 4.
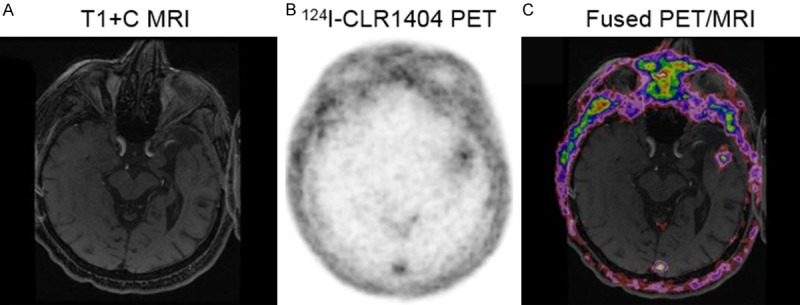
Patient with recurrent low grade tumor (WHO Grade 2 glioma). A: Axial T1 contrast MRI demonstrates a prior resection cavity in the anterior left temporal lobe but no enhancement. B: 124I-CLR1404 axial PET demonstrates mild uptake adjacent to the posterior resection cavity without enhancement on MRI. C: Fused PET/MRI image further depicts these findings.
Figure 5.
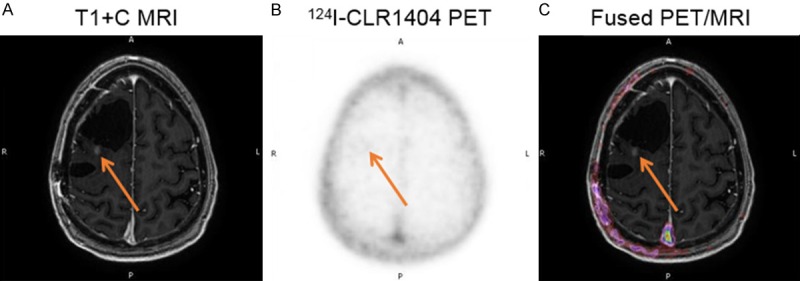
Patient with suspected recurrent high grade tumor (WHO Grade 3 glioma) that was subsequently proven to represent treatment related changes. A: Axial T1 contrast MRI demonstrates 2 prior resection cavities with a small enhancing nodule (closed arrow) posterior to the larger cavity and a larger area of low signal intensity on T1 and high signal intensity on T2 (not shown). B: 124I-CLR1404 axial PET demonstrates no uptake in neither the enhancing nodule nor the high T2 signal. C: Fused PET/MRI image further depicts these findings.
SUV maximum and mean values for tumors, normal cerebral background, and superior sagittal sinus blood pool were measured for all patients with visual 124I-CLR1404 uptake. Tumor to background and tumor to blood pool ratios were calculated and results provided for the 24- and 48-hour time points (Table 3). For all lesions with PET uptake, average (average ± standard deviation) tumor to background ratios (tumor SUVmax to background SUVmean) were 8.25 ± 4.16 (range 3.46 to 15.42) at 24 hours and 8.75 ± 3.60 (range 4.04 to 13.52) at 48 hours. For the 6 confirmed malignant tumors, the average tumor to background ratios were 9.32 ± 4.33 (range 3.46 to 15.42) at 24 hours and 10.04 ± 3.15 (range 5.17 to 13.17) at 48 hours. For the 2 lesions with PET uptake determined to represent treatment related change, the average tumor to background ratios were 5.05 ± 0.4 (range 4.76 to 5.33) at 24 hours and 4.88 ± 1.19 (range 4.04 to 5.72) at 48 hours (Table 3).
Table 3.
Tumor/lesion SUV measurements at 24 and 48 hours, tumor to background (TBR) ratios at 24 and 48 hours, and tumor to blood pool (T:Blood Pool) ratios at 24 and 48 hours
| Tumor Type | Patient no. | Lesion SUVmax 24 hrs | Lesion SUVmax 48 hr | TBR 24 hrs | TBR 48 hrs | T:Blood Pool 24 hrs | T:Blood Pool 48 hrs |
|---|---|---|---|---|---|---|---|
| High Grade | 1 | 1.719 | 1.828 | 7.21 | 9.12 | 1.18 | 1.42 |
| 2 | 5.334 | 5.225 | 13.05 | 13.17 | 3.67 | 3.75 | |
| 4 | 2.107 | 2.214 | 3.46 | 5.17 | 2.28 | 2.44 | |
| 9 | 3.666 | 2.931 | 9.35 | 8.45 | 2.34 | 2.25 | |
| 11 | 2.476 | 2.625 | 7.42 | 10.78 | 6.16 | 7.50 | |
| Low Grade | 3 | - | - | - | - | - | - |
| 6 | 4.816 | 3.629 | 15.42 | 13.52 | 4.27 | 3.34 | |
| Benign treatment related change | 5 | - | - | - | - | - | - |
| 7 | 1.502 | 1.486 | 5.33 | 5.72 | 0.79 | 0.96 | |
| 10 | 2.796 | 2.203 | 4.76 | 4.04 | 1.96 | 1.81 | |
| 12 | - | - | - | - | - | - | |
| Indeterminate | 8 | - | - | - | - | - | - |
TBR is calculated by using tumor/lesion SUVmax and dividing by contralateral normal brain background SUVmean value. T:Bood Pool is calculated by using tumor/lesion SUVmax and dividing by blood pool SUVmean from placing a region of interest in the posterior sagittal sinus near the confluence of sinuses.
For all lesions with PET uptake, average (average ± standard deviation) tumor to blood pool ratios (tumor SUVmax to superior sinus SUVmean) were 2.86 ± 1.77 (range 0.79 to 6.16) at 24 hours and 2.93 ± 2.07 (range 0.96 to 7.50) at 48 hours. For the 6 confirmed malignant tumors, the average tumor to blood pool ratios were 3.35 ± 1.75 (range 1.18 to 6.16) at 24 hours and 3.45 ± 2.15 (range 1.42 to 7.50) at 48 hours. For the 2 lesions with PET uptake determined to represent treatment related changes, the average tumor to blood pool ratios were 1.38 ± 0.82 (range 0.79 to 1.96) at 24 hours and 1.39 ± 0.60 (range 0.96 to 1.81) at 48 hours (Table 3).
Tumors with positive uptake on 124I-CLR1404 PET were compared to MRI to visually identify areas of concordant and discordant 124I-CLR1404 uptake and T1 gadolinium enhancement (Table 2). A total of 9 lesions had gadolinium enhancement on MRI and 89% (8/9) of these had PET uptake. In the 62% (8/13) of lesions with both T1 enhancement on MRI and uptake of 124I-CLR1404, 50% (4/8) had largely concordant 124I-CLR1404 uptake, 0% (0/8) had largely discordant 124I-CLR1404 uptake, and 50% (4/8) had areas of both concordant and discordant 124I-CLR1404 uptake when compared visually. Of interest, all 4 lesions with both concordant and discordant uptake were high grade tumors. Of the 4 lesions with largely concordant uptake, 2 were in lesions with treatment related change, 1 was in a meningioma, and 1 was in a small high grade tumor. One of 9 T1-enhancing lesions without PET uptake was in a patient with a suspected high grade tumor recurrence that was later diagnosed as treatment related change. One of 4 lesions without T1 enhancement on MRI had visual uptake of 124I-CLR1404 above background in a confirmed low grade glioma. All lesions (13/13) had increased T2 signal on MRI and all lesions with visual uptake of 124I-CLR1404 (9/13) had both concordant and discordant areas of 124I-uptake.
Discussion
Currently, MRI is considered the diagnostic standard in brain tumor imaging, though limitations in accurately identifying tumor margins before therapy and specificity in discriminating tumor recurrence from pseudoprogression/treatment-related changes remain. Prior molecular imaging studies of brain tumors with PET have demonstrated improvements in diagnostic accuracy and provided complimentary information compared to MRI [2-14]. A study by Pirotte et al even demonstrated improved survival in high grade gliomas can be attained when all metabolically active tumor is resected [5].
Despite potential improvements of various PET molecular imaging agents, there are also limitations with many PET studies. These include high normal cerebral background that complicates tumor detection and delineation with 18F-FDG, short physical half-life of 11C-MET that limits distribution, and relatively short half-lives of 18F-labeled amino acids and amino acid analogues that would limit distribution to only nearby regional sites.
In this first-in-man pilot study of 124I-CLR1404 in primary and metastatic brain tumors, we demonstrated avid APC uptake in a variety of tumor types. Positive tumor uptake is significantly above the very low background of normal cerebral tissue, facilitating identification of metabolically active tumor borders. There is increasing tumor uptake and decreasing cerebral background and blood pool activity at later time points, thus it may be acceptable in future studies to use only the later time points for image acquisition.
The high tumor to background ratios of 124I-CLR1404 in malignant brain tumors at time of highest tumor uptake, TBR of 10.04 ± 3.15 at 48 hours, compare favorably with other PET molecular imaging agents at time of highest tumor uptake commonly used in malignant brain tumor imaging such as 11C-MET, TBR of 2.28-2.96 [10,14], 18F-FET, TBR of 4.3 [12], 18F-DOPA, TBR of 3.6 [12], and 18F-FDG, TBR of 1.02 [10]. This high tumor-to-normal-background uptake may also allow for better visual demarcation of tumor borders and comparison with MRI images. In this study, we used this visual analysis to qualitatively and quantitatively identify tumor borders and assess for concordance and discordance with areas of MRI enhancement and T2 signal change. Of note, comparison with T2 signal was only qualitative as we could not identify a practical and reproducible method to quantitate T2 signal that was felt to be clinically relevant. Although this study was not designed to compare accuracy of PET versus MRI in delineating tumor margins, this can be explored in the future to determine if the additional 124I-CLR1404 PET information can improve diagnostic accuracy compared to MRI and/or provide an additional information for therapeutic benefit.
124I-CLR1404 uptake was detected in all 5 of 5 high grade tumors and 1 of 2 low grade tumors. There was no uptake in 2 of 4 lesions due to pseudoprogression/treatment related changes and in 1 of 2 low grade tumors. Although the small number of patients in this study with a wide variety of tumor types does not support statistically significant conclusions regarding sensitivity and specificity, these findings suggest that 124I-CLR1404 uptake or lack of uptake may have clinical significance.
The main purpose of this study was to demonstrate the first successful human use of 124I-CLR1404 in brain tumors and to develop a methodology to use in the analysis of larger trials. To that end, we have demonstrated visual uptake in multiple tumor types that can be both qualitatively and quantitatively analyzed. Additionally, we have demonstrated that 124I-CLR1404 compares favorably with other PET molecular imaging agents and possibly provides different imaging information compared to MRI, which can be further evaluated to determine clinical relevance.
The main limitation of this study was the small population of patients with a heterogeneous mix of tumor types. Further compounding this limitation was the use of patients in a clinically complicated post-treatment setting for which current comparison imaging techniques are often inaccurate or incomplete. These limitations do not allow for a meaningful clinical correlation of 124I-CLR1404 tumor uptake and MRI. In the future, a larger patient cohort with a more homogenous tumor type will be analyzed in an ongoing clinical trial to provide clinical correlation to the 124I-CLR1404 PET imaging findings.
Finally, in addition to the PET imaging information being presented in this study, a parallel evaluation is being performed on this data to calculate individualized tumor dosimetry in patients with 124I-CLR1404 PET avid malignant tumors. It is hoped that using a nearly identical PET surrogate biomarker for targeted radiotherapy with 125I- or 131I-CLR1404 can appropriately identify patients a priori who may benefit from a diapeutic personalized approach to radiotherapy.
Conclusion
124I-CLR1404 PET imaging demonstrated avid tumor uptake in a variety of brain tumors with high tumor-to-background ratios which will allow for easier tumor identification and comparison with MRI. There were both regions of concordance and discordance with MRI, which could potentially prove to be clinically relevant. Some patients with suspected tumor recurrence versus pseudoprogression/treatment change on MRI did not have avid 124I-CLR1404 uptake, which may have important clinical implications. Finally, the “diapeutic” potential of CLR1404 may allow patients with 124I-CLR1404 avid tumors to have personalized tumor dosimetry studies performed for possible targeted radiotherapy with 131I- or 125I-CLR1404.
Acknowledgements
Financial support: UL1TR000427, P30CA014520, and R01CA158800.
Disclosure of conflict of interest
None.
References
- 1.Kruser T, Mehta M, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother. 2013;13:389–403. doi: 10.1586/ern.13.7. [DOI] [PubMed] [Google Scholar]
- 2.Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Geist C, Dahlbom M, Silverman DH, Satyamurthy N, Phelps ME, Chen W. 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med. 2012;53:29–36. doi: 10.2967/jnumed.111.092387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirotte BJ, Lubansu A, Massager N, Wikler D, Van Bogaert P, Levivier M, Brotchi J, Goldman S. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5:486–99. doi: 10.3171/2010.1.PEDS09481. [DOI] [PubMed] [Google Scholar]
- 4.Goldman S, Pirotte BJ. Brain tumors. Methods Mol Biol. 2011;727:291–315. doi: 10.1007/978-1-61779-062-1_16. [DOI] [PubMed] [Google Scholar]
- 5.Pirotte BJ, Levivier M, Goldman S, Massager N, Wikler D, Dewitte O, Bruneau M, Rorive S, David P, Brotchi J. Positron emission tomographyguided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery. 2009;64:471–81. doi: 10.1227/01.NEU.0000338949.94496.85. [DOI] [PubMed] [Google Scholar]
- 6.Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56:173–90. [PubMed] [Google Scholar]
- 7.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougère C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabria F, Cascini GL. Current status of 18FDOPA PET imaging in the detection of brain tumor recurrence. Hell J Nucl Med. 2015;18:152–6. doi: 10.1967/s0024499100211. [DOI] [PubMed] [Google Scholar]
- 9.Wray R, Solnes L, Mena E, Meoded A, Subramaniam RM. (18)F-Flourodeoxy-glucose PET/computed tomography in brain tumors: value to patient management and survival outcomes. PET Clin. 2015;10:423–30. doi: 10.1016/j.cpet.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Tomura N, Mizuno Y, Saginoya T. PET/CT findings for tumors in the base of the skull: comparison of 18 F-FDG with 11 C-methionine. Acta Radiol. 2016;57:325–32. doi: 10.1177/0284185115575342. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Yu Y, Zhang H, Xu G, Chen L. A meta-analysis comparing 18F-FLT PET with 18F-FDG PET for assessment of brain tumor recurrence. Nucl Med Commun. 2015;36:695–701. doi: 10.1097/MNM.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 12.Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C, Czernin J, Kessler AF, Homola GA, Ernestus RI, Löhr M, Herrmann K. Comparison of the amino acid tracers 18FFET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55:1611–6. doi: 10.2967/jnumed.114.140608. [DOI] [PubMed] [Google Scholar]
- 13.Lizarraga KJ, Allen-Auerbach M, Czernin J, DeSalles AA, Yong WH, Phelps ME, Chen W. 18F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55:30–36. doi: 10.2967/jnumed.113.121418. [DOI] [PubMed] [Google Scholar]
- 14.Filss CP, Galldiks N, Stoffels G, Sabel M, Wittsack HJ, Turowski B, Antoch G, Zhang K, Fink GR, Coenen HH, Shah NJ, Herzog H, Langen KJ. Comparison of 18F-FET PET and perfusionweighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med. 2014;55:540–5. doi: 10.2967/jnumed.113.129007. [DOI] [PubMed] [Google Scholar]
- 15.Pinchuck AN, Rampy MA, Longino MA, Skinner RW, Gross MD, Weichert JP, Counsell RE. Synthesis and structure activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogs. J Med Chem. 2006;49:2155–2165. doi: 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]
- 16.Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA, Pinchuk AN, Farhoud M, Swanson KI, Floberg JM, Grudzinski J, Titz B, Traynor AM, Chen HE, Hall LT, Pazoles CJ, Pickhardt PJ, Kuo JS. Alkylphosphocholine analogs for broad spectrum cancer imaging and therapy. Sci Transl Med. 2014;6:1–10. doi: 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson KI, Clark PA, Zhang RR, Kandela IK, Farhoud M, Weichert JP, Kuo JS. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery. 2015;76:115–23. doi: 10.1227/NEU.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang RR, Swanson KI, Hall LT, Weichert JP, Kuo JS. Diapeutic cancer-targeting alkylphosphocholine analogs may advance management of brain malignancies. CNS Oncol. 2016;5:223–31. doi: 10.2217/cns-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark PA, Al-Ahmad AJ, Qian T, Zhang RR, Wilson HK, Weichert JP, Palecek SP, Kuo JS, Shusta EV. Analysis of cancer-targeting alkylphosphocholine analogue permeability characteristics using a human induced pluripotent stem cell blood-brain barrier model. Mol Pharm. 2016;13:3341–9. doi: 10.1021/acs.molpharmaceut.6b00441. [DOI] [PMC free article] [PubMed] [Google Scholar]


