Abstract
89Zr-panitumumab is a novel immuno-PET radiotracer. A fully humanized IgG2 antibody, panitumumab binds with high affinity to the extracellular ligand binding domain of EGFR. Immuno-PET with radiolabeled panitumumab is a non-invasive method that could characterize EGFR expression in tumors and metastatic lesions. It might also assist in selecting patients likely to benefit from targeted therapy as well as monitor response and drug biodistribution for dosing guidance. Our objective was to calculate the maximum dosing for effective imaging with minimal radiation exposure in a small subset. Three patients with metastatic colon cancer were injected with approximately 1 mCi (37 MBq) of 89Zr-panitumumab IV. Whole body static images were then obtained at 2-6 hours, 1-3 days and 5-7 days post injection. Whole organ contours were applied to the liver, kidneys, spleen, stomach, lungs, bone, gut, heart, bladder and psoas muscle. From these contours, time activity curves were derived and used to calculate mean resident times which were used as input into OLINDA 1.1 software for dosimetry estimates. The whole body effective dose was estimated between 0.264 mSv/MBq (0.97 rem/mCi) and 0.330 mSv/MBq (1.22 rem/mCi). The organ which had the highest dose was the liver which OLINDA estimated between 1.9 mGy/MBq (7.2 rad/mCi) and 2.5 mGy/MBq (9 rad/mCi). The effective dose is within range of extrapolated estimates from mice studies. 89Zr-panitumumab appears safe and dosimetry estimates are reasonable for clinical imaging.
Keywords: 89Zr-panitumumab, human dosimetry, PET
Introduction
The ability to detect malignancy with high specificity is an area of active research in molecular imaging. Developing imaging probes that specifically attach to unique characteristics found on cancer cells is an ongoing pursuit that could help identify lesions earlier and possibly indicate the most efficacious treatments before the tumor spreads. Monoclonal antibodies (MAbs) have been a popular means of targeting cell surface antigens for therapeutic interventions and diagnostic imaging [1]. MAbs have high affinity for their targets and many have been developed as therapies for human patients. A disadvantage is that their long serum clearance times necessitate protracted imaging spanning multiple days to clear background radioactivity.
Panitumumab is a completely humanized MAb that binds to the epidermal growth factor receptor (EGFR, HER1) and is FDA approved for use in receptor expressing colorectal cancers without KRAS mutations. Many studies have looked at the EGFR pathway and describe potential opportunities for disrupting tumor growth and development by blocking the EGF receptor [2,3]. Abnormal levels of EGFR/HER1 have also been found in other epithelial malignancies, such as lung, head and neck, prostate, breast, glioma, pancreatic and ovarian cancers, and these may also benefit from this targeted approach [4].
89Zr is a radiotracer with favorable attributes for antibody imaging. The radionuclide’s half-life of 78.4 hours is long enough to match the pharmacokinetics of antibody biodistribution. It has good in vivo stability and with a low positron energy of 395 keV, thus PET image resolution is satisfactory. A fully humanized IgG2 antibody, panitumumab binds with high affinity to the extracellular ligand binding domain of EGFR. Immuno-PET imaging with radiolabeled panitumumab is a non-invasive method that could characterize EGFR expression in tumors and metastatic lesions, assist in selecting patients likely to benefit from targeted therapy, monitor responses and establish MAb biodistribution for dosing guidance. As part of a larger study examining treatment effects of sorafenib and cetuximab in metastatic colorectal cancer (NCT00326495), imaging with 89Zr-panitumumab was performed as a correlative for determining tumor distribution of EGFR [5]. However, the dosimetry for 89Zr-panitumumab has been under-studied and here we calculate the maximum dosing with minimal radiation exposure required for effective PET imaging.
Methods
Patient selection
This is a HIPAA compliant, prospective, single institution study, approved by the local institutional review board (IRB) with written informed consent. Patients enrolled on the “Phase II study of Sorafenib in Combination with Cetuximab in EGFR expressing Metastatic Colorectal Cancer” had the option to participate in imaging with 89Zr-panitumumab prior to treatment. Three patients with metastatic colorectal cancer enrolled in the study.
PET/CT imaging
Scanning was performed on a 3D time of flight (TOF) mode Philips Gemini TF PET/CT camera (Philips, Cleveland OH) with an 18 cm coronal and a 57 cm axial Field of View (FOV). Data were reconstructed with an ordered subset expectation maximization (OSEM) algorithm using 3 iterations and 33 subsets. The scanner uses CT based attenuation correction; along with random, normalization, dead time and a model-based scatter correction for anatomical correlation and attenuation correction purposes [6].
The PET/CT imaging design of the clinical trial included a total of three PET/CT scans for each injected dose. Based on preliminary mouse models [7], the dose of 89Zr-panitumumab was set at 37 MBq (1 mCi). Whole body static images were obtained from the base of the skull to the mid-thigh for all scans. The first scan was scheduled at 2-6 hours post injection of the 89Zr panitumumab, the second at 1 to 3 days post injection and the third and final at 5 to 7 days post injection. Three subjects participated in this study, of whom only two underwent the full imaging schedule of three scans. The third subject only underwent the first two PET/CT scans due to scheduling constraints.
89Zr-panitumumab was manufactured as described by Wei et al. [8]. See Figure 4 (https://imaging.cancer.gov/programs_resources/cancertracer-synthesis-resources/Zr-Pan_documentation.htm) and Table 3. PET/CT scans were reviewed using MIM software (version 6.0, MIM Software Inc., Cleveland, OH).
Figure 4.
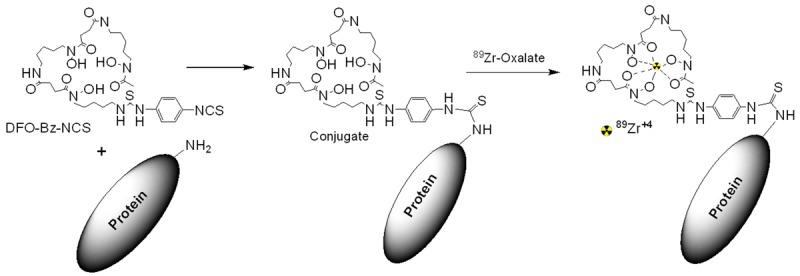
Schematic of the Radiosynthesis of 89Zr-panitumumab.
Table 3.
89Zr-panitumumab Final Product Specifications
| Test | Specification |
|---|---|
| Chemical Purity (particulates) | Clear and Colorless |
| pH | 6-8 |
| Gentistic Acid | ≤5 mg per dose |
| Chemical Purity (HPLC) | Protein <1 mg per injected dose |
| Radiochemical Purity (HPLC) | >95% |
| Radiochemical Purity (TLC) | Rf <0.5, Purity >90% |
| Radionuclidic Purity | Measured half-life 78 hours |
| Residual Solvent Levels | None |
| Bacterial Endotoxin Levels | <175 EU per dose |
| Sterility | No growth observed in 14 days; must also pass filter integrity test prior to injection |
Time activity curves and dosimetry calculation
Volumes of Interest (VOIs) were drawn for each organ on all scans for each patient and included whole body, liver, kidneys, spleen, stomach, lungs, bone, gut, heart, bladder, and psoas regions. Total activity in each organ was recorded in units of Becquerel (Bq). The whole organ was contoured so that the total activity in the organ of interest was measured. For the whole body, a region was drawn using the accompanying CT image and using MIM software’s human subject outline auto VOI generation then transposing this VOI to the PET image.
To estimate the activity in the upper large intestines (ULI), lower large intestines (LLI) and small intestines (SI), from the total activity measured in the gut VOI, a gut ratio based on the weights of the three intestinal types was used.
AULI = AgutWULI/WULI+LLI+SI
ALLI = AgutWLLI/WULI+LLI+SI
ASI = AgutWSI/WULI+LLI+SI
Aχ is the activity for an intestinal region and Wχ is the intestinal region’s weight. For this analysis, the weight of the ULI was taken to be 2.0 Kg, LLI to be 1.6 Kg and SI to be 6.0 Kg.
With the total activity recorded for each organ, and the date and time of each scan, the organ time activity curve was constructed. Once the time activity curves were generated, the total activity in each organ was converted into percent injected dose. Finally, the resident time for each organ was calculated by integrating the percent injected dose time activity curve for each respective organ. For this analysis, the integral between the first and last scan was calculated using a trapezoidal interpolation between data points. An exponential tail estimate was added assuming there was no further biological washout and the 89Zr was trapped in the organ and only underwent radioactive decay. The software package OLINDA 1.1 (Vanderbilt University, Nashville NT) was used to estimate the dosimetry.
Because of the small number of subjects, the dosimetry was analyzed using two different sets of organ residence times. The first set of residence times was determined from the mean residence time for each organ of the two subjects who underwent three scans. These two subjects, having three scans each, provided the best estimate for measuring the activity out to 7 days in each organ. To include the data from the third subject, who was scanned only twice, organ residence times were computed using only the first two scans for all three subjects. In this case, the tail estimation of the integral of the time activity curves starts at day 1 or 2 (depending on the subject) and thus most likely overestimates the residence time. With the two sets of residence times estimated, one using two subjects and all three scans, and the second using all three subjects but only the first two scans, two different series of dose estimates were calculated.
Results
Time activity curves can be viewed in Figure 1 and are plotted in units of Bq and percent injected dose. The measured mean residence times for each organ are plotted as bar charts in Figure 2. The top bar chart shows the mean residence times for the two subjects who had three scans and all three data points in the time activity curve (TAC) were used to measure the mean residence times for each organ. The bottom bar chart shows the mean residence times for all three subjects, but only the first two scans were used to generate a TAC. From these two-scan TACs, the mean residence times were calculated.
Figure 1.
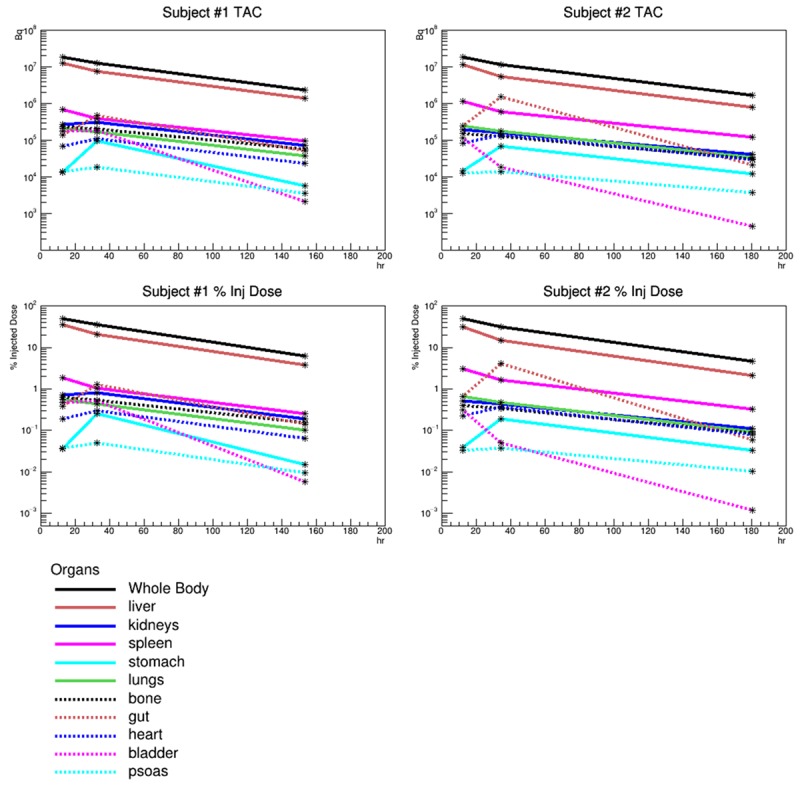
The time activity curves (TAC) for each region contoured on the PET images are plotted in the above figures. The top row is the TAC scaled to total activity in Bq. The bottom row of figures show the TAC scaled to percent injected dose. The left column shows the data for Subject #1 and the right column for Subject #2. Subject #3 was omitted since only two scans were performed.
Figure 2.
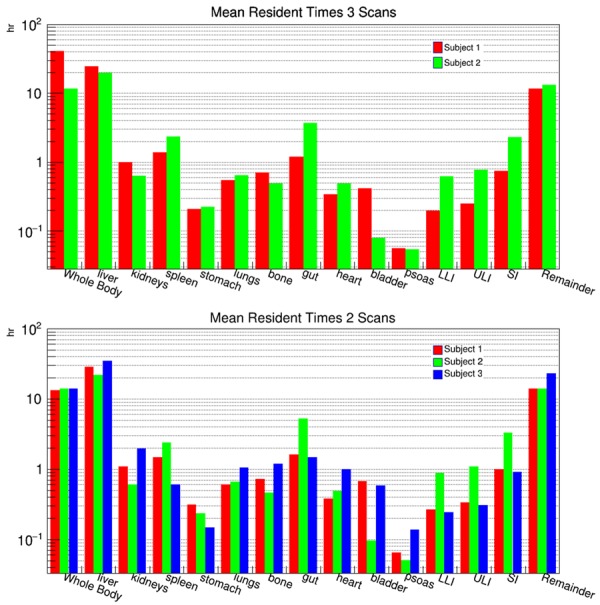
Bar chart plots of the measured mean resident times for each of the regions contoured on the PET images. The top bar chart plots the mean resident times for the two subjects who were scanned three times and all three scan times were used to generate the time activity curves which were then integrated to extract the mean resident times. The bottom bar chart is the mean resident times for the same regions but only using the first two scans to generate the time activity curves.
The result from OLINDA 1.1 are shown in Tables 1 and 2. Table 1 includes the dose estimates using only the first two subjects who completed all three scans. Table 2 includes the dose estimates from all three subjects but only the first two scans were used to measure the mean residence time. Estimating the doses by the two different methods helps account for the systematic errors introduced by having such a small sample of subjects. The whole body effective dose was estimated between 0.264 mSv/MBq (0.97 rem/mCi) and 0.330 mSv/MBq (1.22 rem/mCi). The organ which had the highest dose was the liver which OLINDA estimated between 1.9 mGy/MBq (7.2 rad/mCi) and 2.5 mGy/MBq (9 rad/mCi).
Table 1.
Results from OLINDA 1.1 estimating the dose using the residence times for the two subjects who were scanned three times
| Target Organ | Alpha | Beta | Photon | Total | EDE Cont. | ED Cont. |
|---|---|---|---|---|---|---|
| Adrenals | 0.00E000 | 9.13E-03 | 3.68E-01 | 3.77E-01 | 2.26E-02 | 1.88E-03 |
| Brain | 0.00E000 | 9.13E-03 | 3.77E-02 | 4.68E-02 | 0.00E000 | 2.34E-04 |
| Breasts | 0.00E000 | 9.13E-03 | 9.44E-02 | 1.04E-01 | 1.55E-02 | 5.18E-03 |
| Gallbladder Wall | 0.00E000 | 9.13E-03 | 6.06E-01 | 6.15E-01 | 3.69E-02 | 0.00E000 |
| LLI Wall | 0.00E000 | 8.63E-02 | 1.46E-01 | 2.32E-01 | 0.00E000 | 2.79E-02 |
| Small Intestine | 0.00E000 | 1.07E-01 | 2.18E-01 | 3.25E-01 | 0.00E000 | 1.62E-03 |
| Stomach Wall | 0.00E000 | 3.19E-02 | 2.25E-01 | 2.57E-01 | 0.00E000 | 3.08E-02 |
| ULI Wall | 0.00E000 | 6.83E-02 | 2.91E-01 | 3.59E-01 | 0.00E000 | 1.80E-03 |
| Heart Wall | 0.00E000 | 7.20E-02 | 2.60E-01 | 3.32E-01 | 0.00E000 | 0.00E000 |
| Kidneys | 0.00E000 | 1.46E-01 | 4.04E-01 | 5.50E-01 | 3.30E-02 | 2.75E-03 |
| Liver | 0.00E000 | 6.27E-01 | 1.31E000 | 1.94E000 | 1.16E-01 | 9.69E-02 |
| Lungs | 0.00E000 | 3.20E-02 | 1.93E-01 | 2.25E-01 | 2.70E-02 | 2.70E-02 |
| Muscle | 0.00E000 | 9.13E-03 | 1.10E-01 | 1.19E-01 | 0.00E000 | 5.97E-04 |
| Ovaries | 0.00E000 | 9.13E-03 | 1.51E-01 | 1.60E-01 | 3.99E-02 | 3.19E-02 |
| Pancreas | 0.00E000 | 9.13E-03 | 3.62E-01 | 3.71E-01 | 0.00E000 | 1.86E-03 |
| Red Marrow | 0.00E000 | 6.67E-03 | 1.30E-01 | 1.37E-01 | 1.64E-02 | 1.64E-02 |
| Osteogenic Cells | 0.00E000 | 2.33E-02 | 1.03E-01 | 1.26E-01 | 3.79E-03 | 1.26E-03 |
| Skin | 0.00E000 | 9.13E-03 | 6.67E-02 | 7.58E-02 | 0.00E000 | 7.58E-04 |
| Spleen | 0.00E000 | 5.44E-01 | 6.71E-01 | 1.21E000 | 7.29E-02 | 6.07E-03 |
| Testes | 0.00E000 | 9.13E-03 | 5.00E-02 | 5.91E-02 | 0.00E000 | 0.00E000 |
| Thymus | 0.00E000 | 9.13E-03 | 1.03E-01 | 1.12E-01 | 0.00E000 | 5.59E-04 |
| Thyroid | 0.00E000 | 9.13E-03 | 5.66E-02 | 6.58E-02 | 1.97E-03 | 3.29E-03 |
| Urinary Bladder Wall | 0.00E000 | 9.13E-03 | 8.49E-02 | 9.40E-02 | 0.00E000 | 4.70E-03 |
| Uterus | 0.00E000 | 9.13E-03 | 1.29E-01 | 1.38E-01 | 0.00E000 | 6.88E-04 |
| Total Body | 0.00E000 | 2.94E-02 | 1.44E-01 | 1.73E-01 | 0.00E000 | 0.00E000 |
| Effective Dose Equivalent (mSv/MBq) | 3.86E-01 | |||||
| Effective Dose (mSv/MBq) | 2.64E-01 | |||||
Table 2.
Results from OLINDA 1.1 estimating the dose using all three subjects but only the first two scans to generate the TAC from which average residence times were calculated
| Target Organ | Alpha | Beta | Photon | Total | EDE Cont. | ED Cont. |
|---|---|---|---|---|---|---|
| Adrenals | 0.00E000 | 1.24E-02 | 4.67E-01 | 4.79E-01 | 2.87E-02 | 2.40E-03 |
| Brain | 0.00E000 | 1.24E-02 | 5.11E-02 | 6.35E-02 | 0.00E000 | 3.18E-04 |
| Breasts | 0.00E000 | 1.24E-02 | 1.22E-01 | 1.35E-01 | 2.02E-02 | 6.75E-03 |
| Gallbladder Wall | 0.00E000 | 1.24E-02 | 7.74E-01 | 7.86E-01 | 4.72E-02 | 0.00E000 |
| LLI Wall | 0.00E000 | 9.90E-02 | 1.78E-01 | 2.77E-01 | 0.00E000 | 3.32E-02 |
| Small Intestine | 0.00E000 | 1.23E-01 | 2.69E-01 | 3.92E-01 | 0.00E000 | 1.96E-03 |
| Stomach Wall | 0.00E000 | 3.62E-02 | 2.67E-01 | 3.04E-01 | 0.00E000 | 3.64E-02 |
| ULI Wall | 0.00E000 | 7.98E-02 | 3.58E-01 | 4.38E-01 | 0.00E000 | 2.19E-03 |
| Heart Wall | 0.00E000 | 1.06E-01 | 3.43E-01 | 4.49E-01 | 0.00E000 | 0.00E000 |
| Kidneys | 0.00E000 | 2.18E-01 | 5.30E-01 | 7.49E-01 | 4.49E-02 | 3.74E-03 |
| Liver | 0.00E000 | 8.02E-01 | 1.68E000 | 2.48E000 | 1.49E-01 | 1.24E-01 |
| Lungs | 0.00E000 | 4.17E-02 | 2.47E-01 | 2.89E-01 | 3.47E-02 | 3.47E-02 |
| Muscle | 0.00E000 | 1.24E-02 | 1.41E-01 | 1.53E-01 | 0.00E000 | 7.67E-04 |
| Ovaries | 0.00E000 | 1.24E-02 | 1.87E-01 | 1.99E-01 | 4.99E-02 | 3.99E-02 |
| Pancreas | 0.00E000 | 1.24E-02 | 4.37E-01 | 4.49E-01 | 0.00E000 | 2.25E-03 |
| Red Marrow | 0.00E000 | 9.09E-03 | 1.66E-01 | 1.76E-01 | 2.11E-02 | 2.11E-02 |
| Osteogenic Cells | 0.00E000 | 3.18E-02 | 1.34E-01 | 1.66E-01 | 4.97E-03 | 1.66E-03 |
| Skin | 0.00E000 | 1.24E-02 | 8.62E-02 | 9.87E-02 | 0.00E000 | 9.87E-04 |
| Spleen | 0.00E000 | 4.36E-01 | 5.97E-01 | 1.03E000 | 6.20E-02 | 5.16E-03 |
| Testes | 0.00E000 | 1.24E-02 | 6.66E-02 | 7.90E-02 | 0.00E000 | 0.00E000 |
| Thymus | 0.00E000 | 1.24E-02 | 1.36E-01 | 1.48E-01 | 0.00E000 | 7.40E-04 |
| Thyroid | 0.00E000 | 1.24E-02 | 7.59E-02 | 8.84E-02 | 2.65E-03 | 4.42E-03 |
| Urinary Bladder Wall | 0.00E000 | 1.24E-02 | 1.10E-01 | 1.23E-01 | 0.00E000 | 6.13E-03 |
| Uterus | 0.00E000 | 1.24E-02 | 1.62E-01 | 1.74E-01 | 0.00E000 | 8.71E-04 |
| Total Body | 0.00E000 | 3.77E-02 | 1.83E-01 | 2.21E-01 | 0.00E000 | 0.00E000 |
| Effective Dose Equivalent (mSv/MBq) | 4.65E-01 | |||||
| Effective Dose (mSv/MBq) | 3.30E-01 | |||||
Imaging of the 3 patients failed to demonstrate significant radiotracer accumulation in tumors for all timepoints. All patients had tumors in the liver as well as abdominal lymphadenopathy for the second patient and hilar lymphadenopathy and lung nodules for the third patient. The first patient only had liver metastases. Focal uptake was noted in the lungs at the initial 2-6 hour image in each patient and resolved on subsequent scans (Figure 3). No anatomic correlate was seen on fusion CT to suggest pathologic activity. This likely represented microaggregates of panitumumab, which were entirely asymptomatic and no adverse events were reported.
Figure 3.
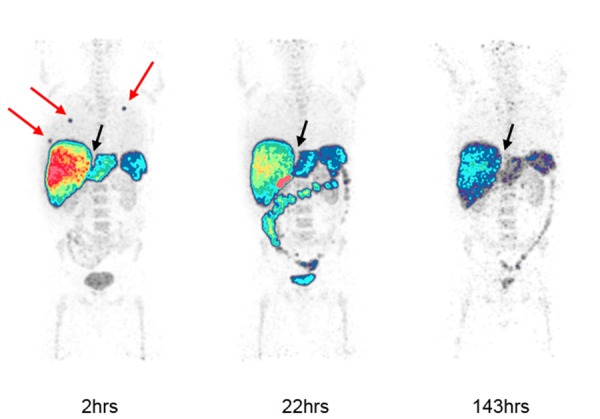
89Zr-panitumumab PET imaging of a patient with metastatic colorectal cancer. Maximum Intensity Projection (MIP) images are shown at 2, 22 and 143 hours post-radiotracer injection. Focal radiotracer accumulation in the lungs noted at 2-hour imaging (red arrows) resolved on subsequent scans. Liver metastases appear photopenic (black arrow). Physiologic activity is noted in the liver, spleen, bowels and bladder.
Discussion
Panitumumab is one of only a few MAbs developed to target EGFR and approved by the FDA for cancer therapy. By binding to the external domain of EGFR, it competitively blocks activation of the receptor and suppresses downstream signaling needed for cancer cell survival [9]. Unfortunately, efficacy has been modest for patients with metastatic colorectal carcinoma even when combined with chemotherapy, but the agent appears more helpful in wild-type KRAS tumors [10]. Panitumumab’s usefulness as treatment has also been explored in other tumors such as in breast cancer and head and neck cancer with similarly limited results [11,12]. Coupled to a radiotracer, panitumumab may find additional application as a marker for EGFR expressing tumors and monitoring related targeted therapies.
With its long half-life of 78.4 hours, 89Zr has favorable characteristics for imaging MAbs and therefore is a good match for panitumumab. This is the first-in-human application of 89Zr-panitumumab as part of an exploratory correlative for a treatment trial of metastatic colorectal cancer [5]. During synthesis, a desferrioxamine derivative (DFO) was conjugated to the MAb and the DFO-MAb conjugate was incubated with 89Zr. The resulting agent, 89Zr-panitumumab, was used in this study but since DFO is not a perfect chelate for 89Zr, a moderate amount of bone uptake was expected.
The whole body effective dose in the present study was estimated between 0.264 mSv/MBq and 0.330 mSv/MBq. Compared to a similar study by Bhattacharyya et al. on 89Zr-panitumumab in HER1 expressing tumors, the whole body effective dose for humans was estimated based on athymic nude mice to be between 0.578 mSv/MBq and 0.769 mSv/MBq [7]. The present study’s whole body effective dose was about a factor of 1.9-2.2 lower than those calculations. Bhattacharyya et al. also estimated dosimetry for 111In-panitumumab and found the whole body effective dose for humans based on athymic nude mice was estimated to be between 0.183 mSv/MBq and 0.246 mSv/MBq; about a factor of 1.4-1.6 smaller than the present study. In a comprehensive mouse-to-human radionuclide comparison study, radiation dosimetry of five 11C-labeled and one 18F-labeled radiotracers in human subjects were compared to murine biodistribution studies [13]. Effective dose estimates from whole body PET in both humans and mice were all within a factor of 2 of each other for all six radiotracers. Thus, the results from the present study are like previously published mouse and human estimated models.
The study is limited by the small patient population which in turn reduced the number of scans acquired. This resulted in a small number of data points. However, only 3-5 patients are typically needed to establish appropriate dosimetry in humans. Additionally, the present study focused on patients with metastatic colon cancer while Bhattacharyya et al. focused on breast cancer xenografts expressing HER1. Furthermore, scaling differences between mice and human models could account for some discrepancies-a factor of 2 is considered acceptable for combined uncertainties in any given radiopharmaceutical dose estimate [7,13,14].
Nontarget binding of MAbs is often a concern that researchers resolve with the addition of cold MAb to saturate non-target-specific binding sites. Previous work with 89Zr-panitumumab in preclinical models demonstrated that addition of cold MAb effectively blocked radiotracer tumor uptake, therefore, we did not add any unlabeled compound [15]. However, a small amount of cold MAb may be necessary to optimize the radiotracer’s ability to identify tumors that overexpress EGFR. This may explain the lack of visibly significant focal uptake of 89Zr-panitumumab in the known metastatic lesions for the 3 patients. Due to physiologic hepatic excretion, uptake in liver lesions was also obscured by high background activity and appeared photopenic. Receptor status could not be verified for every lesion and hence could account for the poor tumor localization if EGFR over-expression was not robust.
In addition, focal radiotracer uptake in the lungs was noted on scans within hours of injection with spontaneous resolution on subsequent images. Thromboembolic events are rare risks that may be associated with therapeutic panitumumab and could be related to possible infusion reactions or other immunogenicity [16]. Microdoses used for tracer imaging typically have negligible adverse effects but this evidence for transient microemboli suggests that a filtered dosing needle may be beneficial during radiotracer injection.
In summary, 89Zr-panitumumab dosimetry was lower than predicted by preclinical trials but still within the scope of previously published extrapolated values [7]. Animal models play an important role in translational medicine, especially murine models as they reasonably correlate to the human body and provide a framework for clinical investigation. Though many mouse model estimates for human dosimetry have been performed, this is the first human study of 89Zr-panitumumab dosimetry. In comparison to other PET agents such as 18F-FDG which has an effective dose of 0.019 MSv/MBq, 89Zr-panitumumab results in higher radiation exposure, consequently, judicious dosing is recommended. 89Zr-panitumumab may be useful as an imaging tool for assessing EGFR over-expression or receptor response to therapies. Further studies in a larger population are necessary to define this radiotracer’s role in EGFR-expressing colorectal cancer.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors wish to thank Dr Gary Griffiths and Dr G. Craig Hill for assistance in editing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Chiavenna SM, Jaworski JP, Vendrell A. State of the art in anti-cancer mAbs. J Biomed Sci. 2017;24:15. doi: 10.1186/s12929-016-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arteaga C. Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol. 2003;30:3–14. [PubMed] [Google Scholar]
- 3.Vallbohmer D, Lenz HJ. Epidermal growth factor receptor as a target for chemotherapy. Clin Colorectal Cancer. 2005;5(Suppl 1):S19–27. [PubMed] [Google Scholar]
- 4.Sasada T, Azuma K, Ohtake J, Fujimoto Y. Immune responses to epidermal growth factor receptor (EGFR) and their application for cancer treatment. Front Pharmacol. 2016;7:405. doi: 10.3389/fphar.2016.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do K, Cao L, Kang Z, Turkbey B, Lindenberg ML, Larkins E, Holkova B, Steinberg SM, Raffeld M, Peer CJ, Figg WD, Eugeni M, Jacobs P, Choyke P, Wright JJ, Doroshow JH, Kummar S. A phase II study of sorafenib combined with cetuximab in EGFR-expressing, KRAS-mutated metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14:154–161. doi: 10.1016/j.clcc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of philips gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med. 2007;48:471–480. [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Kurdziel K, Wei L, Riffle L, Kaur G, Hill GC, Jacobs PM, Tatum JL, Doroshow JH, Kalen JD. Zirconium-89 labeled panitumumab: a potential immuno-PET probe for HER1-expressing carcinomas. Nucl Med Biol. 2013;40:451–457. doi: 10.1016/j.nucmedbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei L, Shi J, Afari G, Bhattacharyya S. Preparation of clinical-grade 89Zr-panitumumab as a positron emission tomography biomarker for evaluating epidermal growth factor receptortargeted therapy. J Lab Comp Radiopharm. 2014;57:25–35. doi: 10.1002/jlcr.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59:1236–1243. [PubMed] [Google Scholar]
- 10.Chen Q, Cheng M, Wang Z, Zhao S. The efficacy and safety of panitumumab plus irrinotecanbased chemotherapy in the treatment of metastatic colorectal cancer: a meta-analysis. Medicine (Baltimore) 2016;95:e5284. doi: 10.1097/MD.0000000000005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringash J, Waldron JN, Siu LL, Martino R, Winquist E, Wright JR, Nabid A, Hay JH, Hammond A, Sultanem K, Hotte S, Leong C, El-Gayed AA, Naz F, Ramchandar K, Owen TE, Montenegro A, O’Sullivan B, Chen BE, Parulekar WR. Quality of life and swallowing with standard chemoradiotherapy versus accelerated radiotherapy and panitumumab in locoregionally advanced carcinoma of the head and neck: a phase III randomised trial from the Canadian cancer trials group (HN.6) Eur J Cancer. 2017;72:192–199. doi: 10.1016/j.ejca.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda N, Lim B, Wang X, Ueno NT. Early clinical development of epidermal growth factor receptor targeted therapy in breast cancer. Expert Opin Investig Drugs. 2017;26:463–479. doi: 10.1080/13543784.2017.1299707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakata M, Oda K, Toyohara J, Ishii K, Nariai T, Ishiwata K. Direct comparison of radiation dosimetry of six PET tracers using human whole-body imaging and murine biodistribution studies. Ann Nucl Med. 2013;27:285–296. doi: 10.1007/s12149-013-0685-9. [DOI] [PubMed] [Google Scholar]
- 14.Stabin MG. Uncertainties in internal dose calculations for radiopharmaceuticals. J Nucl Med. 2008;49:853–860. doi: 10.2967/jnumed.107.048132. [DOI] [PubMed] [Google Scholar]
- 15.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med. 2012;53:113–120. doi: 10.2967/jnumed.111.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miroddi M, Sterrantino C, Simmonds M, Caridi L, Calapai G, Phillips RS, Stewart LA. Systematic review and meta-analysis of the risk of severe and life-threatening thromboembolism in cancer patients receiving anti-EGFR monoclonal antibodies (cetuximab or panitumumab) Int J Cancer. 2016;139:2370–2380. doi: 10.1002/ijc.30280. [DOI] [PubMed] [Google Scholar]


