Highlights
-
•
Brain metastasis is an extremely rare secondary site of ovarian cancer metastasis.
-
•
Serous borderline tumor with brain metastasis has not been previously reported.
-
•
This is a rare case of brain metastasis in a patient with advanced serous borderline tumor.
Keywords: Ovarian cancer, Serous borderline tumor, Serous borderline tumor of the ovary, Brain metastasis
1. Introduction
Ovarian neoplasms are defined on a spectrum that includes borderline, low-grade, and high-grade tumors. A two-tier classification system is used to grade serous neoplasms as low grade or high grade—two tumor types with distinct molecular, pathologic, and clinical features. Serous borderline tumors represent a premalignant lesion with the ability to progress to low-grade serous carcinoma (LGSC) (Bodurka et al., 2012).
Serous borderline tumors and LGSCs are slow-growing tumors, with a relatively indolent course compared to that of high-grade serous carcinomas. Borderline tumors account for approximately 15% of all primary ovarian neoplasms, and 65% of these ovarian borderline tumors are of serous histology (Jones, 2006; Skírnisdóttir et al., 2008). One-third of women diagnosed with serous borderline tumors are younger than 40 years of age. LGSCs also are most commonly seen in young women, with a mean age at diagnosis of 55.5 years (Skírnisdóttir et al., 2008; Kaldaway et al., 2006). Approximately 33% of serous borderline tumors are associated with peritoneal implants, and in the recent 2014 World Health Organization (WHO) classification system, any invasive foci are now considered peritoneal LGSC, as these tumors display similar biological behavior (Shih and Kurman, 2004; Singer et al., 2003; Seidman and Kurman, 2000). Studies aimed at exploring the underlying genetic profiles of these malignancies have found that both serous borderline tumors and LGSCs express BRAF and KRAS mutations (Kaldaway et al., 2006). According to the National Comprehensive Cancer Network, the standard treatment for surgically staged borderline epithelial tumors with non-invasive implants is observation. For those with invasive implants, the standard of care also is observation or consideration of systemic treatment, as is recommended for the treatment of LGSC.
Brain metastasis is an extremely rare secondary site of ovarian cancer metastasis, with an incidence of 1–2.5%, associated with an extremely poor prognosis. While studies have shown a much higher prevalence of brain metastasis in grade 3 (83%) versus grade 1 or 2 tumors (17%), no reported case has been found specifically in patients with serous borderline tumor of the ovary (Cohen et al., 2004). Here, we report on a patient who had been diagnosed with recurrent serous borderline tumor with micropapillary architecture, invasive and non-invasive implants, and metastasis to the brain. According to our institutional policies, this case report has obtained Institutional Review Board exemption.
2. Case description
We report on a 41-year-old female who presented a few months prior to her diagnosis of serous borderline tumor of the ovary. The patient had experienced abdominal bloating for which she was referred to gastroenterology. In May 2008, an ultrasound revealed multiple uterine subserosal fibroids, the largest a right posterior subserosal fibroid measuring 2.3 × 2.6 × 2.2 cm, ascites, and a large heterogeneous solid pelvic mass measuring 11.8 × 7.0 × 8.7 cm.
In June 2008, the patient was referred to Memorial Sloan Kettering Cancer Center (MSK). A computed tomography (CT) scan of the chest, abdomen and pelvis (CAP) revealed extensive peritoneal carcinomatosis, ascites, peritoneal and omental implants measuring up to 1.9 × 1.2 cm in the subdiaphragmatic space, and bilateral complex cystic and solid adnexal masses (6.9 × 5.7 cm on the right, 6.6 × 6.3 cm on the left). In addition, several bilateral pulmonary nodules were noted, along with bilateral hilar lymphadenopathy (2.3 × 1.1 cm on the left, 2.8 × 2.6 cm on the right) and mediastinal lymphadenopathy (2.4 × 1.3 cm). The patient underwent an optimal surgical debulking involving an exploratory laparotomy, with drainage of 10 L of ascites, total abdominal hysterectomy, bilateral salpingo-oophorectomy, en bloc culdesectomy, coloproctostomy, omentectomy, appendectomy, pelvic lymphadenectomy, and the insertion of an intraperitoneal (IP) port. The largest visible mass remaining after surgery measured < 0.5 cm, and there were up to 20 such masses left after surgery.
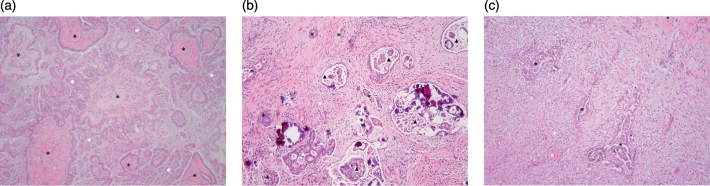
Pathology revealed a stage IIIC serous borderline tumor involving both ovaries. The right ovary exhibited small, scattered foci of micropapillary architecture. There was extensive tumor involvement of the peritoneum in the form of both invasive and non-invasive implants; 3 of 8 left pelvic lymph nodes and 1 of 1 appendiceal lymph nodes showed extensive and large foci of tumor involvement with stromal response. Non-invasive, desmoplastic implants of serous borderline tumor were seen involving the uterine serosa and peritoneal reflection. The cul-de-sac showed implants of serous borderline tumor, indeterminate in nature. The myometrium showed adenomyosis and multiple leiomyomata. The peritoneal fluid was positive for malignant cells (Fig. 1A–C).
Fig. 1.
A. Serous borderline tumor with micropapillary features. The tumor is comprised of large papillae with well-developed fibrovascular cores (black asterisks) surrounded by thin, delicate papillae with length > 5 times the width and scanty fibrovascular support (white asterisks). The area of micropapillary growth pattern spans > 5 mm in diameter. B. Invasive implant. The tumor consists of irregular to round glands of varying size (black asterisks), haphazardly infiltrating dense fibrous stroma (white asterisk), and surrounded by clear spaces. Some glands exhibit micropapillary (black arrowheads) or cribriform architecture (white arrowhead). The glands contain serous and mesothelial-type cells with moderate cytologic atypia. C. Non-invasive desmoplastic implant. Irregular gland-like structures (black asterisks) are embedded in an abundant inflamed and edematous (granulation tissue-like) stroma (white asterisk) in a linear orientation. The gland-like structures are lined by one to several layers of epithelial and mesothelial-type cells displaying abundant eosinophilic cytoplasm and mild cytologic atypia. (hematoxylin & eosin stain; original magnification × 100 [all panels]).
In July 2008, the patient was started on adjuvant intravenous (IV) carboplatin/paclitaxel chemotherapy followed by modified IV/IP cisplatin/paclitaxel chemotherapy. Since then, the patient has received several lines of systemic treatment (Table 1). Additional biopsies to assess for transformation to a high-grade invasive serous carcinoma were not performed at the time of recurrence.
Table 1.
The patient's systemic treatment history.
| Date of treatment | Therapy type | CA-125 level |
|---|---|---|
| July 2008–October 2008 | IV paclitaxel, carboplatin, IV/IP paclitaxel, cisplatin | Pre-Surgical: 1479 Pre-tx: 462 Post-tx: 106 |
| January 2009–March 2009 | Letrozole | Pre-tx: 65 Post-tx: 220 |
| March 2009–April 2009 | Liposomal doxorubicin | Pre-tx: 220 Post-tx:652 |
| May 2009–October 2010 | Gemcitabine, bevacizumab | Pre-tx: 822 Post-tx:132 |
| December 2010–January 2011 | Carboplatin | Pre-tx: 172 Post-tx:1331 |
| February 2011–September 2011 | Bevacizumab, oral metronomic cyclophosphamide | Pre-tx: 658 Post-tx: 156 |
| October 2011–May 2012 | Gemcitabine, bevacizumab | Pre-tx: 156 Post-tx: 133 |
| Treatment break | ||
| January 2013–May 2013 | Pimasertib (MEK1/2 inhibitor), voxtalisib (PI3K, mTOR inhibitor) | Pre-tx: 257 Post-tx: 147 |
| June 2013–January 2014 | Bevacizumab | Pre-tx: 130 Post-tx: 317 |
| February 2014–October 2014 | Gemcitabine, bevacizumab | Pre-tx: 317 Post-tx: 436 |
| Treatment break | ||
| April 2015–October 2016 | Paclitaxel, bevacizumab | Pre-tx: 845 Post-tx: 349 |
| October 2016–January 2017 | Leuprolide | Pre-tx: 349 Post-tx: 1100 |
| February 2017–May 2017 | Bevacizumab, topotecan | Pre-tx: 1225 Post-tx: 994 |
| June 2017-Present | Pemetrexed | Pre-tx: 994 (Most Recent) |
IV, intravenous; tx, treatment.
In November 2014, the patient reported a loss of taste and smell for which she saw an ENT specialist. In March 2015, the patient underwent magnetic resonance imaging (MRI) of the brain, which revealed at least 7 sub-centimeter foci of enhancement within the right paramedian convexity, parietal lobe, right perinsular and right periventricular brain parenchyma, the left temporal lobe, along the ependymal surface of the left frontal horn of the lateral ventricle, and within the infratentorial medial right cerebellum. The largest focus of abnormal enhancement, measuring 1.0 × 0.8 cm, abutted the dural surface of the posterior media left parietal lobe (Fig. 2, Fig. 3). While a biopsy of these lesions to confirm metastasis over primary tumor was not performed, the appearance on MRI was consistent with metastatic disease. The patient was given 20 fractions of whole brain radiation therapy (WBRT) (4000 cGy) from March to April 2015. A post-radiation MRI in May 2015 revealed complete resolution of her brain metastasis, with no new findings. The most recent brain MRI in May 2017 revealed no new metastasis. Most recently, the patient progressed on bevacizumab and topotecan. A CT CAP revealed new metastatic disease extending from the right urinary bladder to the ileum of the right lower quadrant. The patient was started on pemetrexed, and is currently on this treatment regimen.
Fig. 2.
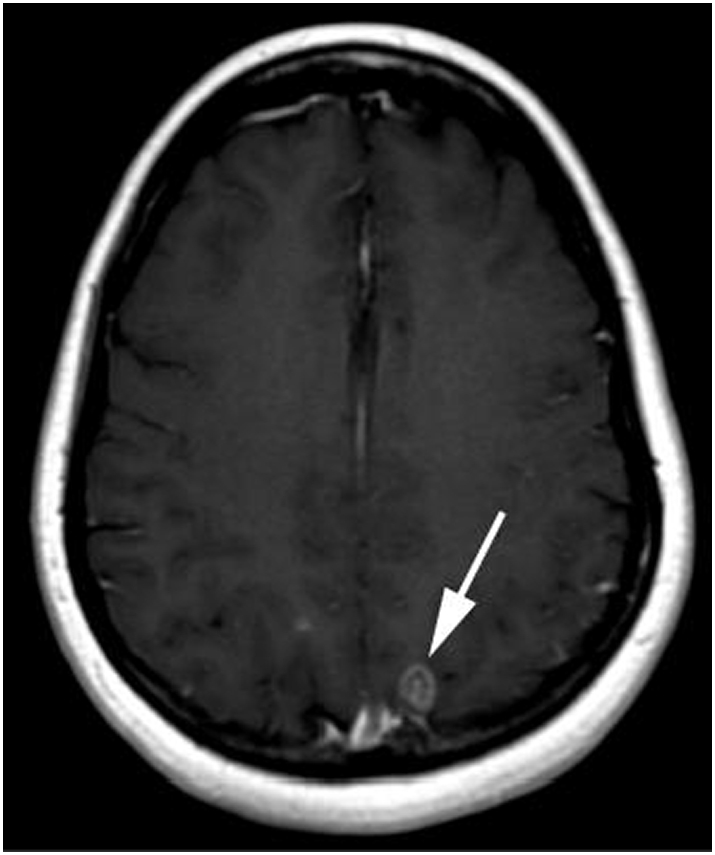
T1 weighted MRI of the brain obtained after administration of intravenous gadolinium demonstrating a ring enhancing metastasis in the posterior left parietal lobe.
Fig. 3.
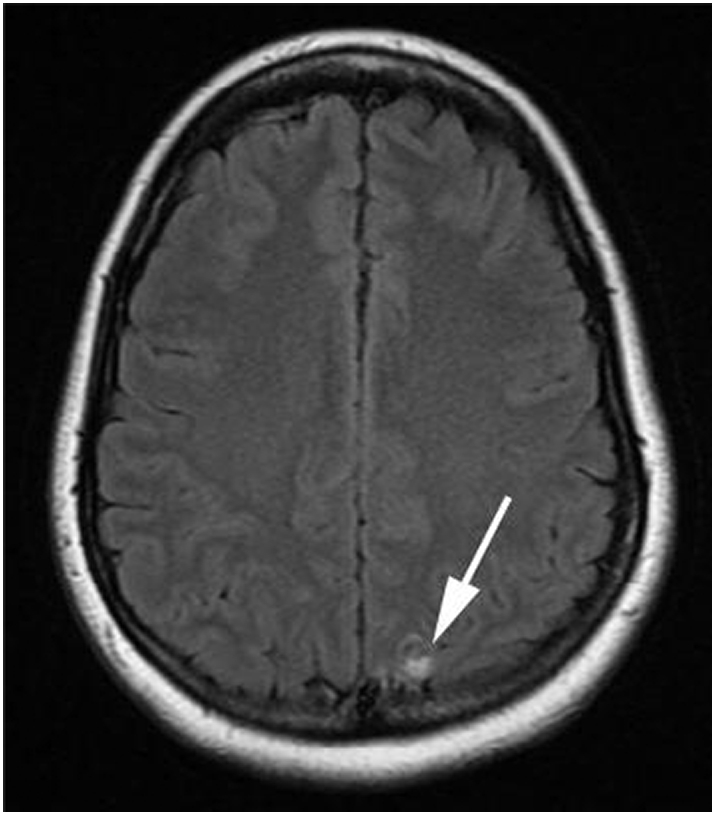
Fluid attenuation inversion recovery MRI image of the brain demonstrating focal high signal in the posterior left parietal lobe in the same location as the ring enhancing metastasis in Fig. 2.
3. Discussion
Brain metastasis from primary ovarian cancer is a rare phenomenon, with fewer than 600 cases reported in the literature. The most common cases of brain metastasis have been seen in patients with advanced-stage (III/IV), poorly differentiated epithelial serous carcinoma; however, to our knowledge, no cases have been reported specifically for serous borderline tumors with invasive implants (Piura and Piura, 2011).
Despite the gradual course of this patient's disease, the presence of invasive implants and lymph node involvement suggests a more aggressive form of disease. In a meta-analysis of 97 studies of 4129 patients with serous borderline tumors, invasive peritoneal disease (LGSC) was associated with shorter overall survival. Results showed an overall survival rate of 95.3% for advanced serous borderline tumors with non-invasive implants versus 66% for patients with invasive peritoneal disease (LGSC) (Seidman and Kurman, 2000). Our patient's clinical course is consistent with the histology of her disease.
Lymph node involvement has also been found to be prevalent in 21–29% of patients with serous borderline tumors. A study by McKenney et al. suggested that lymph nodes with nodular aggregates of epithelium > 1 mm in linear dimension significantly correlated with a decrease in disease-free survival, regardless of implant type (McKenney et al., 2006). In a study of 49 patients, however, Lesiur et al. reported that lymph node involvement in patients with advanced borderline tumors did not significantly correlate with improved prognosis (Lesiur et al., 2011). The comparable rate of recurrence was 25% for those with positive lymph nodes and 23.5% for those with negative lymph nodes. In addition, the 10-year and overall survivals between the two groups was not significantly different. While the McKenney et al. study showed that lymph nodes with nodular aggregates led to reduced disease-free survival, the Lesiur study did not specifically delve into the morphology of lymph nodes. The presence of lymph node involvement in predicting survival requires further study; however, there may be specific lymph node types that pose a high risk to patients (McKenney et al., 2006; Lesiur et al., 2011).
Due to the rarity and complexity of this disease, an understanding of prognostic factors in these patients is an ongoing effort. In a study evaluating KRAS/BRAF mutational status in 70 patients with serous borderline and LGSC, > 50% of patients were found to have a KRAS/BRAF mutation. Specifically, 17 patients were found to have a KRAS mutation (G12D or G12 V), 26 had a BRAF mutation (V600E), and 32 were wild type for KRAS and BRAF. Of 26 patients who had received systemic chemotherapy, 2 had KRAS-mutant tumors, 0 had BRAF-mutant tumors and the remaining had KRAS/BRAF wild-type tumors, suggesting that patients with BRAF mutation tumors are likely to survive longer than patients with KRAS mutant and BRAF wild-type tumors (Grisham et al., 2013). Our patient has a KRAS G12D mutation and has received multiple lines of chemotherapy for her recurrent disease.
To evaluate treatment options for patients with ovarian cancer and brain metastasis, studies have looked at the use of multimodal therapy (surgical resection and WBRT versus WBRT alone) for better survival (Piura and Piura, 2011). A phase I study evaluating the combined use of bevacizumab and WBRT for patients with unresectable brain metastasis from solid tumors concluded that patients who received bevacizumab at 15 mg/kg IV every 2 weeks in combination with WBRT (30 Gy15 fractions) had a better response than those who received lower doses of bevacizumab. The possible treatment response with bevacizumab has been linked to the anti-angiogenesis of bevacizumab, which may disrupt the cycle between angiogenesis-induced hypoxia and radio-resistance (Lévy et al., 2014).
The use of bevacizumab for LGSC has also been supported by the literature. A retrospective study from MSK was one of the first to propose the use of bevacizumab in combination with chemotherapy as a treatment option for patients with LGSC. This study evaluated the response of 17 patients with LGSC and serous borderline tumors. Six patients with LGSC were found to have a partial response, demonstrating a 55% response rate within the group. No complete response was observed for this group. Of the 4 patients with serous borderline tumors, no complete or partial responses were observed (Grisham et al., 2014).
Survival for patients with brain metastasis is poor; however, the literature on prognostics, survival, and treatment options for patients with epithelial ovarian cancer offers limited applicability to patients with serous borderline tumor or LGSC. As a result, it is important to continue our efforts in determining the etiology and disease course of patients with rare disease types and offer empirically supported treatments.
Conflict of interest statement
Dr. Grisham served on an advisory board for Mateon. Outside the submitted work, Dr. Aghajanian reports personal fees from Oxigene Steering Committee Meetings, as well as personal fees from a Cerulean Advisory Board, Bayer Advisory Board, VentiRx Advisory Board, AstraZeneca Advisory Board, ImmunoGen Advisory Board, and Oxigene Advisory Board. She also reports travel reimbursement from Abbvie for investigator meetings.
Dr. Makker reports personal fees from Eisai Pharmaceuticals Advisory Board. The other authors have no conflicts to disclose.
Consent
According to our institutional policies, this case report has obtained Institutional Review Board exemption.
Footnotes
Funding: Supported in part by the MSK Cancer Center Support GrantP30 CA008748.
References
- Bodurka D.C., Deavers M.T., Tian C., Sun C.C., Malpica A., Coleman R.L., KH Lu, Sood A.K., Birrer M.J., Ozols R., Baergen R., Emerson R.E., Steinhoff M., Behmaram B., Rasty G., Gershenson D.M. Reclassification of serous ovarian carcinoma by a 2-tier system: a gynecologic oncology group study. Cancer. 2012;118(12):3087–3094. doi: 10.1002/cncr.26618. [DOI] [PubMed] [Google Scholar]
- Cohen Z.R., Suki D., Weinberg J.S., Marmor E., Lang F.F., Gershenson D.M., Sawaya R. Bain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J. Neuro-Oncol. 2004;66(3):313–325. doi: 10.1023/b:neon.0000014516.04943.38. [DOI] [PubMed] [Google Scholar]
- Grisham R.N., Iyer G., Garg K., DeLair D., Hyman D.M., Zhou Q., Iasonos A., Berger M.F., Dao F., Spriggs D.R., Levine D.A., Aghajanian C., Solit D.B. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham R.N., Iyer G., Sala E., Zhou Q., Iasonos A., DeLair D., Hyman D., Aghajanian C. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int. J. Gynecol. Cancer. 2014;24(6):1010–1014. doi: 10.1097/IGC.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.B. Borderline ovarian tumors: current concepts for prognostic factors and clinical management. Clin. Obstet. Gynecol. 2006;49:517. doi: 10.1097/00003081-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Kaldaway A., Segev Y., Lavie O., Auslender R., Sopik V., Narod S.A. Low-grade serous ovarian cancer: a review. Gynecol. Oncol. 2006;143:433–438. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- Lesiur B., Kane A., Duvillard P., Gouy S., Pautier P., Lhomme C., Morice P., Uzan C. Prognostic value of lymph node involvement in ovarian serous borderline tumors. Am. J. Obstet. Gynecol. 2011;204(5):438.e1–438.e7. doi: 10.1016/j.ajog.2010.12.055. [DOI] [PubMed] [Google Scholar]
- Lévy C., Allouache D., Lacroix J., Dugué A.E., Supiot S., Campone M. Paoletti, X. REBECA: a phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann. Oncol. 2014;25(12):2351–2356. doi: 10.1093/annonc/mdu465. [DOI] [PubMed] [Google Scholar]
- McKenney J.K., Balzer B.L., Longacre T.A. Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors) Am. J. Surg. Pathol. 2006;30(5):614–624. doi: 10.1097/01.pas.0000194743.33540.e6. [DOI] [PubMed] [Google Scholar]
- Piura E., Piura B. Brain metastases from ovarian carcinoma. ISRN Oncol. 2011;2011:1–13. doi: 10.5402/2011/527453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J.D., Kurman R.J. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum. Pathol. 2000;31(5):539–557. doi: 10.1053/hp.2000.8048. [DOI] [PubMed] [Google Scholar]
- Shih Ie-M., Kurman R.J. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004;164(5):1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G., Oldt R., III, Cohen Y., Wang B.G., Sidransky D., Kurman R.J. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003;95(6):484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- Skírnisdóttir I., Garmo H., Wilander E., Holmber L. Borderline ovarian tumors in Sweden 1960-2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int. J. Cancer. 2008;123:1897. doi: 10.1002/ijc.23724. [DOI] [PubMed] [Google Scholar]