Abstract
A few years ago the answer to the question in the title of this review would have been, “unfortunately not much” or even “nothing”, likely eliciting knowing nods of agreement from oncologists. For the last 3 decades, SCLC has been notorious for its lack of progress, as drug after drug, over 60 of them, in fact, including inhibitors of VEGF, IGFR, mTOR, EGFR and HGF has failed and fallen by the wayside due to little or no impact on PFS or OS, while SCLC's cousin, NSCLC, has notched success after success with a spate of targeted treatment and immunotherapy regulatory approvals. However, a paradigm shift or, more appropriately, a ‘paradigm nudge’ is quietly underway in extensive stage SCLC with a series of agents that in early clinical trials have shown the potential to ‘lift the curse’ in SCLC, heretofore referred to as “a graveyard for drug development”. These agents, constituting the “best of what's new” in SCLC, and discussed in this review following a brief overview of the classification, epidemiology, prognosis and current treatment of SCLC, include checkpoint inhibitors, antibody-drug conjugates, PARP inhibitors, epigenetic inhibitor/innate immune activator, and an inhibitor of RNA polymerase II. Compared to NSCLC, the therapeutic options are still limited but with one or more successes to build momentum and drive long-overdue R&D and clinical investment the hope is that the approval floodgates may finally open.
Introduction
As the leading cause of death among men and women in North America, lung cancer has attracted substantial attention and pharmaceutical investment. However, in contrast to the rapidly changing status of non-small cell lung cancer (NSCLC), where significant inroads have been made with targeted agents and immunotherapies, the small cell lung cancer (SCLC) landscape has remained, like its name, disappointingly small and static for over 30 years, with a dearth of effective therapies. Due to the failure of over 60 agents including inhibitors of VEGF, IGFR, mTOR, EGFR, HGF and a P53 cancer vaccine in clinical trials, SCLC, long considered the black sheep of the lung cancer family, has been fittingly referred to as “a graveyard for drug development” [1], [2].
An aggressive neuroendocrine (NE) tumor derived from bronchial epithelial cells, SCLC (also known as oat-cell carcinoma) accounts for about 13–15% [3] of all lung cancers and between 30,000 to 35,000 new cases per year in the U.S. [4] Its rapid doubling time and high growth fraction combined with a propensity to metastasize widely and early on in the disease course (most commonly to the brain, liver, or bone) results in a 95% mortality rate [5], which makes SCLC the most lethal lung cancer subtype. Most cases of SCLC develop in patients aged 60–80 years and the estimated overall death rate is 25,000–30,000 per year [6]. Ancillary factors, which contribute to the high mortality rate include the advanced age of a patient population that is historically difficult to treat secondary to multiple smoking related comorbidities, the dose-limiting cumulative effects of prior, treatment regimens on bone marrow reserves and the lack of responsiveness of the tumor to novel cytotoxic drugs and so-called targeted therapies. This high degree of treatment difficulty combined with a decline in the incidence of SCLC in North America (down from 20–25%) [7], (even while the worldwide incidence, particularly in Central/Eastern and Southern Europe, has continued to rise [8]), and the stigma of it as a self-inflicted “smoker's disease” have led to pharmaceutical disinterest and the relative neglect of a tumor type that accounts for 13–15% of all lung cancers.
Indeed, SCLC is so strongly correlated with a history of smoking, in fact heavy smoking (for example, those with a 30 pack-year history), more so than any other cancer, that the occurrence in a never smoker constitutes an anomaly, worthy of a case report [9]; its rarity has led more than one author [10] to dispute the diagnosis in patients who deny a smoking history. Somewhat surprisingly, then, transformation of NSCLC to SCLC has been reported in non-smoking patients with mutated EGFR when resistance to EGFR tyrosine kinase inhibitors develops [11]. However, due to the use of low-tar filtered and “light” cigarettes, which prompt smokers to inhale more deeply and smoke more intensely/vigorously as a compensatory strategy for the lower delivery of nicotine, thus exposing the higher-order peripheral bronchi to carcinogen-containing smoke, the incidence of centrally-located squamous cell cancers and SCLCs has waned while that of peripheral lung adenocarcinomas has increased [12].
In addition to pulmonary SCLC, another entity is extrapulmonary small cell carcinoma (EPSCC), thought to represent up to 5% of all cases of small cell carcinoma, and to most commonly occur in the gastrointestinal tract and genitourinary system [13]. However, failure to account for high levels, up to 25% [14], of secondary or treatment-emergent small cell/neuroendocrine prostate cancer after the development of anti-androgen resistance may underestimate the true rate of EPSCC.
Classification of SCLC—Limited and Extended Stage
The most commonly used staging system for SCLC is the Veterans Administration Lung Study Group (VALG) staging system that classifies patients into two categories, limited stage (LS), which is confined to one hemithorax and one radiation field or extensive stage (ES) which extends beyond one hemithorax. Current standard of care is concurrent chemoradiation for LS disease and chemotherapy alone for ES disease [15].
Without treatment, extensive stage small cell lung cancer (ES-SCLC), representing approximately two-thirds of all cases (LS-SCLC comprises the other one-third), is rapidly and invariably fatal within 2 to 4 months [16]. With combination chemotherapy, responses are dramatic but sadly short-lived: SCLC inevitably relapses and relapse is associated with a median OS often <6 months [17] (the median OS for SCLC patients in the third line setting is 4.7 months [18], a survival rate which has scarcely improved over the last 40 years [19].
Extensive-Stage SCLC Classification by First Line Response and Chemotherapy-Free Interval
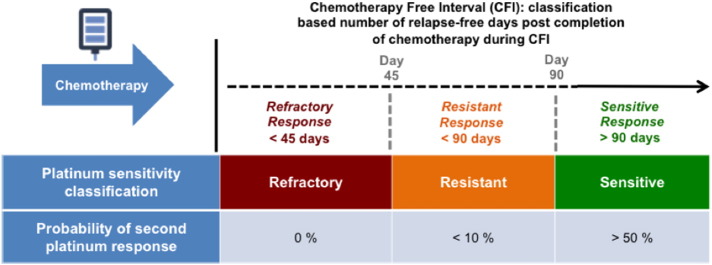
ES-SCLC patients are further classified according to their response to first line platinum doublets. The relapse-free interval of sensitive patients exceeds 90 days after treatment with platinum doublets is completed, resistant disease relapses within 90 days of treatment completion during a chemotherapy-free interval (CFI) and refractory disease either never responds at all or relapses within 45 days of treatment completion during a CFI. The practical value of this classification scheme is that platinum sensitivity or lack thereof correlates with prognosis and dictates secondary treatment strategies; the longer the chemotherapy free interval (CFI) the greater the likelihood of a re-response to platinum (Figure 1).
Figure 1.
Platinum sensitivity is classified as refractory, resistant, or sensitive, according to the time elapsed during a chemotherapy-free interval since finishing first-line treatment. Probability of re-treatment response is shown for each group of patients.
To the extent that all patients with small cell lung cancer, whatever their initial classification, will eventually develop resistance to all conventional therapies, the incidence of relapsed and refractory/resistant SCLC (as well as platinum sensitive third line or beyond SCLC) is probably roughly equivalent to that of SCLC as a whole; however, unlike platinum sensitive SCLC, where the response rate to second-line topotecan is 14% to 38%, the response rate with topotecan for resistant or refractory SCLC (rSCLC) is a dismal 2% to 8%, which makes rSCLC (and, by extension, platinum sensitive third line and beyond SCLC, which may be considered at this late stage to be pan-treatment refractory) a recalcitrant and neglected tumor subtype [20].
SCLC is an Orphan Disease Based on Prevalence and Historical Neglect
SCLC meets the criterion for an Orphan Disease on the basis of prevalence (<200,000 patients in the United States of America) as well as historical neglect since (a) the treatment landscape has not changed in over 30 years; (b) no new drugs have been approved for 19 years; (c) the only officially defined second-line treatment in the USA, topotecan (Hycamtin) (amrubicin is registered for administration only in Japan), is ineffective in resistant/refractory SCLC; and (d) new potential treatment options such as immunotherapy and Rova-T are unlikely to have activity specifically in relapsed refractory or resistant SCLC (rSCLC), for reasons discussed below.
Having been understudied, underfunded, and without effective therapeutic options for so many decades, SCLC has been referred to as the “forgotten cancer” [21], an inevitable consequence of its reputation as a “graveyard for drug development”. The NCI has referred to SCLC as a “poster child” for cancers [22] classified as recalcitrant on the basis of relative five-year survival rates that are less than 20%. The 5-year relative survival rate of SCLC is around 5% with the loss of approximately 30,000 lives per year.
Therefore, a significant improvement in overall response rate and survival in SCLC with would constitute the first real progress in this rare and recalcitrant disease subtype in decades.
Treatment for Extensive-Stage (ES) SCLC
The current standard of first-line care for ES-SCLC is platinum based (cisplatin-etoposide or cisplatin-irinotecan or cisplatin-topotecan) with or without concurrent radiation therapy, followed in general by topotecan (Hycamtin), the sole agent with FDA approval specifically for the second line setting [23]. Topotecan binds to the topoisomerase I-DNA complex and prevents the religation of DNA after single strand cleavage has taken place, which results in cell death [24].
A standard third line treatment is lacking although single agent paclitaxel, irinotecan, gemcitabine and vinorelbine are commonly tried in patients with acceptable performance status. The prognosis for relapsed SCLC is poor with a median survival of weeks to months [15]. The standard treatment algorithm is shown in Figure 2.
Figure 2.
Standard treatment of ES-SCLC.
New Treatments in Development
-
1.
Immunotherapy
The data with immunotherapy in relapsed SCLC is mixed. In two Phase I/II trials for relapsed SCLC, KEYNOTE-028 [25] with the anti-PD-1 antibody, pembrolizumab, and CheckMate 032 study with the anti-PD-1 antibody, nivolumab plus/minus the CTLA-4 inhibitor, ipilimumab, durable response rates were observed. In the randomized CheckMate 032 study [26] objective response rates were 11% for nivolumab (13% and 8% in platinum sensitive and resistant disease, respectively) and 25% for nivolumab + ipilimumab (25% and 24% in platinum sensitive and resistant disease, respectively), while the median overall survival was 4.1 months for nivolumab and 7.9 months for the combination.
The risk of toxicity, however, is not insignificant especially in combination: 16% of patients in the CheckMate 032 study discontinued due to treatment-related adverse events and 3 immune-related deaths occurred (from pneumonitis, myasthenia gravis, and renal failure).Any grade treatment-related adverse events occurred in 82% of the combination vs. 60% for nivolumab alone; Grade 3/4 serious adverse events occurred in 33% for the combination vs. 14% for nivolumab alone.
Moreover, in a double-blind Phase 3 study of ipilimumab plus etoposide and a platinum agent overall survival was not increased, when compared with chemotherapy alone [27].
Overall, the challenge with immunotherapy in relapsed SCLC and, in particular, in platinum resistant or refractory relapsed SCLC, is two-fold: (1) that due to the high burden of disease and the rapidity of progression the time that it takes to mount an effective anti-tumor response, typically up to 3–6 months, may exceed the overall survival of the patient and (2) that checkpoint inhibitor-treated patients may be at higher risk of developing life-threatening immune-related toxicities since a variety of paraneoplastic immune-mediated syndromes such as encephalitis and myasthenia gravis [28] are already associated with SCLC.
-
2.
Antibody-Drug Conjugates (ADCs)
Similar to Erlich's magic bullet concept, antibody-drug conjugates, which are intended to decrease toxicity and improve the therapeutic index, comprise (1) an antibody directed at a defined antigen on cancer cells, (2) a linker, and (3) a cytotoxic agent.
-
a)
Rovalpituzumab Tesirine (Rova-T)
Rova-T is an antibody drug conjugate that recognizes delta-like protein 3 (DLL3), a NOTCH ligand, which is expressed on the surface of approximately two-thirds of SCLC cells but is absent from healthy cells; in a trial of 79 patients with SCLC [29] that relapsed after first or second line therapy, high expression of DLL3 correlated with response, irrespective of platinum sensitivity; however, the median overall survival of 5.8 months in the Rova-T trial was 1.1 months better than the historical median overall survival of 4.7 months for 3rd line SCLC [18], which suggests that patients with harder-to-treat resistant/refractory SCLC in third line or beyond may actually fare worse than 5.8 months. Moreover, Grade 3 and above toxicity occurred in 26% of Rova-T-treated patients.
-
b)
Sacituzumab Govitecan (IMMU-132)
IMMU-132 is an antibody drug conjugate (ADC) that comprises approximately 8 molecules of SN-38, the active metabolite of the topoisomerase-1 inhibitor, camptothecin (irinotecan), conjugated to an antibody that binds Trop-2, a calcium-transducing transmembrane glycoprotein widely expressed (>80% with the caveat that no standardized method for the immunohistochemical assessment of TROP2 expression in tumors exists) in most epithelial cancers, including SCLC [30]. In Phase II trial of 49 evaluable patients with recurrent metastatic SCLC, both platinum-sensitive and platinum-resistant, and a median of two prior therapies, an overall response rate of 14% (7/49 confirmed partial responses), a clinical benefit rate (PR + SD >4 mos) of 35% (17/49) and a median OS of 7.5 months were reported [31]. As expected for SN-38, Grade 3/4 toxicities included neutropenia (34%), fatigue (13%) and diarrhea (9%). IMMU-132 has received FDA Fast Track Designation in SCLC.
-
3.
PARP inhibitors
Since SCLC is known to overexpress PARP protein, PARP inhibitors, which include veliparib, have been investigated in SCLC. In a phase II 93 patient relapsed SCLC study of temozolomide (TMZ) plus veliparib vs. TMZ alone, the response rate for veliparib/TMZ compared to TMZ alone was significantly different (39% vs. 14% P = .016). However, the median overall survival was not significantly different between the two arms (8.2 months vs. 7.0 months P = .50) and Grade 3/4 thrombocytopenia and neutropenia more commonly occurred in the veliparib/TMZ arm: 50% vs. 9% and 31% vs. 7%, respectively [32]. High levels of SLFN11, a marker for defective DNA damage repair, obtained at original diagnosis, were associated with better overall survival only in the veliparib/TMZ arm.
-
4.
Transcription inhibitor—Lurbinectedin
The combination of doxorubicin + lurbinectedin (PM1183), a novel marine-derived RNA II polymerase inhibitor structurally-related to FDA approved trabectedin (Yondelis), vs. topotecan or the combination VCR (cyclophosphamide, doxorubicin, and vincristine) is currently under investigation in a Phase III 600 patient study (ATLANTIS NCT02566993) for 2nd line SCLC patients after failure of 1st line platinum doublets. In addition to RNA II inhibition, lurbinectedin also disrupts the nucleotide excision repair (NER) pathway (its activity is attenuated in NER-deficient cells) [33], and is further associated with depletion of tumor-associated macrophages [34]. The Phase III trial follows on the heels of a Phase I dose escalation study in which the overall response rate and complete response rate, among 21 patients with SCLC, were 67% and 10%, respectively [35]. However, toxicity is an issue: due to a 96% rate of Grade 3/4 neutropenia on the Phase I trial as well as 24% Grade 3 febrile neutropenia and 5% Grade 4 febrile neutropenia, prophylactic G-CSF support is required on the Phase III trial.
-
5.
Epi-immune agent—RRx-001
RRx-001 is a minimally toxic tumor associated macrophage (TAM) and tumor associated neutrophil (TAN) repolarizing agent and pan-epigenetic inhibitor with orphan drug status in SCLC [36]. In over 170 patients treated to date over all studies in multiple tumor types including metastatic colorectal cancer, cholangiocarcinoma, as well as neuroendocrine, SCLC, EGFR mutated NSCLC and epithelial ovarian cancer, only one serious adverse event has been possibly attributed to RRx-001 as a single agent [37].
In an ongoing Phase II proof-of-principle clinical study called QUADRUPLE THREAT (NCT02489903), RRx-001 is dosed as a single agent until RECIST version 1.1-defined progression, at which point first line platinum doublets are sequentially reintroduced in third line or beyond resistant and refractory small cell lung cancer (rSCLC) as well as three other tumor types. To date, in over 50% of evaluable patients, RRx-001 has demonstrated reversal of chemoresistance to reintroduced first-line platinum doublets.
Patient biopsies have correlated response with a high density of infiltrated TAMs and, according to the literature, tumor-infiltrating macrophages are abundant in SCLC [38].
Moreover, on the basis of preclinical evidence, RRx-001 may be protective against the bone marrow and renal toxicities of cisplatin [39].
Conclusion
The success of cancer treatment in solid tumors is continuously eroded by the occurrence of drug resistance, innate or acquired and the extensive stage SCLC tumor type is no exception. While initially exquisitely chemoradiosensitive, relapse in SCLC is the rule and the efficacy of treatment beyond first line dramatically dwindles—along with therapeutic options— as it becomes increasingly resistant to treatment.
Unlike many other solid-tumor malignancies, NSCLC in particular, where advances in diagnosis and treatment have resulted in improved survival, SCLC has remained in a holding pattern at best, with only a two month improvement in median survival times shown in Phase III trials over the last 30 years [40], engendering an ingrained therapeutic skepticism in the oncology community that borders on—if not outright devolves into—nihilism [41]. Such is the sense of futility after so many clinical failures with single- or combination chemotherapies and targeted agents (one oncologist compared the implacability of SCLC to a “brick wall” [42]) that for the last 3 decades it has been largely written off by the pharmaceutical industry as too formidable of a challenge, resulting in a vicious circle of treatment stagnation and inaction, which, in turn, has reinforced the perception of futility.
However, to continue the oncologist's comparison from the preceding sentence, the hope is that the newest molecular chisels described in this review i.e., checkpoint inhibitors, antibody-drug conjugates, PARP inhibitors, epigenetic inhibitors and transcription inhibitors will be used to chip/pry away at different sections of the formerly impenetrable SCLC brick wall until finally the whole of it crumbles. Nevertheless, like actual chisels (or any set of tools for that matter), which come in different sizes and styles i.e. broad or flat vs. narrow-pointed, depending on their intended purpose, one size never fits all and the use of these agents, all of which have distinct limitations or challenges associated with them, is only justified if limited to a defined subset of patients whose tumors are prescreened or monitored during treatment for certain favorable characteristics. These characteristics, which may serve as the basis for an individualized treatment plan, are summarized in Table 1.
Table 1.
Matching Tumor and Patient Characteristics, some of which are Obvious and some of which are not, to the Individual Treatments Described in this Review
| Agent or Class | Tumor Characteristics |
|---|---|
| Checkpoint inhibitors | Platinum sensitive disease Slower growing disease No history of paraneoplastic syndromes |
| Rova-T | Platinum sensitive disease Presence of DLL3 |
| IMMU-132 | Presence of TROP-2 receptor |
| PARP inhibitor + temozolomide | Presence of SLFN11 |
| Lurbinectedin | No bone marrow suppression Presence of NER-related genes |
| RRx-001 | High infiltration of tumor associated macrophages |
In conclusion, then, to return to the question posed in the title of this review, “What's New in SCLC?” the short answer, as of July 2017, hopefully infusing some much-needed optimism into the treatment of a tumor type long-neglected by researchers, physicians and drug companies is “quite a lot actually”.
Acknowledgments
Acknowledgement
Dedication from C. Carter: To my father, Larry Carter, who died of SCLC well before anything was new in it.
Acknowledgments
Funding
None.
References
- 1.Khanna P, Blais N, Gaudreau PO, Corrales-Rodriguez L. Immunotherapy Comes of Age in Lung Cancer. Clin Lung Cancer. 2017;18(1):13–22. doi: 10.1016/j.cllc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res. 2015;4(5):533–544. doi: 10.3978/j.issn.2218-6751.2015.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H. National Cancer Institute; 2010. SEER Cancer Statistics Review, 1975–2007. [http://seer.cancer.gov/csr/1975-2007/, Ref Type: Internet Communication. Jan 22, 2017] [Google Scholar]
- 5.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 6.Navada S, Lai P, Schwartz AG, Kalemkerian GP. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End Results database (abstract 7082) J Clin Oncol. 2006;24(18_suppl):7082-7082. [Google Scholar]
- 7.Small Cell Lung Cancer Treatment (PDQQR): Health Professional Version. National Cancer Institute; 2015. Available at http://www.ncbi.nlm.nih.gov/books/NBK65909/#CDR00000629451. [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Pokharel K, Gilbar P, Sorour N. Case Report: Small-cell lung cancer in a young, female, never-smoker. Lung Cancer Manage. 2015;4(4):159–162. [Google Scholar]
- 10.Casciato DA, Territo MC. Lippincott Williams & Wilkins; 2009. Manual of Clinical Oncology. [Medical - 794 pages] [Google Scholar]
- 11.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dela Cruz CS, Tanoue LT, Matthat RA. Lung Cancer: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2011;32(4) doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116(4):888–895. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]
- 14.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, Rubin MA, Nanus DM. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30:e386–9. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 15.Allen J, Jahanzeb M. Extensive-stage small-cell lung cancer: evolution of systemic therapy and future directions. Clin Lung Cancer. 2008;9(5):262–270. doi: 10.3816/CLC.2008.n.041. [DOI] [PubMed] [Google Scholar]
- 16.Pelayo Alvarez M, Gallego Rubio O, Bonfill Cosp X, Agra Varela Y. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD001990. [DOI] [PubMed] [Google Scholar]
- 17.Davies AM, Evans WK, Mackay JA, Shepherd FA. Treatment of recurrent small cell lung cancer. Hematol Oncol Clin North Am. 2004;18(2):387–416. doi: 10.1016/j.hoc.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Simos D, Sajjady G, Sergi M, Liew MS, Califano R, Ho C, Leighl N, White S, Summers Y, Petrcich W. Third-line chemotherapy in small-cell lung cancer: an international analysis. Clin Lung Cancer. 2014;15(2):110–118. doi: 10.1016/j.cllc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2(12):1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 20.Von Pawel J, Schiller J, Shepherd F, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 21.Gazdar AF, Minna JD. Developing New, Rational Therapies for Recalcitrant Small Cell Lung Cancer. J Natl Cancer Inst. 2016;108(10) doi: 10.1093/jnci/djw119. djw119, http://dx.doi.org/10.1093/jnci/djw119. [DOI] [PubMed] [Google Scholar]
- 22.Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl. 5):S565–78. doi: 10.3978/j.issn.2072-1439.2013.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC) Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Mattern MR, Mong SM, Bartus HF, Mirabelli CK, Crooke ST, Johnson RK. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 1987;47:1793–1798. [PubMed] [Google Scholar]
- 25.Ott PA, Elez Fernandez ME, Hiret S, Kim Dong-Wan, Moss RA, Winser T, Yuan S, Cheng JD, Piperdi B, Mehnert JM. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol. 2015;33(15_suppl):7502-7502. [Google Scholar]
- 26.Hellmann MD, Antonia SJ, Ponce S, Ott PA, Calvo E, Taylor M, Ready N, Hann CL, De Braud F, Eder JP. World Conference on Lung Cancer. Abstract MA09.05. Presented December 6, 2016. 2016. Nivolumab alone or with ipilimumab in recurrent small cell lung cancer: 2-year survival and updated analyses from the CheckMate 032 trial. [Google Scholar]
- 27.Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu Y, Zielinski C, Thomas M. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;25:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, Zhang G. A Mini-Review for Cancer Immunother- apy: Molecular Understanding of PD-1/PD-L1 Pathway & amp;amp; Translational Blockade of Immune Checkpoints. Int J Mol Sci. 2016;17(7) doi: 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietanza C. Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein3 (DLL3)-targeted anibody drug conjugate (ADC), in small cell lung cancer (SCLC) [abstract LBA 7] Presented at the ECC 2015. 2015 [Google Scholar]
- 30.Starodub A, Camidge DR, Ronald J, Scheff RJ, Thomas SS, Guarino MJ, Masters GA, Kalinsky K, Gandhi L, Bardia A. Trop-2 as a therapeutic target for the antibody-drug conjugate (ADC), sacituzumab govitecan (IMMU-132), in patients (pts) with previously treated metastatic small-cell lung cancer (mSCLC) J Clin Oncol. 2016;34:8559-8559. [abst 8559] [Google Scholar]
- 31.Gray JE, Heist RS, Starodub AN, Camidge DS, Kio E, Masters G, Purcell WT, Guarino MJ, Misleh J, Schneider CJ, et al. CT155-Phase 2 study of sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate (ADC), in patients with pretreated metastatic small-cell lung cancer (mSCLC). [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017;77(13 Suppl):Abstract nr CT155. http://dx.doi.org/10.1158/1538-7445.AM2017-CT155.
- 32.Byers LA, Krug L, Waqar S, Dowlati A, Hann C, Chiappori A, Owonikoko T, Woo K, Bensman Y, Hurtado B. MA11.07 Improved Small Cell Lung Cancer (SCLC) Response Rates with Veliparib and Temozolomide: Results from a Phase II Trial. J Thorac Oncol. 2017;12(1):S406–S407. [Google Scholar]
- 33.Soares DG, Machado MS, Rocca CJ, Poindessous V, Ouaret D, Sarasin A, Galmarini CM, Henriques JA, Escargueil AE, Larsen AK. Trabectedin and its C subunit modified analogue PM01183 attenuate nucleotide excision repair and show activity toward platinum-resistant cells. Mol Cancer Ther. 2010;10:1481–1489. doi: 10.1158/1535-7163.MCT-11-0252. [DOI] [PubMed] [Google Scholar]
- 34.Céspedes MV, Guillén MJ, López-Casas PP, Sarno F, Gallardo A, Alamo P, Cuevas C, Hidalgo M, Galmarini CM, Allavena P. Lurbinectedin induces depletion of tumor-associated macrophages, an essential component of its in vivo synergism with gemcitabine, in pancreatic adenocarcinoma mouse models. Dis Model Mech. 2016;9(12):1461–1471. doi: 10.1242/dmm.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forster M, Calvo E, Olmedo Garcia ME, Lopez Criado MP, Moreno V, Soto-Matos A, Holgado E, Brown NF, Flynn M, Boni V. Lurbinectedin(PM1183)with doxorubicin (DOX), an active treatment as second-line therapy in small cell lung cancer (SCLC). Presented at: 2015 ASCO Annual Meeting; May 29–June 2, 2015. Chicago, IL. 2015 Abstract 7509. [Google Scholar]
- 36.Oronsky B, Scicinski J, Ning S, Peehl D, Oronsky A, Cabrales P, Bednarski M, Knox S. Rockets, radiosensitizers, and RRx-001:an origin story part I. Discov Med. 2016;21(115):173–180. [PubMed] [Google Scholar]
- 37.Oronsky B, Paulmurugan R, Foygel K, Scicinski J, Knox SJ, Peehl D, Zhao H, Ning S, Cabrales P, Summers TA., Jr. Carter CA.RRx-001: a systemically non-toxic M2-to-M1 macrophage stimulating and prosensitizing agent in Phase II clinical trials. Expert Opin Investig Drugs. 2017;26(1):109–119. doi: 10.1080/13543784.2017.1268600. [DOI] [PubMed] [Google Scholar]
- 38.Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126(7):2610–2620. doi: 10.1172/JCI81603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oronsky B, Reid TR, Larson C, Carter CA, Brzezniak CE, Oronsky A, Cabrales P. RRx-001 protects against cisplatin-induced toxicities. J Cancer Res Clin Oncol. 2017 doi: 10.1007/s00432-017-2416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chute JP, Chen T, Feigal E, Simon R, Johnson BE. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol. 1999;17(6):1794–1801. doi: 10.1200/JCO.1999.17.6.1794. [DOI] [PubMed] [Google Scholar]
- 41.Popat S, O'Brien M. Chemotherapy strategies in the treatment of small cell lung cancer. Anticancer Drugs. 2005;16:361–372. doi: 10.1097/00001813-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Bassiri K, Cairns L, McVie G, Seckl M. Highlights from the ecancer Future Horizons in Lung Cancer conference, 1–2 September 2016: Focusing on the future of treatment for NSCLC and SCLC. Ecancermedicalscience. 2017;11:729. doi: 10.3332/ecancer.2017.729. [DOI] [PMC free article] [PubMed] [Google Scholar]