Abstract
Optoacoustic imaging (OAI) can detect haemoglobin and assess its oxygenation. However, the lack of a haemoglobin signal need not indicate a lack of perfusion. This study uses a novel method to assist the co-registration of optoacoustic images with dynamic contrast enhanced ultrasound (DCE-US) images to demonstrate, in preclinical tumour models, the value of combining haemoglobin imaging with a perfusion imaging method, showing that a lack of a haemoglobin signal does not necessarily indicate an absence of perfusion. DCE-US was chosen for this particular experiment because US is extremely sensitive to microbubble contrast agents and because microbubbles, like red blood cells but unlike currently available optical contrast agents, do not extravasate. Significant spatial correlations were revealed between the DCE-US properties and tumour blood-oxygen saturation and haemoglobin, as estimated using OAI. It is speculated that DCE-US properties could be applied as surrogate biomarkers for hypoxia when planning clinical radiotherapy or chemotherapy.
Keywords: CEUS, Microbubbles, Optoacoustic, Photoacoustic, MSOT, Registration
1. Introduction
Hypoxia is prevalent in most malignant human cancers because the functionally abnormal tumour vasculature is not able to meet the excessive oxygen consumption needs of the proliferating tumour cells. This stimulates several compensatory biological processes, which alter gene expression profiles [1], [2] that lead to metastasis and cancer progression [3], [4], and enable a tumour to circumvent chemo- [5] and radiotherapy [6]. It has been widely established that the hypoxic state of a tumour can influence treatment outcome [7], [8], [9], [10]. In addition, hypoxia-targeted therapies are under development and in clinical trial [11]. Therefore, for a number of reasons, there is an urgent need for a diagnostic method for determining whether a tumour is hypoxic, so as to enable hypoxia-based treatment individualisation [11].
Commonly used methods for assessing tissue hypoxia in patients are needle electrode polarography, for direct measurement of tumour oxygen saturation, and needle biopsies, for quantifying biomolecules expressed under hypoxic conditions [11]. Both these techniques are invasive and do not represent the whole tumour, which is likely to be crucial for planning treatment. Non-invasive imaging techniques for assessing hypoxia are therefore under development and investigation. However, tracer-based hypoxia imaging methods such as phosphorescence quenching [12], electron paramagnetic resonance [13] and positron emission tomography [11] are of limited value due to restricted access of the imaging probes to the hypoxic regions. Other imaging modalities provide ways to infer hypoxia and its spatial and temporal variation by quantifying the haemodynamics of tissue perfusion, total haemoglobin (HbT) or blood oxygen saturation (SaO2), each with its own limitations [11].
Quantitative SaO2 imaging is particularly challenging. For example, blood oxygen level dependent magnetic resonance imaging (BOLD MRI) is only sensitive to differences between two states of the proportion of deoxy-haemoglobin (Hb) present, rather than an absolute level of oxygenation. In tumour studies, the change of state is achieved by changing the level of oxygen in the gas breathed [14]. Diffuse optical spectroscopic imaging [15] is in principle able to overcome this difficulty by using recognisable properties of the wavelength dependence of optical absorption in Hb and oxy-haemoglobin (HbO2) to make absolute measurements of Hb and HbO2. However, it is accompanied by depth-dependent low spatial resolution due to scattering of photons by tissue, leading to difficulty in interpretation of blood SaO2 or HbT values. This method works best when one can transmit light entirely through the body part to be imaged and therefore its application has largely been limited to breast imaging [16], [17], [18].
Multispectral optoacoustic imaging also uses the spectroscopic absorption signatures of HbO2 and Hb to make absolute measurements of HbT and SaO2 but is able to do this with a high spatial resolution (determined by acoustic wave focusing) without the problems associated with photon scattering and without needing to transmit light entirely through the body [19], [20], [21]. The measurements do, however, depend on the system’s sensitivity to identify Hb and HbO2, which is affected by depth-dependent signal to noise ratio, the spectral dependence of light attenuation in intervening tissue and the presence of other chromophores (for example oxy- and deoxy-myoglobin [22]). Additionally, “discrete sampling issues” and “imperfectness in the reconstruction algorithm”, as reported by Ding et al. [23], could also result in the appearance of ambiguous negative values that would make the spectral recognition of Hb and/or HbO2 more difficult. An absence of intra-tumoural optoacoustic haemoglobin signal is ambiguous in that it cannot be known whether such regions are truly avascular or whether they are perfused but with a tissue blood volume that is below the threshold of detection.
We hypothesise, therefore, that there would be value in combining multispectral optoacoustic imaging with a co-registered sensitive perfusion imaging method for resolving such ambiguity. In addition, such a combinative imaging approach may be useful for gaining a more complete understanding of the hypoxic state of the tumour. For example, tumour vascular perfusion characteristics might one day be combined with local blood oxygenation estimates using an oxygen transport model [24], to enable estimation of the local partial pressure of oxygen within the tumour cell environment.
To test this hypothesis we chose to combine multispectral optoacoustic imaging with microbubble based dynamic contrast enhanced ultrasound (DCE-US). It is practical to combine these modalities since they both use ultrasound and can be implemented on the same hardware. Moreover, microbubbles can be used to image the vasculature at the microscopic scale [25], [26] and their size makes them behave like erythrocytes, effectively preventing their extravasation from the fenestrated microvasculature, making DCE-US sensitive solely to the vascular perfusion characteristics, whereas alternatives such as contrast enhanced (CE-) MSOT [27], DCE-MRI [28] or DCE computed tomography (CT) [28] use contrast agents (indocyanine green, iodine or gadolinium, respectively) [29] that move between the vascular and interstitial compartments. The study might also have been performed using CE-MSOT, or even DCE-MRI or DCE-CT, utilising contrast agents that would remain intravascular, such as microbubbles loaded with dye molecules [30], gadolinium [31] and gold nanoparticles [32], respectively. Such agents, however, remain in the research domain and are not readily available, whereas microbubbles are conveniently and commercially available, licensed for use in clinical ultrasound, and modern nonlinear signal processing makes ultrasound scanners extremely sensitive to their presence (even detecting a single microbubble at a depth of many centimetres in tissue [26]). These provided the main reasons why DCE-US was chosen for the present study, knowing that the findings should be applicable to other contrast enhanced imaging modalities given a suitable contrast agent. A further advantage of studying the relationship between MSOT and DCE-US, rather than between MSOT and contrast enhanced MSOT, MRI or CT, is the potential for unanticipated discoveries which have useful consequences. Specifically, although it was not an aim of this study, if correlations between MSOT and DCE-US features were to be discovered, this may suggest a way to translate the use of valuable MSOT image information into the clinic in situations, such as when imaging deep within the body, where MSOT can not be employed.
Although previous studies have compared DCE-US with multispectral optoacoustic imaging in animal tumours [33], [34], [35], [36], none have investigated the above hypothesis nor explored the full range of microbubble-based perfusion properties such as time of arrival, time to peak, wash-in time, peak contrast, area under the curve (AUC), wash-in rate and wash-out rate with SaO2 and HbT. The aims of this study were twofold: (i) to assess whether regions in optoacoustic images lacking an identifiable blood spectral signature are indeed avascular, (ii) to determine whether there are relationships between the properties obtained using the two imaging approaches.
An MSOT inVision 256-TF™ (iThera Medical, Munich) was used for optoacoustic imaging because of its real-time full spectral and whole mouse cross-sectional imaging ability, and a clinical scanner (Aplio XG™, Toshiba, Tokyo) was used for DCE-US so that findings from the study may have the potential for direct clinical translation and full advantage can be taken of commercially available microbubble agents, which as yet are not available for high-frequency ultrasound imaging [37]. As a consequence, the study required the development of a novel experimental apparatus and scanning method to assist the process of co-registering image data between the two systems, which is also described in this paper.
2. Methods
2.1. Cell lines
Two pancreatic tumour models were chosen because they possess a heterogeneous vasculature with hypoxic regions [38], [39], [40]. The cell line, PDA-KPC-1 GEMM, derived from KPC mice, a genetically engineered pancreatic ductal adenocarcinoma model, was obtained from the University of Pennsylvania (Philadelphia, USA) [41]. The carcinoma cell line, MIA PaCa-2, was obtained from ATCC. Cells were grown in Dulbecco's Modified Eagle Medium, supplemented with 10% foetal bovine serum, in a humidified atmosphere of 5% CO2 in air at 37 °C.
2.2. Tumour model
Groups of female CrTac:NCr-Fox1nu athymic nude mice, approximately 6 weeks of age, were injected subcutaneously on the right flank with either 3 million PDA-KPC-1-GEMM cells (n = 2) or 5 million MIA PaCa-2 cells (n = 3). The animals were imaged when the tumour volume reached about 500 mm3.
2.3. Optoacoustic imaging
Optoacoustic imaging was performed using a commercially available pre-clinical tomographic whole-body imaging system, MSOT inVision 256-TF™. As stated by the manufacturer, the imaging system contains a partial ring array of 256 transducer elements, each with a centre frequency of 5 MHz and a bandwidth of greater than 60%. The array covers a reception angle of 270°. The animal to be imaged was anaesthetised using an intraperitoneal injection of fentanyl, midazolam and medetomidine in distilled water (ratio of 2:2:1:5, 10 mL/kg) and the tail vein was catheterised using a catheter fabricated in the laboratory. The animal was then placed in acoustic coupling gel in an animal holder consisting of a nose cone for air supply and a polyethylene membrane, which was then submerged in the water tank of the system, maintained at 34 °C. The animal in the holder was illuminated with multiple 10 ns pulses of laser light, at a pulse repetition interval of 100 ms, each pulse at a different wavelength ranging from 710 nm to 950 nm, at intervals of 10 nm. In order to create a cross-sectional image at one wavelength, optoacoustic signals generated by the tissue from 10 pulses were averaged. The signals were reconstructed using a model-based inversion algorithm [42] (ViewMSOT™ v 3.6). The in-plane resolution of such images has been measured to be of the order of 150 μm (measured by iThera Medical). The animal was then translated along its long axis in steps of 1 mm to obtain additional cross-sectional images which together covered the whole tumour. The slice-thickness resolution has been measured to be about 800 μm (measured by iThera Medical).
2.4. Ultrasound imaging
After optoacoustic imaging, for DCE-US imaging, the anaesthetised animal in its holder was transferred undisturbed to a purpose-built gantry, designed to replicate the way that the holder is supported in the MSOT imaging system. As shown in Fig. 1 the gantry consisted of a water-bag standoff replicating the water tank setting of the animal holder in the MSOT system. The animal in the animal holder was submerged in the water warmed to a temperature of 34°C. Maintaining the animal in its original holder, and submerged in water, restricted changes in posture and orientation of the tumour. The animal’s body temperature was maintained during the imaging process using a heating lamp. A 1204BT linear array probe (Toshiba Aplio XG™ clinical US scanner) was mounted beneath the animal holder and water bag on a mechanical stage, pointing upwards to image the animal in cross-section. The ultrasound probe was translated mechanically along the long axis of the animal in steps of 1 mm to cover the whole tumour. After an initial full mechanical scan to record the tumour’s echo anatomy, the transducer was aligned to the cross-section of the tumour having maximum area. 100 μL of Sonazoid™ microbubbles [43] was then injected through the catheter. Interleaved non-linear contrast mode and fundamental B-mode images were recorded at a frame rate of 10 Hz for up to 40 s. Non-linear contrast mode images were obtained by the scanner’s proprietary pulse-subtraction coded harmonic technique with a mechanical index of less than 0.3 and a dynamic range setting of 65 dB.
Fig. 1.
The DCE-US imaging apparatus which replicates the arrangement used to support the animal holder in the MSOT inVision 256™ and thus provides a good basis for subsequent registration between ultrasound and optoacoustic images. The red cast in the image is due to the heating lamp in place to maintain the animals core temperature. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. Data analysis
Prior to optoacoustic image analysis, any negative value pixels arising as a result of noise or reconstruction errors were assigned a value of zero. Under the assumption of no position or wavelength dependent fluence variation due to light attenuation, the reconstructed data were spectrally unmixed using linear regression (ViewMSOT™ v 3.6) to resolve the Hb and HbO2 components in the optoacoustic image. For every pixel in the reconstructed image, the unmixing algorithm models the observed optoacoustic spectrum as a linear combination of the known spectra of Hb and HbO2, to compute Hb and HbO2 images as the spatial distribution of the relative concentration of each absorber [21], [44], [45]. Mean SaO2 and mean HbT were then calculated for user-defined regions of interest (ROIs) within the tumour (see below) using the Eq. (1) [46], [47] and Eq. (2),
| (1) |
| (2) |
where and are the means over the ROIs of the MSOT calculated concentrations of HbO2 and Hb respectively. An “oxymap” image, showing the calculated SaO2 values for each pixel, was also obtained, using the ViewMSOT software. This was not used for calculating the mean SaO2 values in ROIs because it was observed to be subject to pixel dropout and the software did not account for the zero value pixels for calculating the mean values of SaO2 in a region of interest.
The MSOT and DCE-US images having maximum tumour cross-sectional area were assumed to correspond to a similar plane and were registered manually as follows, utilising rigid body transformations within Microsoft’s Expression Encoder 4™ (Microsoft, Washington). First, the spectrally unmixed and oxymap images were spatially rescaled, maintaining the aspect ratio, to match the 10 mm scale bar to that of the DCE-US image. After rescaling, the MSOT images were rotated and translated so that the tumour boundary and any major blood vessels observed in the single wavelength image at 800 nm (isosbestic point of Hb and HbO2) matched those of the non-contrast and contrast ultrasound images. Blood vessels were made more visible in the contrast mode ultrasound images by subtracting the pre- and post-microbubble injection sequences (examples are shown later in Figs. 4(E) and 5(E), encircled by a red boundary). The spectrally unmixed and oxymap images were translated and rotated to the same degree. Rigid body-transformations were assumed sufficient for registering the two imaging modalities because the animal was maintained in its original holder and submerged in water, which restricted changes in animal posture and orientation of the tumour.
Fig. 4.
(A) An ultrasound image of the M1 (PDA-KPC) tumour before microbubble injection. B) The ultrasound image of the tumour 40 s after microbubble injection. C) An overlay of the spectrally unmixed optoacoustic image from Fig. 2B on the ultrasound image from (A) after rigid registration of the two images. D) The graphic overlay on (C) to indicate the regions of interest analysed, colour coded as red (blood vessel), dashed purple (upper rim), orange (upper body), dashed light green (core), dark green (lower body) and dashed cyan (lower rim). (E) Microbubble post-injection contrast-mode US images at time points of 10, 15 and 40 s respectively, after subtraction from the pre-injection image. (F) The time intensity curves obtained by analysing the DCE-US data according to the regions of interest shown in (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
A) An ultrasound image of the M3 (MIA PaCa-2) tumour before microbubble injection. B) The ultrasound image of the tumour 40 s after microbubble injection. C) An overlay of the spectrally unmixed optoacoustic image from Fig. 3B on the ultrasound image from (A) after rigid registration of the two images. D) The graphic overlay on (C) to indicate the regions of interest analysed, colour coded as red (blood vessel), dashed purple (upper rim), green (top left dark region), brown (top right dark region), dashed yellow (inner blood vessel), pink (bottom dark region) and dashed cyan (lower rim). E) Microbubble post-injection contrast-mode US images at time points of 10, 15 and 40 s respectively, after subtraction from the pre-injection image. (F) The time intensity curves obtained by analysing the DCE-US data according to the regions of interest shown in (D). (G) The time intensity curves obtained by analysing the DCE-US data in the three black regions according to the regions of interest shown in (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Regions of the tumour showing an absence of HbO2 and Hb on the spectrally unmixed optoacoustic images were selected by manual outlining to assess their perfusion characteristics with DCE-US. Time intensity curves (TICs) were calculated as the mean contrast signal within each ROI verses time, after background echo image subtraction, using a program written in Matlab (2010b, MathWorks, Natick, MA).
In order to further assess any potential relationship between HbT and SaO2 values and DCE-US perfusion characteristics, ROIs were selected in the periphery and in the body of the tumour where the optoacoustic images showed a presence of blood, and the same regions were selected on the DCE-US image sequences. Seven properties were calculated from the TICs and compared with SaO2 and HbT, as calculated optoacoustically. The peak contrast (maximum contrast value attained by the TIC), time to peak (time required for the microbubbles to reach the peak contrast) and the time of arrival (the intersection point between the baseline and a tangent of the wash-in rise) of microbubbles for different regions of the tumour were obtained by visually fitting a smooth curve to each TIC. The wash-in time was obtained as a difference between the time to peak and time of arrival. The wash-in rate was determined by measuring the slope of a straight line between the peak contrast and contrast at the time of arrival. The wash-out rate of microbubbles was calculated as the decay constant of an exponential decay curve that was fit to the TIC data between the time of peak contrast and the 40 s time point.
To estimate and assess the relationships of SaO2 and HbT to DCE-US properties, two types of analyses were carried out. First, a within-tumour analysis was conducted in which, for each tumour, the correlation between each optoacoustic and each DCE-US image property was evaluated. For each tumour and each pair of properties, the mean property value was calculated in each ROI, after which the Pearson correlation coefficient was calculated over the number of ROIs in that tumour. This allowed the relationships between the optoacoustic and the DCE-US properties to be assessed without the confounding effect of inter-tumour variation. However, the significance of any such correlations, if present, would inevitably be low because the number of ROIs in each tumour was never more than seven, and was sometimes as few as four. Therefore the significance of the within-tumour correlation coefficients was assessed by studying their consistency across all of the tumours, as revealed by a Student’s t-test applied to the means and standard deviations (across tumours) of the within-tumour correlation coefficients for each pair of properties.
Second, the correlation between each optoacoustic and each DCE-US property was evaluated using all ROIs for all tumours. Here, the correlations were expected to be low, and of low significance, because of the confounding influence of inter-tumour variations. Therefore, this analysis was repeated after applying an approximate correction method, the objective of which was to estimate the values of the correlation coefficients that would be expected if there were no inter-tumour variation. This correction method began by calculating for each mouse the slope aMi and intercept bMi in the least squares best fit of the linear model (y = aMix + bMi) for the variation of image property y with image property x over the tumour ROIs for that mouse. The mean slope and mean intercept over all such fits for all mice were then calculated using:
| (3) |
where N is the total number of mice.
A corrected data point, corrected for the departure of a given mouse’s best fit linear relationship from the average relationship, was computed for each data point corresponding to each ROI using Eq. (4):
| (4) |
where and yMi = aMixk + bMi, and k is the ROI number for the mouse. New correlation coefficients rcorrected, and their corresponding p values, were calculated using these corrected data points.
The AUC, correlation coefficients and corrections for between-tumour variations were calculated using Prism™ version 7 (GraphPad, San Diego). Means and standard deviations, and statistical assessments using Student’s t-test, were calculated using Microsoft Excel® 2007™.
3. Results
3.1. Optoacoustic imaging
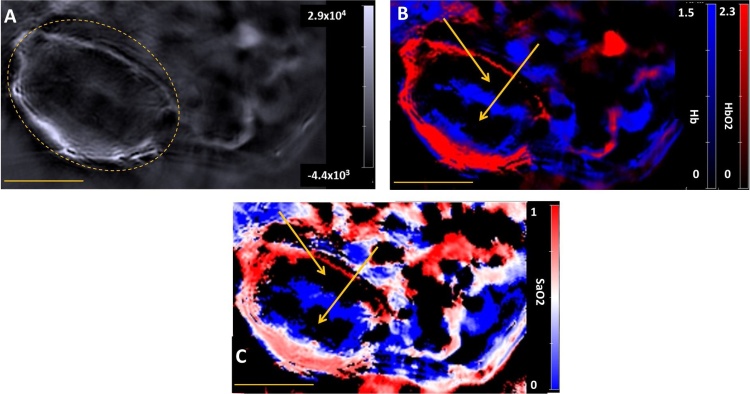
For both tumour models, the optoacoustic signals found at the periphery of the tumours were typically stronger than those found in the tumour body; examples are shown in Figs. 2 A and 3 , A. In order to allow the variations in image appearance between mice to be better appreciated, image sets from two additional mice (M2 and M4) are provided in Figs. S1 and Fig. S2, respectively, in the supplementary section. Spectral unmixing revealed a strong presence of HbO2 in the periphery of the tumours (Figs. 2B and 3B), corresponding to a high SaO2 value (Figs. 2C and 3C). The body of the tumours had a higher proportion of Hb in comparison to the periphery (Figs. 2B and 3B), corresponding to a low SaO2 value. The PDA-KPC tumour showed an absence of Hb/HbO2 signal in its core, as evidenced by black regions and marked by arrows in Fig. 2B and C. The MIA PaCa-2 tumour illustrated showed heterogenous vascularisation in its body, with an absence of blood signal in three segments, as indicated by arrows in Fig. 3C.
Fig. 2.
A) A reconstructed single wavelength MSOT image of mouse M1 (example of a PDA-KPC tumour) at 800 nm. The dashed yellow line indicates the tumour. B) The corresponding spectrally unmixed image, showing the distribution of Hb and HbO2 components in blue and red respectively. C) The oxymap image, showing the distribution of the SaO2 values. The yellow scale bar at the lower-left in each of these images is 5 mm. The yellow arrows in images B and C indicates the centre of a region that lacks any spectral signature of blood. The background, Hb, HbO2 and oxymap colour bars reflect the magnitude of the photoacoustic signals, deoxy-haemoglobin, oxy-haemoglobin and SaO2, respectively, as calculated by the MSOT system. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
A) A reconstructed single wavelength MSOT image of mouse M3 (example of MIA PaCa-2 tumour) at 800 nm. The dashed yellow line indicates the tumour. B) The corresponding spectrally unmixed image, showing the distribution of Hb and HbO2 components in blue and red respectively. C) The oxymap image, showing the distribution of the SaO2 values. The yellow scale bar at the lower-left in each of these images is 5 mm. The yellow arrows in images B and C indicates the centre of a region that lacks any spectral signature of blood. The background, Hb, HbO2 and oxymap colour bars reflect the magnitude of the photoacoustic signals, deoxy-haemoglobin, oxy-haemoglobin and SaO2, respectively, as calculated by the MSOT system. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Contrast mode imaging and registration with optoacoustic imaging
In order to assess whether tumour regions having no blood signal on optoacoustic imaging also lacked observable perfusion on DCE-US, ROIs were drawn around black regions on the optoacoustic Hb:HbO2 image, such as the core of the PDA-KPC tumour in Fig. 4(D) (dashed green and dark green) and in the three different segments of the tumour MIA PaCa-2, as seen in Fig. 5(D) (top-left dark region (green colour), top-right dark region (brown) and bottom dark region (pink)). The same ROIs were applied to the DCE-US image sequences to obtain TICs (Figs. 4F and 5F) and evaluate the perfusion characteristics.
Figs. 4C and 5C show the spectrally unmixed MSOT images as transparent overlays on the DCE-US images of the same example PDA-KPC and MIA PaCa-2 tumours as shown in Fig. 2, Fig. 3, respectively. The contrast mode ultrasound images of the tumours before and after microbubble injection are shown in Fig. 4A and B, for the PDA-KPC tumour case, and Fig. 5A and B, for the MIA PaCa-2 tumour case.
In order to assess whether the microbubble perfusion properties were correlated in general with SaO2 and HbT values, ROIs were also selected in other parts of the tumour and in a feeding blood vessel. Example ROIs are illustrated in Fig. 4D in red (blood vessel), dashed purple (upper rim), orange (upper body), and dashed cyan (lower rim), and Fig. 5D in red (blood vessel), dashed purple (upper rim), dashed yellow (inner blood vessel), and dashed cyan (lower rim).
Figs. 4E and 5E show the overlays of the selected ROIs on the microbubble post-injection contrast-mode US images at time points of 10, 15 and 40 s, after subtraction from the pre-injection image. As seen in Figs. 4E and 5E, the microbubbles arrive first at the feeding blood vessel at a time point t ∼ 10 s, and subsequently arrive in other parts of the tumour. As seen in mouse M1 (PDA-KPC) in Fig. 4, the tumour core, lacked a blood signal in the MSOT image (Fig. 4D) and was echogenic on US before contrast injection (Fig. 4A), was perfused on DCE-US, albeit with a low blood volume and speed (Fig. 4E (t = 40s) and 4F, dashed green curve). Similarly, in mouse M3 (MIA PaCa-2) in Fig. 5, the three black regions (Fig. 5D) were highly perfused, as can be seen from the background subtraction image at t = 40 s (Fig. 5E) and TICs of the dark regions in Fig. 5F. Similar results were observed in all tumour cases. These results clearly demonstrate the value of combining the two imaging modalities, to differentiate the poorly perfused regions of the tumour from those having no perfusion, which is difficult to observe with MSOT alone.
3.3. Relationships between microbubble dynamic properties and blood content and oxygenation
For the within-tumour analysis, as seen in Fig. 6A and C, there was a consistent negative correlation of SaO2 and HbT with the time of arrival, time to peak and wash-in time. Similarly, SaO2 and HbT were positively correlated in almost all mice with peak-contrast, wash-out rate, wash-in rate and AUC, there being one exception, a negative correlation of HbT with AUC in mouse M1 (PDA-KPC). Since a limited number of ROIs (4–7) were selected for calculating these correlation coefficients, only a few were significant at the p = 0.05 level (indicated by an asterisk in Fig. 6A and C). However, significance is established because of the consistency of the within-tumour behaviour across all of the tumours; as shown in Fig. 6B and D, the mean correlation coefficients were significantly different from zero for all but one optoacoustic and DCE-US combination.
Fig. 6.
(A) The correlation coefficients for SaO2 with each DCE-US characteristic for individual tumour cases (B) The means and standard deviations of the intra-tumour correlations shown in (A). (C) The correlation coefficients for HbT compared with each DCE-US characteristic for individual tumour cases. (D) The means and standard deviations of the intra-tumour correlations shown in (C). The asterisk signs indicate the significance level for there being no correlation (* (p ≤ 0.05), ** (p ≤ 0.01), *** (p ≤ 0.001), **** (p ≤ 0.0001)).
Results for the cross-tumour analysis without accounting for the inter-tumoural variation of the measurements are shown in Fig. 7, Fig. 8, Fig. 9. These results have been grouped so as to show the observed negative correlations in Fig. 7, positive correlations involving DCE-US blood volume measures in Fig. 8 and positive correlations involving DCE-US rate measures in Fig. 9.
Fig. 7.
Variation of SaO2 (left column) and HbT (right column) with microbubble time of arrival (top), time to peak (middle) and wash-in time (bottom). Correlations between SaO2 and these DCE-US TIC properties were stronger and more significant than those between HbT and the DCE-US TIC properties. The p values indicate the probabilities that the correlation coefficients (r) are zero. Symbols represent individual mice:  M1(PDA-KPC),
M1(PDA-KPC),  M2(PDA-KPC),
M2(PDA-KPC),  M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),
M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),  M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
Fig. 8.
Variation of SaO2 (left column) and HbT (right column) with microbubble AUC (top) and peak contrast (bottom). Correlations involving HbT were stronger and more significant than those involving SaO2. The p values indicate the probabilities that the correlation coefficients (r) are zero. Symbols represent individual mice:  M1(PDA-KPC),
M1(PDA-KPC),  M2(PDA-KPC),
M2(PDA-KPC),  M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),
M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),  M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
Fig. 9.
Variation of SaO2 and HbT with microbubble wash-in and wash-out rates. Significant correlations were observed for SaO2 and HbT with wash-in rate. The correlations of SaO2 and HbT with wash-out rate were not significant at the 0.05 level. The p values indicate the probabilities that the correlation coefficients (r) are zero. Symbols represent individual mice:  M1(PDA-KPC),
M1(PDA-KPC),  M2(PDA-KPC),
M2(PDA-KPC),  M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),
M3(MIAPaCa-2), ▼ M4(MIAPaCa-2),  M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
M5(MIAPaCa-2). The indicative linear regression line is to aid visual interpretation of the strength of correlation and is not intended to imply that a linear relationship exists.
Referring to Fig. 7, significant negative correlation was observed between SaO2 and DCE-US derived microbubble time of arrival (r = −0.5629, p = 0.0015), time to peak (r = −0.597, p = 0.0006) and wash-in time, (r = −0.4966, p = 0.0061). HbT also showed a negative correlation with time of arrival (r = −0.4743, p = 0.0093), time to peak (r = −0.3625, p = 0.0533) and wash-in time (r = −0.239, p = 0.2117). However, the correlation coefficients involving HbT were consistently lower than those involving SaO2, and were not significant for time to peak and wash-in time.
Significant positive correlation was observed between HbT and the DCE-US blood volume TIC properties (AUC and peak contrast). As seen in Fig. 8, the correlations between HbT and AUC (r = 0.4059, p = 0.0289) and peak contrast (r = 0.4944, p = 0.0064) were stronger and more significant than those involving SaO2 with AUC (r = 0.3411, p = 0.0702) and with peak contrast (r = 0.3083, p = 0.1038).
Significant positive correlations were observed between microbubble wash-in rate and both SaO2 (r = 0.5294, p = 0.0031) and HbT (r = 0.4313, p = 0.0195). Although positive correlations were also seen between microbubble wash-out rate and SaO2 (r = 0.2854, p = 0.1334) and HbT (r = 0.3584, p = 0.0563), neither was significant at the p = 0.05 level.
A summary of the correlation coefficients from Fig. 7, Fig. 8, Fig. 9, and their significance, is shown in Fig. 10. The significance levels after correcting for inter-tumoural variations (Section 2.5) are indicated as red asterisks in brackets in Fig. 10. This approximate attempt to account for inter-tumour variability increased the significance of 7 of the 14 correlations (particularly the time based TIC properties), had no effect on the significance of 5 and decreased the significance in 2. The scatter plots of the relationships between the optoacoustic imaging and the microbubble DCE-US characteristics, after correcting for the inter-tumoural variability (Figs. S3, S4 and S5) have been included in the supplementary section.
Fig. 10.
Summary of the observed correlations of optoacoustic imaging properties SaO2 (A) and HbT (B) with DCE-US characteristics for all ROIs in all tumours. Asterisks indicate statistically significant correlations before (black) and after (red bracket) accounting for inter-tumoural variation at levels: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. A non-significant correlation at the p = 0.05 level is denoted by (ns). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge, this is the first time that a combinative imaging approach of optoacoustic imaging and DCE-US has been applied to an automated whole-body mouse tomographic optoacoustic imaging system, the MSOT inVision 256–TF, which lacks the capability to perform microbubble contrast enhanced ultrasound imaging. For all five tumours, it was observed that certain tumour regions that showed no optoacoustic blood signal were perfused on DCE-US. Similar findings can be expected with CE-MSOT using a contrast agent that does not extravasate. A low blood volume (as indicated by a low microbubble uptake on DCE-US), below the optoacoustic detection threshold of the imaging system, seems to be a plausible explanation. These tumours are not prone to contain confounding absorbers; nor does it seem likely that the light attenuation in the subcutaneous tumours would be a contributing factor, that would obscure the recognition of haemoglobin. Nevertheless, to improve the spatial homogeneity and quantification of tumour SaO2 and HbT, incorporating an algorithm to estimate the wavelength and depth-dependent light attenuation would be helpful. Tzoumas et al. [21] recently developed an approach to incorporate wavelength-dependent light attenuation to estimate blood SaO2 within deep tissue with more accuracy, using the MSOT system. Work in this area is underway. An alternative (hypothetical) explanation for the above observation is that the microbubbles, given their smaller size than red blood cells, were reaching areas of the tumour not accessible to the blood cells.
The SaO2 values were found to be negatively correlated with DCE-US time of arrival and time to peak with a high level of significance (Fig. 7). This implies that the intravascular microbubbles follow the path of the red blood cells. As the oxygenated blood travels from the feeding vessels to the microvasculature of the tumour, the blood SaO2 should decrease on the path taken by the red blood cells. The extent of the decrease in SaO2 would depend on the metabolic activity and demand for oxygen, which would be considerable for the periphery of the tumour in comparison with its hypoxic core [48]. A similar correlation was also observed with HbT, although with a lower correlation coefficient and a lower level of significance.
HbT was positively correlated with AUC and peak contrast (Fig. 8). These correlations were expected as HbT, peak contrast and AUC are all dependent on the local blood volume. This finding broadly confirms the results (a) of Zion et al. [33], who observed a positive correlation of AUC with HbT in PC3 human prostate carcinoma xenograft models, and (b) of Eisenbrey et al. [36], who had observed a positive correlation of peak contrast with HbT (r = 0.49, p = 0.021). Given that Zion et al. and Eisenbrey et al. used different tumours, a different microbubble contrast agent and different ultrasound and optoacoustic imaging systems to those employed here, this confirmation of findings lends credance to their results and ours, and increases confidence in other findings reported only here.
A significant positive correlation of SaO2 and HbT with wash in rate of microbubbles was observed (Fig. 9). The wash-in and the wash-out rates are related to blood velocity. The red blood cells would have a higher velocity in the peripheral tumoural blood vessels offering less resistance than the tortuous tumour vasculature accompanied by a high interstitial pressure, commonly present in the interior of the tumour [49]. Regions of the tumour showing a high SaO2 and HbT value, predominantly in the periphery, showed a higher wash-in rate in comparison to the regions having low SaO2 and HbT values. A moderately positive correlation of SaO2 and HbT with the wash-out rate was also observed, although it was not statistically significant. The transit time, the total time for the bubbles to pass a particular position, was not measured because we did not allow time for the bubble contrast signal to return to baseline. This suggests the need for further work to observe microbubbles for longer periods. However, the sum of wash-in rate and wash-out rate could be regarded as directly related to the inverse of transit time, and a negative correlation between SaO2 and HbT with the microbubble transit time can therefore be expected.
The correlations observed between the optoacoustic and DCE-US properties of different tumour regions suggest that a combination of low microbubble signal, late microbubble arrival and slow microbubble transit should be predictive of low oxygen saturation. This remains to be tested but suggests new roles for DCE-US in providing surrogate measures of oxygenation levels, which may be of value for example in predicting radiation and drug treatment effectiveness in clinical situations where a tumour to be treated is too deep to be imaged optoacoustically.
In order to assess whether the correlation coefficients, along with their significance, may have been decreased by inter-tumoural variations in the functional relationship between image properties, a difference between the overall mean relationship for a pair of image properties (over all ROIs and all tumours) and that for all ROIs for a tumour was obtained, and used to obtain a correction factor that was subtracted from each dependent image property value in a pair, for each individual tumour. The resulting changes in correlation coefficients and their significance, as indicated in brackets (red) in Fig. 10, suggest that inter-tumoural variation was important in some relationships and not others. It would therefore be worthwhile continuing such studies with increased statistical power as described in the following paragraph.
A limitation of the present study was that the registration of the MSOT and US images was dependent on the author’s judgement of the matching features in the two sets of images, and of finding an MSOT image that matched the DCE-US imaging plane. Such registration may vary from user to user. Importantly for the present study, its limited accuracy prevented a pixel by pixel correlation between the optoacoustic and DCE-US image properties of the type achieved by Zion et al. [33], which was possible in their case because they used hardware in which optoacoustic and ultrasound imaging were implemented with the same transducer. This limited the statistical power of our intra-tumoural analyses since large ROIs had to be used, restricting the number of ROIs per tumour to between four and seven. Repeating this study using simultaneous optoacoustic and DCE-US imaging with the same transducer should significantly reduce this source of variation, allowing greatly improved statistical power when assessing the intra-tumoural relationships between image characteristics. It also provides motivation for investigating the use of automated registration methods, implementing DCE-US on the MSOT system and the development of 3D DCE-US. The study is also limited by the absence of histological confirmation of regions of the tumours lacking a blood signature on MSOT. From this study, no conclusions can be drawn regarding the histological nature of such regions, e.g. the condition of the endothelium, or whether the cells there are hypoxic, necrotic or apoptotic. In fact, there is a general need for studies that use histological markers as a reference standard for assessing blood and oxygen saturation using optoacoustic images; work in this area has just begun [50]. However, the absence of such confirmation in the present study does not detract from our finding that in some dark regions, as imaged by MSOT, the tissue was perfused.
Finally, for preclinical combined modality research using the iThera MSOT inVision™, the novel registration system described may be of more general interest, for registering MSOT images to various ultrasound images such as B-mode, microbubble, Doppler and elastography, as well as for registering to high intensity ultrasound and other therapeutic devices. Additional uses may include the optoacoustic localisation (e.g. for targeting with physical therapies) of orthotopic tumours grown from cell lines that have been genetically modified to express proteins that are spectroscopically identifiable by MSOT.
5. Conclusion
There is value in combining optoacoustic spectral imaging with dynamic contrast ultrasound imaging when studying tumour hypoxia. For example, combining the two imaging modalities allows DCE-US to assist interpretation of the optoacoustic images, as demonstrated here where DCE-US helped to answer the question of whether a region that lacks an optoacoustic blood signal is perfused. Eventually, data from a vascular haemodynamic property measurement method such as microbubble ultrasound may be combined with blood oxygenation and haemoglobin concentration measures from a method such as optoacoustic imaging, in a model-based estimation of the hypoxic status of cancer cells in specific regions of a tumour [23].
If further work confirms that DCE-US properties are predictive of low blood oxygen saturation, this finding would suggest potential to use DCE-US characteristics as surrogates for blood oxygen saturation, in predicting radiation and drug treatment effectiveness in clinical situations when tumours are located too deep for optoacoustic imaging to be used.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This research was supported by the EPSRC, the Cancer Research UK Cancer Imaging Centre at the Institute of Cancer Research and Royal Marsden NHS Foundation Trust, and the Research Council of Norway. We acknowledge funding (SE, GB) from Cancer Research UK [grant number C309/A11566], EPSRC strategic equipment grant EP/NO15266/1 and NHS funding to the NIHR Biomedical Research Centre. We are grateful to Phoenix Solutions AS, for kindly supplying the Sonozoid™.
Biographies

Anant Shah received his Ph.D. degree in biophysics in 2014 from the Institute Of Cancer Research, for his work on photoacoustic imaging of molecular markers of cancer prognosis and response using gold nanoparticles. He is currently working as a postdoctoral researcher at the Institute of Cancer Research. His research is oriented towards assessing the potential of photoacoustic imaging for cancer treatment planning and treatment response.

Nigel Bush is a member of the Ultrasound and Optics team in the Division of Radiotherapy and Imaging at The Institute of Cancer Research (Sutton,UK). Since 1979 he has been involved in a broad range of ultrasound imaging research projects applied to clinical and preclinical studies. Research fields include ultrasound skin imaging, elastography, tissue property characterisation, high frequency array development and US contrast agents. He is currently using ultrasound and photoacoustic imaging to monitor novel contrast agent- drug therapies to treat pre-clinical tumour xenografts.

Gary Box has worked at the Institute of Cancer Research for over thirty years and is now currently working within the Cancer Research UK Cancer Therapeutics Unit at the ICR. He has many years of experience in developing and optimizing various in vitro models (e.g. invasion and migration assays); evaluating novel compounds for potential efficacy studies; ex vivo culture and genetically tagging cell lines for bioluminescence imaging and fluorescence imaging.

Sue Eccles was until her recent retirement Professor of Experimental Cancer Therapeutics within the CRUK Cancer Therapeutics Unit, The Institute of Cancer Research and now holds the position of Honorary Professor of Tumour Biology in the Cancer Therapeutics Division. She was responsible for directing the preclinical evaluation of compounds emerging from the CTU’s molecularly targeted drug discovery programme. Research interests include the cellular and molecular mechanisms of metastasis, focussing on 3D in vitro assays and in vivo tumour model systems and the role of the microenvironment (including hypoxia) in tumour progression and resistance to therapy.

Jeff Bamber is Professor in Physics Applied to Medicine, leader of the Ultrasound and Optical Imaging Team and a Senior Tutor at the Institute of Cancer Research (ICR), London. He holds honorary appointments at the Royal Marsden and other hospitals and colleges in London. He has contributed to subjects such as understanding the acoustic characteristics of normal and malignant tissues, ultrasound image formation, speckle and clutter reduction, elastography and photoacoustic imaging. His current research aims to increase the functional and molecular imaging capability of ultrasound, providing new tools to experimental cancer biology and helping to personalise cancer treatment by bringing the cost-effectiveness, safety, speed and convenience of ultrasonic methods to clinical problems such as assessing tumour aggressiveness and response, and guiding treatment. He is past president of the International Association for Breast Ultrasound and past vice-president of the International Society of Skin Imaging.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.pacs.2017.08.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Nelson D.A., Tan T.-T., Rabson A.B., Anderson D., Degenhardt K., White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luoto K.R., Kumareswaran R., Bristow R.G. Tumor hypoxia as a driving force in genetic instability. Genome Integr. 2013;4:5. doi: 10.1186/2041-9414-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graeber T.G., Osmanian C., Jacks T., Housman D.E., Koch C.J., Lowe S.W. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 4.Harris A.L. Hypoxia – a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 5.Shannon A.M., Bouchier-Hayes D.J., Condron C.M., Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 6.Gray L.H., Conger A.D., Ebert M., Hornsey S., Scott O.C.A. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 7.Jubb A.M., Buffa F.M., Harris A.L. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J. Cell. Mol. Med. 2010;14:18–29. doi: 10.1111/j.1582-4934.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaupel P., Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 9.Knocke T.-H., Weitmann H.-D., Feldmann H.-J., Selzer E., Pötter R. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother. Oncol. 1999;53:99–104. doi: 10.1016/s0167-8140(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 10.Brizel D.M., Scully S.P., Harrelson J.M., Layfield L.J., Bean J.M., Prosnitz L.R. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 11.Walsh J.C., Lebedev A., Aten E., Madsen K., Marciano L., Kolb H.C. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid. Redox Signal. 2014;21:1516–1554. doi: 10.1089/ars.2013.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X., Wang X., Mao H., Wu W., Liu B., Jiang X. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015;6:5834. doi: 10.1038/ncomms6834. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K., Subramanian S., Devasahayam N., Aravalluvan T., Murugesan R., a Cook J. Electron paramagnetic resonance imaging of tumor hypoxia: enhanced spatial and temporal resolution for in vivo pO2 determination. Magn. Reson. Med. 2006;55:1157–1163. doi: 10.1002/mrm.20872. [DOI] [PubMed] [Google Scholar]
- 14.Baudelet C., Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn. Reson. Med. 2002;48:980–986. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 15.Cerussi A.E., Tanamai V.W., Hsiang D., Butler J., Mehta R.S., Tromberg B.J. Diffuse optical spectroscopic imaging correlates with final pathological response in breast cancer neoadjuvant chemotherapy. Phil. Trans. A. Math. Phys. Eng. Sci. 2011;369:4512–4530. doi: 10.1098/rsta.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carp S.A., Sajjadi A.Y., Wanyo C.M., Fang Q., Specht M.C., Schapira L. Hemodynamic signature of breast cancer under fractional mammographic compression using a dynamic diffuse optical tomography system. Biomed. Opt. Express. 2013;4:2911–2924. doi: 10.1364/BOE.4.002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswal N.C., Xu Y., Zhu Q. Imaging tumor oxyhemoglobin and deoxyhemoglobin concentrations with ultrasound-guided diffuse optical tomography. Technol. Cancer Res. Treat. 2011;10:417–429. doi: 10.7785/tcrt.2012.500219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson P.G., Kainerstorfer J.M., Sassaroli A., Krishnamurthy N., Homer M.J., Graham R.A. Broadband optical mammography: chromophore concentration and hemoglobin saturation contrast in Breast cancer. PLoS One. 2015;10:e0117322. doi: 10.1371/journal.pone.0117322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M.-L., Oh J.-T., Xie X., Ku G., Wang W., Li C. Simultaneous molecular and hypoxia imaging of brain tumors In vivo using spectroscopic photoacoustic tomography. Proc. IEEE. 2008;96:481–489. [Google Scholar]
- 20.Burton N., Morscher S., Sardella T., Razansky D., Ntziachristos V., Driessen W. Novel approaches for dynamic tumor microenvironment imaging by multispectral optoacoustic tomography (MSOT) Soc. Nucl. Med. Annu. Meet. Abstr. 2014;55:1079. [Google Scholar]
- 21.Tzoumas S., Nunes A., Olefir I., Stangl S., Symvoulidis P., Glasl S. Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues. Nat. Commun. 2016;7:12121. doi: 10.1038/ncomms12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L., Yao J., Li L., Wang L.V. In vivo photoacoustic tomography of myoglobin oxygen saturation. J. Biomed. Opt. 2015;21:61002. doi: 10.1117/1.JBO.21.6.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L., Luís Deán-Ben X., Lutzweiler C., Razansky D., Ntziachristos V. Efficient non-negative constrained model-based inversion in optoacoustic tomography. Phys. Med. Biol. 2015;60:6733–6750. doi: 10.1088/0031-9155/60/17/6733. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.-W., Stantz K.M. Development of a mathematical model to estimate intra-tumor oxygen concentrations through multi-parametric imaging. Biomed. Eng. Online. 2016;15:114. doi: 10.1186/s12938-016-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen-Jeffries K., Browning R.J., Tang M.X., Dunsby C., Eckersley R.J. In vivo acoustic super-Resolution and super-Resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging. 2015;34:433–440. doi: 10.1109/TMI.2014.2359650. [DOI] [PubMed] [Google Scholar]
- 26.Errico C., Pierre J., Pezet S., Desailly Y., Lenkei Z., Couture O. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 2015;527:499–502. doi: 10.1038/nature16066. [DOI] [PubMed] [Google Scholar]
- 27.Burton N.C., Patel M., Morscher S., Driessen W.H.P., Claussen J., Beziere N. Multispectral opto-acoustic tomography (MSOT) of the brain and glioblastoma characterization. Neuroimage. 2013;65:522–528. doi: 10.1016/j.neuroimage.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor J.P.B., Tofts P.S., Miles K.A., Parkes L.M., Thompson G., Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br. J. Radiol. 2011;84 doi: 10.1259/bjr/55166688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sessa C., Guibal A., Del Conte G., Rüegg C. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat. Clin. Pract. Oncol. 2008;5:378–391. doi: 10.1038/ncponc1150. [DOI] [PubMed] [Google Scholar]
- 30.Jeon M., Song W., Kim J., Kim J., Oh J., Lovell J. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. J. Biomed. Opt. 2014;19:16005–16009. doi: 10.1117/1.JBO.19.1.016005. [DOI] [PubMed] [Google Scholar]
- 31.Ao M., Wang Z., Ran H., Guo D., Yu J., Li A. Gd-DTPA-loaded PLGA microbubbles as both ultrasound contrast agent and MRI contrast agent—a feasibility research. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010;93B:551–556. doi: 10.1002/jbm.b.31614. [DOI] [PubMed] [Google Scholar]
- 32.Teraphongphom N., Chhour P., Eisenbrey J.R., Naha P.C., Witschey W.R.T., Opasanont B. Nanoparticle loaded polymeric microbubbles as contrast agents for multimodal imaging. Langmuir. 2015;31:11858–11867. doi: 10.1021/acs.langmuir.5b03473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Zion A., Yin M., Adam D., Foster F.S. Functional flow patterns and static blood pooling in tumors revealed by combined contrast-enhanced ultrasound and photoacoustic imaging. Cancer Res. 2016;76:4320–4331. doi: 10.1158/0008-5472.CAN-16-0376. [DOI] [PubMed] [Google Scholar]
- 34.Gerling M., Zhao Y., Nania S., Norberg K.J., Verbeke C.S., Englert B. Real-time assessment of tissue hypoxia in vivo with combined photoacoustics and high-frequency ultrasound. Theranostics. 2014;4:604–613. doi: 10.7150/thno.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raes F., Sobilo J., Le Mée M., Rétif S., Natkunarajah S., Lerondel S. High resolution ultrasound and photoacoustic imaging of orthotopic lung cancer in mice: new perspectives for onco-pharmacology. PLoS One. 2016;11:e0153532. doi: 10.1371/journal.pone.0153532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenbrey J.R., a Merton D., Marshall A., Liu J.-B., Fox T.B., Sridharan A. Comparison of photoacoustically derived hemoglobin and oxygenation measurements with contrast-enhanced ultrasound estimated vascularity and immunohistochemical staining in a breast cancer model. Ultrason. Imaging. 2015;37:42–52. doi: 10.1177/0161734614527435. [DOI] [PubMed] [Google Scholar]
- 37.Daeichin V., van Rooij T., Skachkov I., Ergin B., Specht P.A.C., Lima A. Microbubble composition and preparation for high-Frequency contrast-Enhanced ultrasound imaging: in vitro and in vivo evaluation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017;64:555–567. doi: 10.1109/TUFFC.2016.2640342. [DOI] [PubMed] [Google Scholar]
- 38.Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eklund L., Bry M., Alitalo K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol. Oncol. 2013;7:259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey K.M., Cornnell H.H., Ibrahim-Hashim A., Wojtkowiak J.W., Hart C.P., Zhang X. Evaluation of the steal phenomenon on the efficacy of hypoxia activated prodrug TH-302 in pancreatic cancer. PLoS One. 2014;9:e113586. doi: 10.1371/journal.pone.0113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal A., Razansky D., Ntziachristos V. Fast semi-analytical model-based acoustic inversion for quantitative optoacoustic tomography. IEEE Trans. Med. Imaging. 2010;29:1275–1285. doi: 10.1109/TMI.2010.2044584. [DOI] [PubMed] [Google Scholar]
- 43.Sontum P.C. Physicochemical characteristics of sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med. Biol. 2008;34:824–833. doi: 10.1016/j.ultrasmedbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Morscher S., Driessen W.H.P., Claussen J., Burton N.C. Semi-quantitative multispectral optoacoustic tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics. 2014;2:103–110. doi: 10.1016/j.pacs.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho C.J.H., Balasundaram G., Driessen W., McLaren R., Wong C.L., Dinish U.S. Multifunctional photosensitizer-based contrast agents for photoacoustic imaging. Sci. Rep. 2014;4:5342. doi: 10.1038/srep05342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laufer J., Elwell C., Delpy D., Beard P. In vitro measurements of absolute blood oxygen saturation using pulsed near-infrared photoacoustic spectroscopy: accuracy and resolution. Phys. Med. Biol. 2005;50:4409–4428. doi: 10.1088/0031-9155/50/18/011. [DOI] [PubMed] [Google Scholar]
- 47.Hochuli R., Beard P.C., Cox B. Effect of wavelength selection on the accuracy of blood oxygen saturation estimates obtained from photoacoustic images. Proc. SPIE. Photons Plus Ultrasound Imaging Sens. 2015;9323:93231V–93238. [Google Scholar]
- 48.Nakajima E.C., Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol. Carcinog. 2013;52:329–337. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter L.T., Jain R.K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 1989;37:77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 50.Tomaszewski M.R., Gonzalez I.Q., J.P.B.O Connor, Abeyakoon O., Parker J.M., Williams K.J. Oxygen enhanced optoacoustic tomography (OE-OT) reveals vascular dynamics in murine models of prostate cancer. Theranostics. 2017;7 doi: 10.7150/thno.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.