Abstract
Transformation of Waldenström’s macroglobulinemia (WM) to diffuse large B-cell lymphoma (DLBCL) occurs in up to 10% of patients and is associated with an adverse outcome. Here we performed the first whole-exome sequencing study of WM patients who evolved to DLBCL and report the genetic alterations that may drive this process. Our results demonstrate that transformation depends on the frequency and specificity of acquired variants, rather than on the duration of its evolution. We did not find a common pattern of mutations at diagnosis or transformation; however, there were certain abnormalities that were present in a high proportion of clonal tumor cells and conserved during this transition, suggesting that they have a key role as early drivers. In addition, recurrent mutations gained in some genes at transformation (for example, PIM1, FRYL and HNF1B) represent cooperating events in the selection of the clones responsible for disease progression. Detailed comparison reveals the gene abnormalities at diagnosis and transformation to be consistent with a branching model of evolution. Finally, the frequent mutation observed in the CD79B gene in this specific subset of patients implies that it is a potential biomarker predicting transformation in WM.
Introduction
Waldenström’s macroglobulinemia (WM) is a neoplastic disease characterized by bone marrow infiltration with lymphoplasmacytic lymphoma and the presence of an IgM monoclonal component.1 Most patients show an indolent clinical course, and survival outcome has improved in recent years.2 However, this long-term evolution has led to an inherent increased risk of developing other malignancies including acute leukemia3 or non-Hodgkin lymphoma.4 Transformation into more aggressive histologies, in this case to diffuse large B-cell lymphoma (DLBCL), has been reported in up to 10% of WM patients.5, 6 Moreover, the prognosis of these patients appears to be worse than that for patients with de novo DLBCL and survival from the time of transformation is usually poor (median survival of ~2 years).6, 7, 8
The biological process of transformation in follicular lymphoma has been thoroughly studied.9, 10, 11, 12, 13, 14, 15 However, data from other indolent B-cell lymphoproliferative disorders are limited. No causes of WM transformation have yet been described, so understanding this process would be of great interest and would facilitate the development of new therapeutic strategies for improving the outcome of these patients. Recent advances in determining the WM mutational profile have revealed the presence of recurrent mutations, such as those of MYD88, CXCR4 and ARID1A.16, 17 However, it is not known whether they are part of the mechanisms underlying the transformation to aggressive lymphoma. This event is of particular interest, especially when it involves such genetically distinct entities in appearance. Accordingly, WM seems to be a very homogeneous disease with a monotonous recurrent driver mutation (MYD88 L265P),18, 19 and infrequent secondary alterations.20 Conversely, DLBCL harbors a wide range of diverse somatic mutations and genetic lesions that affect numerous intracellular pathways, thus making it intrinsically much more complex.21, 22, 23 However, the two entities share some alterations, such as MYD88 L265P (observed in ~90% of WM and in 29% of ABC-type DLBCL),18, 19, 21, 24 CD79A/CD79B (15% WM and 10–15% DLBCL),17, 20, 21, 23, 25, 26 or copy number variations affecting 6q (40–60% WM and 20–40% DLBCL).27, 28, 29, 30
Transformation to DLBCL can occur at any time during the course of WM: at diagnosis, before treatment, during therapy and even 20 years after the initial diagnosis.6 In addition, no indicative clinicopathological feature or risk factor for developing DLBCL has so far been identified. No genomic studies of transformed WM patients have been carried out, so nothing is known about the biological signals that might explain particular susceptibilities. For this reason, the identification of genetic changes driving transformation is essential for a comprehensive understanding of the transition from WM to DLBCL that could help in the development of new diagnostic strategies and targeted therapies.
Against this background, we decided to perform the first whole-exome sequencing study focused on WM patients who experienced histological transformation. Integrating our new findings into our existing knowledge about the transformation of indolent lymphoproliferative syndromes to aggressive diseases should enable a transformational biological model to be derived, help define the molecular risk criteria for monitoring the course of the disease, and develop new preventive strategies. Our results revealed a higher incidence of mutations in the CD79B gene than in non-transformed WM patients, which could be interpreted as being a potential mechanism contributing to transformation. Finally, the comparison between diagnosis and transformation allowed us to establish an evolutionary pattern associated with the transforming event.
Subjects and methods
Subjects
Four patients diagnosed with transformed WM were included in the study. In three of them (patients 1, 2 and 4), matched tumor samples from diagnosis and transformation to DLBCL, as well as germline DNA were available for comparative study. One extra sample from patient 2 (corresponding to an event of WM progression without transformation) was also included. For patient 3, only DNA from germinal and transformed tumor cells was available. Cases were diagnosed using standard WHO classification criteria,31 including the new concepts that appear in the most recent review.32 The study and all procedures were performed in accordance with the Helsinki Declaration and were reviewed and approved by the Institutional Review Board of the Research Biomedical Institute of Salamanca. Informed consent was obtained from all patients.
The study group included one woman and three men, with a median age of 76 years (range, 62–82 years). The median time from diagnosis of WM to histological transformation was 52 months (range, 42–153 months). As an uncommon fact in this short series, we have to remark that two of the four patients had received treatment prior to histological transformation, and the other two (3 and 4) were chemo-naive. The complete patient characteristics are shown in Table 1.
Table 1. Clinical characteristics of patients.
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| WM | ||||
| Age at diagnosis | 62 (2009) | 82 (2010) | 81 (2003) | 72 (2002) |
| Clinical symptoms | ||||
| Anemia | Yes | Yes | No | Yes |
| Polyadenopathies | Yes | No | No | No |
| LDH elevation | Yes | No | No | No |
| Hyperviscosity | No | No | Yes | No |
| Others | No | No | No | No |
| BM infiltration (FCM) | 12% | 22% | 16% | 11% |
| Frontline therapy (year) | R-VD (2009) | R-CD (2010) | None | W&W |
| Therapies at relapse (year) | FC (2011) | FC (2013)RB (2013/2014)R-VD (2015) | None | None |
| Transformation to DLBCL | ||||
| Time to transformation (years) | 3 | 5 | 3 | 13 |
| Clinical symptoms | ||||
| Anemia | Yes | No | Yes | Yes |
| Polyadenopathies | No | Yes | No | No |
| LDH elevation | Yes | Yes | Yes | Yes |
| B symptoms | Yes | No | Yes | Yes |
| Others | Splenomegaly | |||
| Tumor infiltration (FCM) | 40% (Spleen) | 50% (Adenopathy) | 16% (BM)a | 43% (BM) |
| DLBCL therapy | None | GemOx (2015) Ibrutinib (2016) | None | RCOP (2015) (reduced) |
| Status | Dead | Palliative care | Dead | Dead |
Abbreviations: BM, bone marrow; DLBCL, diffuse large B-cell lymphoma; FC, fludarabine, cyclophosphamide; FCM, flow cytometry; GemOx, gemcitabine, oxaliplatin; LDH, lactate dehydrogenase; RB, rituximab, bendamustine; R-CD, rituximab, cyclophosphamide, dexamethasone; RCOP, rituximab, cyclophosphamide, vincristine, prednisone; R-VD, rituximab, bortezomid, dexamethasone; WM, Waldeström macroglobulinemia; W&W, watch and wait (observation).
BM: 8% WM and 8% DLBCL.
DNA extraction and quality assessment
The sample origins were as follows: (1) for WM tumor cells: bone marrow at diagnosis in all patients; (2) for DLBCL cells: spleen (patient 1), inguinal lymph node (patient 2) and bone marrow (patients 3 and 4) at transformation; and (3) for germline cells: peripheral blood samples in which no infiltration (<0.1%) was demonstrated by flow cytometry.
DNA was extracted by conventional methods: manually with the DNAzol reagent (MRC, Cincinnati, OH, USA) or automatically with the Maxwell system (Promega Corporation, Madison, WI, USA). Quantification and quality control of DNA were evaluated before enrichment and library preparation. DNA concentrations were measured using the Qubit fluorometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). DNA sample quality was assessed by gel electrophoresis. At least 1 μg of DNA was required for library preparations.
Flow cytometry studies
Immunophenotypic evaluation was done by conventional methods, using panels of monoclonal antibodies previously described by our group33 and following the general recommendations of the EuroFlow group for the immunophenotypic evaluation of hematological malignancies.34
Fluorescence in situ hybridization studies
Simple interphase fluorescence in situ hybridization was performed on cell nuclei from whole bone marrow samples using our previously published techniques.35 Del(6q), MYC and BCL6 translocations t(14;18) (q21; q32) and del(17p) were analyzed with the probes Vysis CEP 6, LSI MYC Dual Color Break Apart Rearrangement (8q24), LSI BCL6 Dual Color Break Apart Rearrangement (3q27), Vysis IGH/BCL2 Dual Color Dual Fusion Translocation Probe t(14;18)(q32;q21) and LSI TP53 Probe (Abbott Molecular, Des Plaines, IL, USA), respectively. At least 100 cells were analyzed in all patient samples, applying Vysis scoring criteria. The cutoff point for the identification of alteration was set at ⩾10% cells with abnormal signal.
V(D)J clonal rearrangements
V(D)J clonal rearrangements of WM and DLBCL matched samples were amplified and sequenced as described by the BIOMED-2/Euroclonality strategy.36
Whole-exome sequencing
All diagnostic and transformation samples, as well as those for normal DNA matching, were sent for library construction and whole-exome sequencing at Macrogen, Inc. (Seoul, South Korea). Enrichment and generation of libraries were performed with SureSelectXT2 Human All Exon V5 of 51 MB (Agilent Technologies, Santa Clara, CA, USA) that uses cRNA probes of 120 nt. Paired-end sequencing was carried out in the Illumina HiSeq 2000 platform (Illumina, Inc., San Diego, CA, USA). The number of reads was set up according to each sample tumor infiltration defined by flow cytometry. Germinal samples were sequenced with a mean depth of 100 × and tumor DNA with a depth of 150 × or 200 ×, depending on whether the infiltration was greater or less than 40%, respectively.
Sequencing data processing and bioinformatic analysis
DreamGenics (Oviedo, Spain) supervised the pre-processing and performed the initial bioinformatics analysis using algorithms and non-commercial pipelines to call variants, analyze and compare them. Briefly, data generated by the sequencer were converted to FastQ with the Illumina Consensus Assessment of Sequence and Variation version 1.8 software (https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html) and aligned to the human reference genome (Genome Reference Consortium human build 37, human genome 19) with BWA software.37 Variants were called using Atlas-SNP and Atlas-indel,38 discarding those with suboptimal quality indexes according to the pipeline criteria.
Interpretation of variants
Only non-synonymous protein-coding alterations were considered. Criteria for filtering out single nucleotide polymorphisms were a frequency lower than 2% in the germinal sample and a population allele frequency <1% (according to the Human Gene Mutation Database).39 Some mutations were excluded on the basis of their distance with respect to the Agilent V5 probes. All samples were analyzed in pairs, with their corresponding germinal sample taken as reference.
Variant allele frequencies (VAFs) were corrected according to their tumor content defined by flow cytometry, in order to better estimate the percentage of tumor cells affected by each mutation. Variants with a corrected VAF of <10% were not included in the analysis.
Enrichment analysis
Gene-set enrichment analysis was performed with the WEB-based GEne SeT AnaLysis Toolkit (WebGestalt) (http://www.webgestalt.org/webgestalt_2013/) to highlight categories and pathways present in the Gene Ontology and Pathway Commons databases. The statistical method employed was the hypergeometric test, with P-values adjusted by the Benjamini and Hochberg (1995) method.40
Results
Global non-synonymous variations
Overall, we found 421 non-synonymous variations (NSVs) at diagnosis and transformation in the four patients, distributed among 355 genes. Of these, only 39 were mutated exclusively in WM (at diagnosis, n=29; on progression, n=10) and 49 were present at the time of both events (diagnosis and transformation). All other genes (n=267) were mutated only in the DLBCL samples.
Variant allelic frequency and percentage of tumor cells
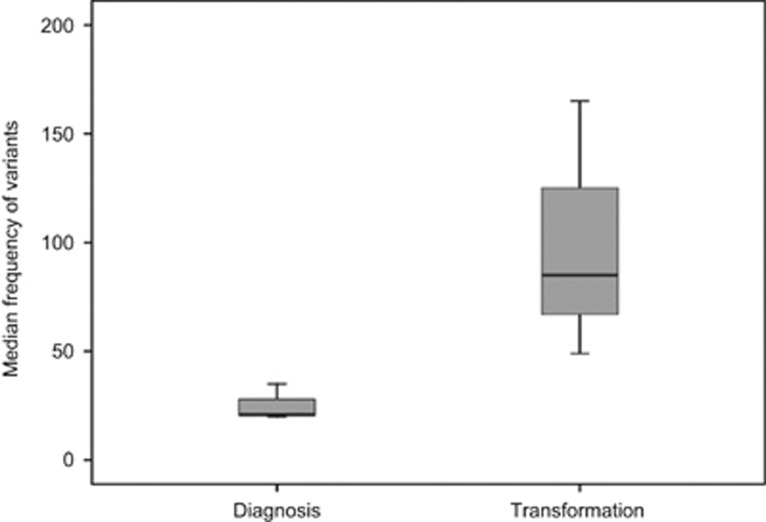
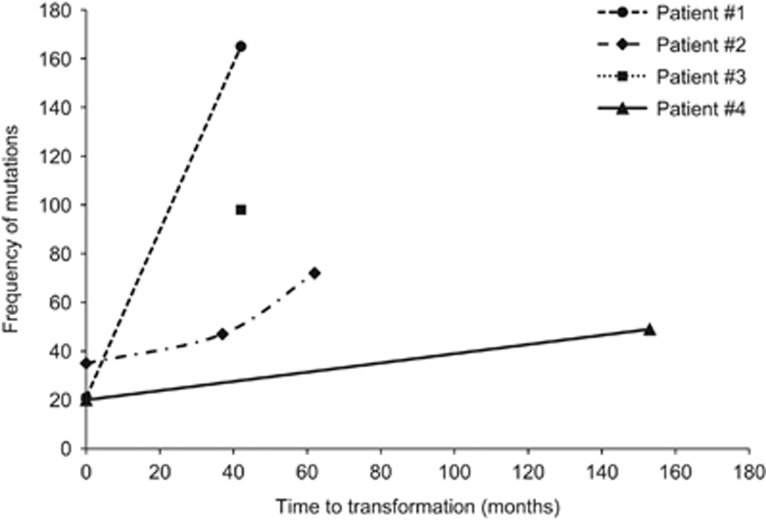
To understand the possible mechanisms leading to the transformation event, we compared the results in tumor cells of the two events studied. First, we observed a much higher frequency of mutated genes at transformation (median 85, range 49–165) than at diagnosis (median 21, range 20–35) (Figure 1). Accordingly, there was a median gain of 70 variants (range 29–144) per case during the transition from WM to DLBCL. Interestingly, the number of gains was not closely correlated with the interval between diagnosis and transformation (R2=0.51). However, we noticed that patients 1 and 3, who presented the fastest transformation (~3 years), contained more variants (165 and 98, respectively) than did patient 2, who transformed in 5 years and had 72 mutations, and patient 4, who exhibited the smoothest transformation, lasting 13 years, and acquired only 49 alterations (Figure 2).
Figure 1.
Frequency of mutations at diagnosis (WM) and transformation (DLBCL). Comparison of the median number of mutations of the four patients at diagnosis (n=21) and upon transformation (n=85).
Figure 2.
Representation of the total number of alterations at each moment (diagnosis, progression and transformation) versus the time to transformation. Patients 1 and 3 presented the fastest transformation (~3 years) and the highest frequency of variants (165 and 98, respectively). Patient 2 transformed in 5 years and had 72 mutations. Patient 4 took 13 years to transform and acquired only 49 alterations.
NSV present at diagnosis and transformation
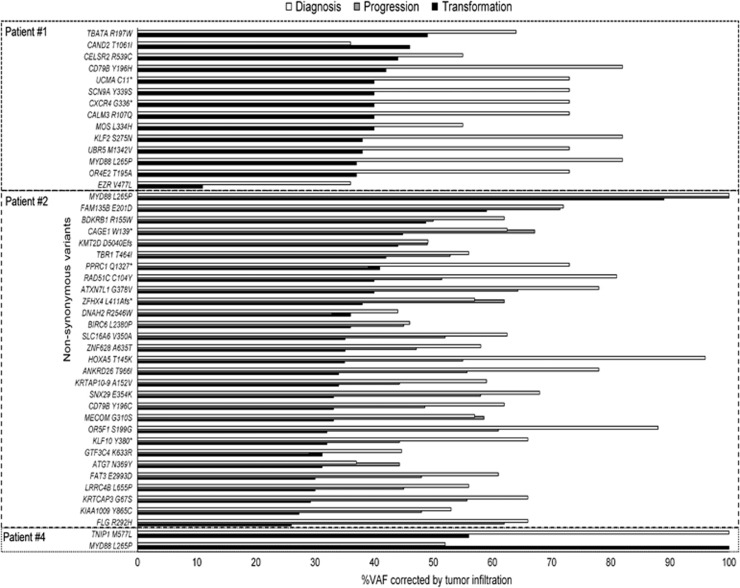
We found that the VAF was increased at transformation for almost all these common mutations, but this was attributed to the higher tumor infiltration present in the DLBCL compared with the corresponding WM sample (1, 40 vs 12% 2, 51.3 vs 22.4% 4, 54.6 vs 11%). Thus, the corrected VAF using flow cytometry showed the opposite pattern and VAFs were greater at diagnosis than at transformation (Figure 3). This means that, although there were more alterations at transformation, the percentage of tumor cells affected by each alteration was usually lower. This was confirmed using the globally corrected mean VAF for all NSVs present at diagnosis and at transformation (Table 2).
Figure 3.
VAF of common mutations at diagnosis, progression and transformation for each patient. The percentage of tumor cells affected by a mutation decreased from diagnosis to transformation in most of the cases.
Table 2. Mean VAF of common and exclusive mutations at diagnosis and transformation.
|
Patient 1 |
Patient 2 |
Patient 4 |
||||
|---|---|---|---|---|---|---|
| Diagnosis | Transformation | Diagnosis | Transformation | Diagnosis | Transformation | |
| Mean VAF of common mutations | 76% | 78% | 64% | 37% | 66% | 38% |
| Mean VAF of exclusive mutations | 68% | 51% | 30% | 22% | 45% | 16% |
Abbreviation: VAF, variant allele frequency.
Not all patients had the same number of potential early drivers. Patients 2 and 4 had NSVs in 29 and 14 genes in common at diagnosis and transformation, respectively. However, patient 1 only shared two variants at the time of both events: MYD88 and TNIP1 (the latter having a higher VAF at diagnosis). This gene codes for a TNFAIP3-interacting protein that plays a role in regulating nuclear factor-κB activation, which is a frequent mechanism of alteration of cell cycle control in all lymphoid neoplasias.
Recurrent NSV among different patients
MYD88 L265P was present in all patients at both times (3/3 at diagnosis and 4/4 at transformation). The second most frequent was CD79B Y196C/H, which was found in 3/4 patients at transformation (75%) and in 2/3 at diagnosis (67%). VAFs of CD79B mutations were half of the MYD88 in two patients and the same as MYD88 in one patient, and kept that relation at baseline and transformation. CELSR2, FAM135B, IGFN1 and ZFHX4 also had recurrent variations (in at least two patients).
We then considered variants that could be detected at the time of only one of the events, that is, exclusively at diagnosis, progression or transformation (Supplementary Table I). Variations exclusively detected at diagnosis were not present in the lymphoma clone, implying that it does not provide any evolutionary advantage (passenger mutations). In contrast, recurrent variations exclusively present at the transformation stage (or that appeared late in the Waldenström’s clone) may be considered as temporally intermediate or late drivers that confer some advantage to the tumor clone. Five genes had recurrent NSVs acquired at the transformation stage: FRYL (MLL fusion partner in lymphoid leukemia), HNF1B (transcription factor whose expression is altered in some cancers), PER3 (checkpoint protein that plays an important role in checkpoint activation, cell proliferation and apoptosis), PIM1 (proto-oncogene with serine/threonine kinase activity involved in the pathogenesis of lymphoma) and PTPRD (tumor suppressor that contributes to the development of multiple cancers). All these data analyzed without inclusion of patient 3 are included in Supplementary Table II.
At this point, we carried out gene-set enrichment analysis of the genes modified at transformation (n=314) to search for cellular processes and the pathways affected. Genes were classified into the following functional categories: histone modification (KMT2D, HDAC9), chromatin modification (PAX5, UBR5), cell–cell adhesion (CELSR2, TNIP1), cell development (WT1, CXCR4), cell differentiation (KIT, EZR), protein modification (KMD5C, KMD1B), chromatin organization (ARID1A, SOX1), protein autophosphorylation (PIM1, PRKD1), chromosome organization (HIST1H2BC, TP53), transcription regulation (RARB, PRDM1) and protein kinase activity (BCR, TGFA). Focusing on pathways, those most significantly affected were: insulin growth factor-1 pathway (CD79B, PPM1D), interleukin-3-mediated signaling events (TGFBRAP1, IFNA14), insulin pathway (COPA, CAD), vascular endothelial growth factor and vascular endothelial growth factor receptor signaling network (ACTN1, PELP1), and nectin adhesion pathway (ROBO1, NOS3).
Patterns of evolution from WM to DLBCL
As mentioned above, all patients shared some NSVs at both stages, but some others were exclusively detected at diagnosis or transformation. Accordingly, certain subclones present at diagnosis would have evolved by acquiring new mutations responsible for the transformation event (or the progression), whereas others would have been reduced (disappearing or becoming undetectable). Therefore, and despite both tumors (WM and DLBCL) having the same V(D)J rearrangement and an identical CDR3 region, suggesting a common progenitor cell, their evolution is consistent with a branching model. These findings should be considered with caution because of the low level of infiltration of diagnostic samples, which may lead to the underdetection of variants that are present at a low level in the tumor population.
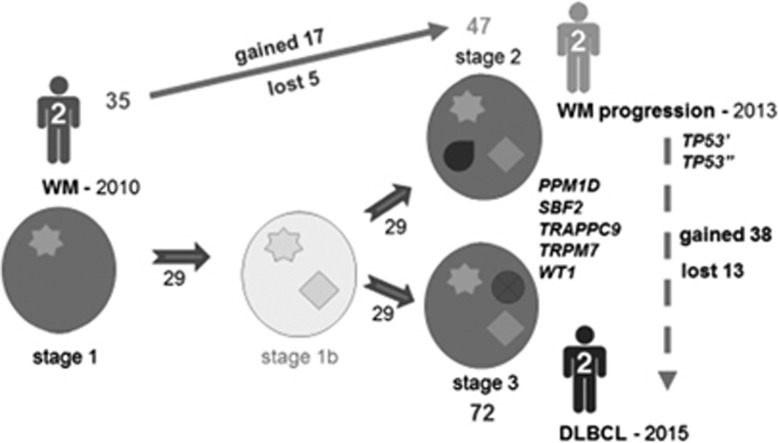
This nonlinear evolutionary pattern was well illustrated by patient 2, who had a symptomatic progression of WM before transformation, and was studied at the time of all three events (Figure 4). During the evolution of his condition, we found mutations that were conserved from diagnosis to transformation (n=29). However, many other novel alterations not present at diagnosis appeared at progression (n=17) and transformation (n=38). Almost all of these were different, with five exceptions: PPM1D, SBF2, TRAPPC9, TRPM7 and WT1. In the same way, some mutations present at diagnosis or acquired at progression (that is, two TP53 mutations) disappeared or became undetectable (at the level of sensitivity used) by the time of disease transformation. This would mean that the transformed final clone did not come from the intermediate subclone responsible for progression, but from a previous minor subclone that only grew after progression.
Figure 4.
Evolution of WM in patient 2. This patient was diagnosed with WM in 2010 and transformed to DLBCL in 2015, with a symptomatic progression in 2013 before the transformation. We observed 35 mutations at diagnosis, 47 at relapse and 72 at transformation, including 29 alterations that were conserved at the times of the three events. The PPM1D, SBF2, TRAPPC9, TRPM7 and WT1 genes were mutated either at progression or transformation. By contrast, the two mutations found in TP53 were seen at relapse but were lost by the time of transformation. This implies that the transformed final clone did not evolve from the same subclone as was responsible for progression, but from a previous one that would not yet have acquired the TP53 mutations (among others).
Discussion
WM patients may eventually experience histological transformation to DLBCL (2.4% transformation rate at 10 years), being at risk of poor outcome and short survival.6, 7 Understanding the biology and mechanisms underlying this process is important for identifying susceptible patients and for developing therapeutic strategies aimed at cancer control. In this study, we have carried out for the first time a whole-exome sequencing study of four cases with paired WM and transformed DLBCL samples in order to evaluate the genetic basis of this transition and to find genomic alterations and pathways that could be therapeutically targeted.
Maybe due to the small sample size, we could not find a unique genetic event responsible for WM transformation to DLBCL. In fact, our findings revealed extensive genetic heterogeneity with a large number of aberrations affecting many genes and pathways, reflecting the complexity associated with the transformation process. Even asymptomatic and chemotherapy-naive patients, such as patients 3 and 4, may develop an aggressive disease after more than 12 years (in the case of patient 4) with an untreated indolent WM. Many more alterations were associated with aggressive lymphoma development, although the number was inversely related to the time to transformation. Thus, patients showing the fastest transition (1 and 3) presented the greatest number of mutations. This could be explained by the onset of new mutations conferring a proliferative advantage on the harboring cell, leading to stronger competition during the evolution of the cancer.41, 42, 43
However, not all the events would be of equal importance in the pathogenesis of the disease. Mutations present at both events are likely to be spread in nearly all clones and to remain stable over time. In our WM cases, these variants had a higher VAF, so targeting these presumed early genetic events could lead to the elimination of all oncogenic clones. An example of these mutations could be the MYD88 L265P, the only one present in all patients and at all times, a mutation with a well established role in both WM and DLBCL.16, 24, 44 However, as it is encountered in over 90% of WM cases at diagnosis, and only a small proportion of them will transform, no inference can be made about its role in transformation. In patient 1, it was the only alteration, together with TNIP1, recognized at both times. TNIP1 is an essential gene for nuclear factor-κB activation, same as MYD88, which could highlight the need of more than one alteration in the nuclear factor-κB pathway to be significant for the transformation process, where nuclear factor-κB signaling is known to be involved.12 Furthermore, this patient also showed other differences with respect to the other cases, such as a much higher frequency of mutations at transformation (n=165), diffuse spleen infiltration by aggressive lymphoma cells, and an unmutated CD79B.
Now considering CD79B, this B-cell receptor-associated gene was frequently mutated, appearing in 2/3 cases (67%) at diagnosis and in 3/4 cases (75%) at transformation. VAFs of this mutation in relation to MYD88 indicated that it was present in half of the tumor clone in two patients and in the whole clone in the other patient, staying the same at both moments. This variant affects the first tyrosine ITAM kinase domain of the receptor and has been described in 12% of ABC-type DLBCLs,45 in connection with the acquisition of the lymphoma phenotype. However, a limited oncogenic potential has been associated with CD79B mutations, since other alterations must occur in order to facilitate the transition to the aggressive lymphoma.46, 47, 48 In conventional WM, CD79B has been found to be mutated in ~10%,17, 20, 25, 26 so the high frequency reported here (3/4 of cases) is intriguing. Accordingly, we suggest that CD79B mutations identify a subgroup of WM with aggressive clinical evolution and a high risk of transformation.
With the exception of MYD88 and CD79B, we observed a notable diversity in the mutational spectrum across samples, with few recurrent genes. Only CELSR2 (growth factor), FAM135B, IGFN1 and ZFHX4 (transcription factor) were present in more than one patient. This recurrence should be taken into account, since another commonly mutated gene in WM (30% patients), CXCR4,17, 20 appeared in just one patient. Regardless of their incidence, relevant mutations seem to be present in the initial WM cell that will transform, suggesting that they may be involved in tumor initiation. Conversely, genes exclusively involved in transformation may represent cooperating events that interact with these pathogenic mutations. The most frequently altered genes were FRYL, HNF1B, PER3, PIM1 and PTPRD (50% of patients). PIM1 could be targeted with the PIM kinase inhibitors that have already shown activity in myeloma and acute myeloid leukemia.49, 50 However, some of the non-recurrent alterations could have been randomly acquired and may not be causally related to the pathogenesis of the disease.
Aberrations acquired at transformation probably cooperate with early initiating events in the selection of tumor clones responsible for disease progression. Nonetheless, considering the diversity of the alterations, many of them will not confer any advantage on the malignant cell. An illustrative case could be the presence of mutations in TP53. Deletions and mutations of this gene have been found to be related to poor prognosis in chronic lymphocytic leukemia,51 multiple myeloma52 and probably in WM.53 However, in our cases this gene appeared mutated at transformation in patient 1 and at progression in patient 2. Interestingly, this abnormality disappeared at transformation in the latter case, suggesting the loss of any advantage. Therefore, it is not easy to determine what prompts aggressive behavior: the genes, the number of alterations, the clone in which they arise, the pathway affected, the cell function deregulated or, most likely, a combination of all of these factors. These matters should be addressed in further studies with the ultimate aim of developing therapeutic strategies that may disrupt these mechanisms, thereby completely preventing transformation.
Finally, we observed that the transformation process seemed to be consistent with part of a branching model of evolution in which only clones containing driver mutations evolve to more aggressive populations by acquiring new aberrations. Identical scenarios have been reported in multiple myeloma,54 follicular lymphoma,12 chronic lymphocytic leukemia,55 acute myeloid leukemia,42 acute lymphoblastic leukemia56 and even solid tumors.57 Nevertheless, this needs to be confirmed in further analyses of single cells. Likewise, it would also be interesting to establish whether there is a progenitor tumor cell that is common to both diseases and that is responsible for their pathogenesis. In this study, the analysis of the V(D)J rearrangement and CDR3 region of the immunoglobulin heavy chain gene in the WM and their matched lymphoma samples, confirmed that they belonged to the same clone. The concept of the tumor-initiating cell is already established for leukemia58 and follicular lymphoma,59, 60 and it would be supported in our case by the presence from the outset of certain mutations that are shared by the entire tumor population, as they remain clonally stable throughout the entire course of the disease. DLBCL would then arise from these precursors, showing a higher (as in patients 2 and 4) or lower (patient 1) degree of genetic similarity to the WM clone.
In conclusion, although this is merely the first step and we were not able to identify a unique genetic event responsible for WM transformation to DLBCL, it appears that certain alterations may contribute to the onset of aggressive disease. Those genes frequently mutated at diagnosis in this subset of patients who suffer disease transformation compared to conventional WM (CD79B) may be considered as potential biomarkers for predicting the risk of transformation in prospective studies. Additional research is needed to better understand the biology of this process and to facilitate the design of preventive therapies targeted during the early evolutionary pathways that are responsible for transformation. This knowledge will enable us to improve the outcome of these patients.
Acknowledgments
We thank Alicia Antón, Rebeca Maldonado and Montserrat Hernández for their technical assistance and Phil Mason for revising the English language of the manuscript. This work was supported by research grants from the Foundation Memoria Don Samuel Solórzano Barruso (FS/26–2015), the Asociación Castellano-Leonesa de Hematología y Hemoterapia (FUCALHH 2015) and the Gilead Sciences (GILEAD) Fellowship Program (GLD16/00162), as well as funds from the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Economy and Competitiveness, CIBERONC-CB16/12/00233.
Author contributions
CJ, SAA and RGS designed the initial study. CJ, SAA and MA selected the patients and prepared the samples. GRO preprocessed the data for analysis. CJ, SAA, MIPC and MGA analyzed the data and interpreted the results. MCC, MES, RC and AB helped collect the data. LAM helped with the statistical analysis. RGS was the clinician responsible for patients, ensuring the protocols were correctly followed, sampling and collecting clinical data. NP and NCG were responsible for the immunophenotyping and cytogenetic analysis, respectively, of the patients included in this series. CJ and SAA prepared the initial version of the paper. RGS reviewed the conception and design of most of the work, and corrected the manuscript. MG was the head of the group, supervised the final revision of the draft and gave final approval of the version to be published.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
The authors declare no conflict of interest
Supplementary Material
References
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30: 110–115. [DOI] [PubMed] [Google Scholar]
- Kyrtsonis MC, Vassilakopoulos TP, Angelopoulou MK, Siakantaris P, Kontopidou FN, Dimopoulou MN et al. Waldenström’s macroglobulinemia: clinical course and prognostic factors in 60 patients. Experience from a single hematology unit. Ann Hematol 2001; 80: 722–727. [DOI] [PubMed] [Google Scholar]
- Leleu X, Soumerai J, Roccaro A, Hatjiharissi E, Hunter ZR, Manning R et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol 2009; 27: 250–255. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Olszewski AJ, Hunter ZR, Kanan S, Meid K, Treon SP. Incidence of secondary malignancies among patients with Waldenström macroglobulinemia: an analysis of the SEER database. Cancer 2015; 121: 2230–2236. [DOI] [PubMed] [Google Scholar]
- Lin P, Mansoor A, Bueso-ramos C, Hao S. Diffuse large B-cell lymphoma occurring in patients with lymphoplasmacytic lymphoma/Waldenström macroglobulinemia clinicopathologic features of 12 cases. Am J Clin Pathol 2003; 120: 246–253. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Gustine J, Meid K, Dubeau T, Hunter ZR, Treon SP. Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia. Am J Hematol 2016; 91: 1032–1035. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Olszewski AJ, Kanan S, Meid K, Hunter ZR, Treon SP. Survival outcomes of secondary cancers in patients with Waldenström macroglobulinemia: an analysis of the SEER database. Am J Hematol 2015; 90: 696–701. [DOI] [PubMed] [Google Scholar]
- Durot E, Tomowiak C, Michallet A-S, Dupuis J, Lepretre S, Toussaint E et al. Retrospective analysis of 56 cases of transformed Waldenström macroglobulinemia. a study on behalf of the French Innovative Leukemia Organization (FILO). Blood 2016; 128: 2982 (abstract). [Google Scholar]
- Martinez-Climent JA, Alizadeh AA, Segraves R, Blesa D, Rubio-Moscardo F, Albertson DG et al. Transformation of follicular lymphoma to diffuse large cell lymphoma is associated with a heterogeneous set of DNA copy number and gene expression alterations. Blood 2003; 101: 3109–3117. [DOI] [PubMed] [Google Scholar]
- Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol 2007; 25: 2426–2433. [DOI] [PubMed] [Google Scholar]
- Bouska A, McKeithan TW, Deffenbacher KE, Lachel C, Wright GW, Iqbal J et al. Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood 2014; 123: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okosun J, Bödör C, Wang J, Araf S, Yang C-Y, Pan C et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 2013; 46: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualucci L, Khiabanian H, Fangazio M, Vasishtha M, Messina M, Holmes AB et al. Genetics of follicular lymphoma transformation. Cell Rep 2014; 6: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AJ, Rosenwald A, Wright G, Lee A, Last KW, Weisenburger DD et al. Transformation of follicular lymphoma to diffuse large B-cell lymphoma proceeds by distinct oncogenic mechanisms. Br J Haematol 2007; 136: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Berra E, Cerri M, Deambrogi C, Barbieri C, Franceschetti S et al. Aberrant somatic hypermutation in transformation of follicular lymphoma and chronic lymphocytic leukemia to diffuse large B-cell lymphoma. Haematologica 2006; 91: 1405–1409. [PubMed] [Google Scholar]
- Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367: 826–833. [DOI] [PubMed] [Google Scholar]
- Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014; 123: 1637–1646. [DOI] [PubMed] [Google Scholar]
- Jiménez C, Sebastián E, Chillón MC, Giraldo P, Mariano Hernández J, Escalante F et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, waldenström’s macroglobulinemia. Leukemia 2013; 27: 1722–1728. [DOI] [PubMed] [Google Scholar]
- Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y, Liu X et al. MYD88 L265P in Waldenstrom’s macroglobulinemia, IgM monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific PCR. Blood 2013; 121: 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain S, Roumier C, Venet-Caillault A, Figeac M, Herbaux C, Marot G et al. Genomic landscape of CXCR4 mutations in Waldenström macroglobulinemia. Clin Cancer Res 2016; 22: 1480–1488. [DOI] [PubMed] [Google Scholar]
- Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A 2012; 109: 3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, Mendez-lago M, Mungall AJ, Goya R, Karen L, Corbett R et al. Frequent mutation of histone modifying genes in non-Hodgkin lymphoma. Nature 2012; 476: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 2011; 43: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011; 470: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain S, Roumier C, Galiègue-Zouitina S, Daudignon A, Herbaux C, Aiijou R et al. Genome wide SNP array identified multiple mechanisms of genetic changes in Waldenstrom macroglobulinemia. Am J Hematol 2013; 88: 948–954. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Prieto-Conde I, García-Álvarez M, Chillón MC, García-Mateo A, Escalante F et al. Genetic characterization of Waldenstrom macroglobulinemia by next generation sequencing: an analysis of fouteen genes in a series of 61 patients. Blood 2015; 126: 2971 (abstract). [Google Scholar]
- Braggio E, Keats JJ, Leleu X, Van Wier S, Jimenez-Zepeda VH, Valdez R et al. Identification of copy number abnormalities and inactivating mutations in two negative regulators of nuclear factor-kB signaling pathways in Waldenström’s macroglobulinemia. Cancer Res 2009; 69: 3579–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schop RFJ, Van Wier SA, Xu R, Ghobrial I, Ahmann GJ, Greipp PR et al. 6q deletion discriminates Waldenström macroglobulinemia from IgM monoclonal gammopathy of undetermined significance. Cancer Genet Cytogenet 2006; 169: 150–153. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Compagno M, Houldsworth J, Monti S, Grunn A, Nandula SV et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med 2006; 203: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit K, Jhanwar SC, Ladanyi M, Filippa DA, Chaganti RS. Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin’s lymphoma: correlations between recurrent aberrations, histology, and exposure to cytotoxic treatment. Genes Chromosomes Cancer 1991; 3: 189–201. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2008. [Google Scholar]
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva B, Montes MC, García-Sanz R, Ocio EM, Alonso J, de las Heras N et al. Multiparameter flow cytometry for the identification of the Waldenström’s clone in IgM-MGUS and Waldenström’s Macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia 2014; 28: 166–173. [DOI] [PubMed] [Google Scholar]
- van Dongen JJM, Lhermitte L, Böttcher S, Almeida J, van der Velden VHJ, Flores-Montero J et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012; 26: 1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocio EM, Schop RFJ, Gonzalez B, Van Wier SA, Hernandez-Rivas JM, Gutierrez NC et al. 6q deletion in Waldenström macroglobulinemia is associated with features of adverse prognosis. Br J Haematol 2007; 136: 80–86. [DOI] [PubMed] [Google Scholar]
- van Dongen JJM, Langerak AW, Brüggemann M, Evans PAS, Hummel M, Lavender FL et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17: 2257–2317. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wan Z, Coarfa C, Drabek R, Chen L, Ostrowski EA et al. A SNP discovery method to assess variant allele probability from next-generation resequencing data. Genome Res 2010; 20: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson PD, Mort M, Ball EV, Howells K, Phillips AD, Thomas NS et al. The Human Gene Mutation Database: 2008 update. Genome Med 2009; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses 1995; 45: 486–490. [DOI] [PubMed] [Google Scholar]
- Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012; 120: 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Ley TJ, Larson DE, Miller CA, Daniel C, Welch JS et al. Clonal evolution in relapsed acute myeloid leukemia revealed by whole genome sequencing. Nature 2012; 481: 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor L, Brioli A, Wardell CP, Murison A, Potter NE, Kaiser MF et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 2014; 28: 1705–1715. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhou Y, Liu X, Xu L, Cao Y, Manning RJ et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood 2013; 122: 1222–1232. [DOI] [PubMed] [Google Scholar]
- Kraan W, Horlings HM, van Keimpema M, Schilder-Tol EJM, Oud MECM, Scheepstra C et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 2013; 3: e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010; 463: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K-H, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies. Immunol Rev 2012; 246: 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 2013; 23: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino T, Garcia-Gomez A, Gonzalez-Mendez L, San-Segundo L, Hernandez-Garcia S, Lopez-Iglesias A-A et al. The novel Pan-PIM kinase inhibitor, PIM447, displays dual antimyeloma and bone-protective effects, and potently synergizes with current standards of care. Clin Cancer Res 2017; 23: 225–238. [DOI] [PubMed] [Google Scholar]
- Harada M, Benito J, Yamamoto S, Kaur S, Arslan D, Ramirez S et al. The novel combination of dual mTOR inhibitor AZD2014 and pan-PIM inhibitor AZD1208 inhibits growth in acute myeloid leukemia via HSF pathway suppression. Oncotarget 2015; 6: 37930–37947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Martinez P, Wade R, Hockley S, Oscier D, Matutes E et al. Mutational status of the TP53 gene as a predictor of response and survival in patients with chronic lymphocytic leukemia: results from the LRF CLL4 trial. J Clin Oncol 2011; 29: 2223–2229. [DOI] [PubMed] [Google Scholar]
- Mateos M-V, Gutierrez NC, Martin-Ramos M-L, Paiva B, Montalban M-A, Oriol A et al. Outcome according to cytogenetic abnormalities and DNA ploidy in myeloma patients receiving short induction with weekly bortezomib followed by maintenance. Blood 2011; 118: 4547–4553. [DOI] [PubMed] [Google Scholar]
- Poulain S, Roumier C, Bertrand E, Renneville A, Tricot S, Caillault Venet A et al. TP53 mutation in Waldenstrom macroglobulinemia. Blood 2016; 128: 4092 (abstract). [Google Scholar]
- Bolli N, Avet-Loiseau H, Wedge DC, Van Loo P, Alexandrov LB, Martincorena I et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun 2014; 5: 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau DA, Tausch E, Taylor-weiner AN, Stewart C, Reiter JG, Bahlo J et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015; 526: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 2011; 469: 356–361. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014; 506: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Climent JA, Fontan L, Gascoyne RD, Siebert R, Prosper F. Lymphoma stem cells: enough evidence to support their existence? Haematologica 2010; 95: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti E, Wrench D, Matthews J, Iqbal S, Davies A, Norton A et al. Transformation of follicular lymphoma to diffuse large B-cell lymphoma may occur by divergent evolution from a common progenitor cell or by direct evolution from the follicular lymphoma clone. Blood 2009; 113: 3553–3557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.