Abstract
Osteonecrosis is a debilitating toxicity associated with acute lymphoblastic leukemia (ALL) treatment. A recent report associated inter-individual differences in hip anatomy with the development of idiopathic osteonecrosis in adults. To evaluate the impact of hip anatomy on the development of therapy related osteonecrosis, we retrospectively evaluated the femoral neck-shaft angle, femoral neck offset, and lateral center-edge angle using x-rays of 18 osteonecrosis cases and 46 control children treated for newly diagnosed ALL on a single protocol. Despite adequate statistical power, we found no association between hip anatomy and osteonecrosis. Investigation of other factors contributing to ALL associated osteonecrosis is warranted.
Keywords: osteonecrosis, imaging, acute lymphoblastic leukemia
Introduction
With modern therapy, as many as 90% of children with acute lymphoblastic leukemia (ALL) can be cured.1 However, the intensification of glucocorticoids and asparaginase needed to achieve this high cure rate has been associated with an increased incidence of osteonecrosis.2,3 Osteonecrosis of the femoral head is particularly problematic, as most patients with involvement of ≥ 30% of the articular surface progressing to femoral head collapse.4 The greatest risk factor for the development of osteonecrosis is age, with patients 10–20 years old at diagnosis at higher risk than either older or younger patients.3,5 However, the reason for this age-related increased susceptibility remains unclear.
Although magnetic resonance imaging (MRI) is very sensitive for the early detection of osteonecrosis,6 it is resource intensive and not universally available. A recent report suggested that inter-individual differences in hip anatomy may increase the risk of developing idiopathic osteonecrosis of the femoral head.7 Hip anatomy is known to vary by age,8 gender, and ancestry,9 all of which have also been associated with altered risks of osteonecrosis.2,3,5,10,11 Because hip anatomy can be readily assessed by plain x-ray which is universally available, we evaluated the association of hip anatomy with the development of osteonecrosis in children treated for ALL.
Results
All patients were treated for newly diagnosed ALL as part of Total Therapy Study XV (ClinicalTrials.gov, number NCT00137111).1 With institutional review board approval, patients with hip x-rays were identified through chart review. Because of their higher rate of osteonecrosis,3,5 our study focused on patients over the age of 10 years. Osteonecrosis of the femoral head was defined as femoral head lesions on MRI involving ≥ 30% of the epiphysis as previously described.6 If multiple x-rays were available for a single patient, the one closest to the osteonecrosis-defining MRI was evaluated.
Anatomic measurements of the hip, including femoral neck-shaft angle (NSA), femoral neck offset (FNO), and lateral center-edge angle (LCEA), were performed as previously described7,12,13 using two independent reviewers (M.V.P. and S.C.K.). All measurements were made using InteleViewer™ (Intelerad, Montreal, QC) with statistical analysis performed using R.14 P values were obtained by linear or logistic regression as appropriate. Power analyses were performed in R using the “pwr” library and assumed previously demonstrated between-group differences would be replicated in our population.
Hip x-rays were available for 114 of 462 patients treated on protocol. After restricting our analysis to patients older than 10 at the time of x-ray with available MRI evaluations of the hips, 18 had MR-demonstrated hip osteonecrosis and 46 served as unaffected controls. All x-rays taken on patients without osteonecrosis were taken to assess local pain. The patients were diagnosed at a median age of 13.8 and 12 years (P=0.06) and had a median age of 14 and 14 years at the time of x-ray (P=0.53) for cases and controls, respectively. There were 28 females and 36 males. Patients were primarily white (CEU>90% as determined by STRUCTURE, N=49)2,15 and male (N=36). Fifty-four patients (including 15 cases) were treated with standard-/high-risk therapy and 10 (three cases) low-risk therapy.
Increasing age at the time of x-ray was associated with a greater FNO (P=2.2×10−5, Table 1). There was a trend toward smaller NSA with increasing age (P=0.056, Figure 1a) and in females (P=0.053). There was no association between ancestry or protocol treatment arm (low- vs. standard/high-risk) and any anatomic measurement; LCEA was not associated with any patient characteristics (P>0.05 for all variables).
TABLE 1.
Association of hip anatomy with clinical covariates and osteonecrosis risk
| Age at x-ray | Female Gender | White ancestry | Osteonecrosis | Osteonecrosis (multivariate) | |
|---|---|---|---|---|---|
| Femoral Neck Offset | 2.2×10−5 1.1 (1.1–1.2) |
0.13 1.3 (0.93–1.8) |
0.74 0.93 (0.62–1.4) |
0.21 1.7 (0.76–4.4) |
0.27 1.7 (0.67–4.9) |
| Neck Shaft Angle | 0.056 0.71 (0.5–1) |
0.053 7.6 (0.98–58) |
0.31 3.5 (0.3–41) |
0.09 0.88 (0.75–1.01) |
0.11 0.88 (0.75–1.02) |
| Lateral Center-edge Angle | 0.15 1.5 (0.85–2.8) |
0.92 1.2 (0.033–43) |
0.45 4.9 (0.076–320) |
0.15 1.1 (0.98–1.2) |
0.17 1.1 (0.98–1.2) |
Values indicated are linear regression P values with hazard ratios (including 95% confidence
FIGURE 1.
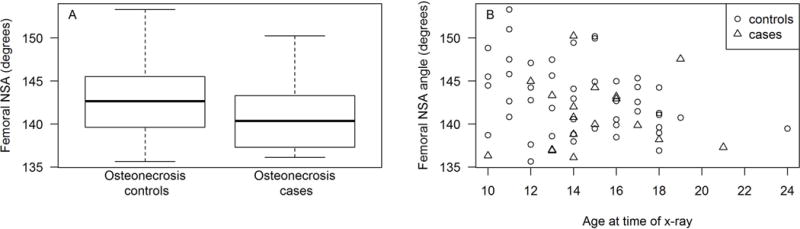
Association of femoral neck shaft angle (NSA) with osteonecrosis. Neck shaft angle trended smaller with increasing age (P=0.056, Figure 1a). Children who developed osteonecrosis had a trend toward smaller femoral neck shaft angles (NSA) than did controls (141° in cases vs. 143° in controls, P=0.09, Figure 1b), although there was considerable overlap between the groups. The association between neck shaft angle and osteonecrosis was further weakened when age was considered as a covariate (P=0.11).
There were no associations between the measured anatomic parameters and the development of hip osteonecrosis in our cohort [P=0.2 for FNO, P=0.09 for NSA (Figure 1b), and P=0.15 for LCEA, Table 1]. Because five patients had unilateral hip osteonecrosis, we evaluated intra-patient variability in hip measurements and found these to be less than inter-patient variability. There was no trend toward a greater incidence of osteonecrosis in the more “high-risk” hip as defined by anatomical measurements. Because of the marginal P values associating anatomic parameters and osteonecrosis, we also evaluated the association of osteonecrosis with hip measurements in a multivariable analysis including age at the time of x-ray. Inclusion of age as a covariate further weakened the association between anatomic variability and osteonecrosis (P=0.27 for FNO, P=0.11 for NSA, and P=0.17 for LCEA, Table 1).
Discussion
Osteonecrosis has become a therapy limiting side-effect in recent pediatric ALL regimens.5 Although factors such as age,2,3,10,11 gender,2,10 ancestry,2,10,11 and therapy intensity with dexamethasone2 and asparaginase10,11 have been associated with the development of osteonecrosis, the inter-individual variations resulting in osteonecrosis among patients remains incompletely understood. This is the first evaluation of associations between hip anatomy and therapy-related osteonecrosis in children with ALL. In contrast to published findings in adults with idiopathic osteonecrosis,7 we identified no such association in our patient population, despite being adequately powered to identify the previously described differences in our population. At an alpha of 0.05 and coefficients of variation of 0.15, 0.03, and 0.15 for FNO, NSA, and LCEA, respectively, we had an 80% power to detect a difference in means between the cases and controls of 0.54 cm for the FNO, 3.29° for NSA, and 5.57° for LCEA, differences comparable to those previously reported. However, the differences we observed in FNO, NSA, and LCEA were all smaller than the within-group differences among the cases or controls (Table 1, Figure 1a and 1b). This suggests that while hip anatomy contribute to excess risk of developing idiopathic osteonecrosis in adults, alternative factors drive the development of therapy related osteonecrosis in children with ALL.
There are several limitations to our study related to its retrospective design. Because children with ALL do not routinely undergo x-rays of their hips during therapy, it is possible that the population with x-rays performed does not represent the ALL population as a whole. As we focused on patients older than 10 at the time of x-ray, these results should be applied only to that group. Additionally, because x-rays were taken in response to patient symptoms (either osteonecrosis or hip pain of other etiologies), patients were allowed to find positions of comfort while undergoing radiographs instead of being positioned in an identical pose. Due to skeletal immaturity, consistent identification of the needed landmarks was challenging. These factors resulted in an increase in inter-reviewer variability in the measurements of hip anatomy. It is also possible that smaller anatomic differences than were previously shown7 may contribute to the risk of osteonecrosis. If this is true, our study would be underpowered to detect such risk. However, even with the larger differences seen in our study, there is significant overlap between the anatomy of osteonecrosis cases and controls. This suggests that smaller differences would poorly discriminate between patients at standard or elevated risk of develop osteonecrosis, further arguing against prospective screening.
Although a prospective trial is the ideal setting to evaluate correlations between risk factors and therapy toxicity, our study suggests that prior knowledge of a patient’s hip anatomy is unlikely to increase the ability to predict osteonecrosis during ALL therapy. Efforts to further minimize this side effect should focus on early detection6 and an improved understanding of the pathophysiology through clinical and genomic studies.
Acknowledgments
This work was supported by the American Lebanese-Syrian Associated Charities and the National Institutes of Health P30-CA021765, 5R25-CA023944, U01-GM115279, and R01-CA142665.
NIH grants: P30 CA021765; R25 CA023944; P50 GM115279; U01 GM115279; R01 CA142665
Abbreviations
- ALL
acute lymphoblastic leukemia
- MRI
magnetic resonance imaging
- FNO
femoral neck offset
- NSA
femoral neck-shaft angle
- LCEA
lateral center-edge angle
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karol SE, Yang W, Van Driest SL, et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126(15):1770–1776. doi: 10.1182/blood-2015-05-643601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattano LA, Jr, Devidas M, Nachman JB, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. The lancet oncology. 2012;13(9):906–915. doi: 10.1016/S1470-2045(12)70274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karimova EJ, Rai SN, Howard SC, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25(12):1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 5.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(18):3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 6.Kaste SC, Pei D, Cheng C, et al. Utility of early screening magnetic resonance imaging for extensive hip osteonecrosis in pediatric patients treated with glucocorticoids. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(6):610–615. doi: 10.1200/JCO.2014.57.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollivier M, Lunebourg A, Abdel MP, Parratte S, Argenson JN. Anatomical Findings in Patients Undergoing Total Hip Arthroplasty for Idiopathic Femoral Head Osteonecrosis. The Journal of bone and joint surgery American volume. 2016;98(8):672–676. doi: 10.2106/JBJS.14.01099. [DOI] [PubMed] [Google Scholar]
- 8.Szuper K, Schlegl AT, Leidecker E, Vermes C, Somoskeoy S, Than P. Three-dimensional quantitative analysis of the proximal femur and the pelvis in children and adolescents using an upright biplanar slot-scanning X-ray system. Pediatric radiology. 2015;45(3):411–421. doi: 10.1007/s00247-014-3146-2. [DOI] [PubMed] [Google Scholar]
- 9.Lavy CB, Msamati BC, Igbigbi PS. Racial and gender variations in adult hip morphology. Int Orthop. 2003;27(6):331–333. doi: 10.1007/s00264-003-0507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karol SE, Mattano LA, Jr, Yang W, et al. Genetic risk factors for the development of osteonecrosis in children under age 10 treated for acute lymphoblastic leukemia. Blood. 2016;127(5):558–564. doi: 10.1182/blood-2015-10-673848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husmann O, Rubin PJ, Leyvraz PF, de Roguin B, Argenson JN. Three-dimensional morphology of the proximal femur. J Arthroplasty. 1997;12(4):444–450. doi: 10.1016/s0883-5403(97)90201-1. [DOI] [PubMed] [Google Scholar]
- 13.Rubin PJ, Leyvraz PF, Aubaniac JM, Argenson JN, Esteve P, de Roguin B. The morphology of the proximal femur. A three-dimensional radiographic analysis. The Journal of bone and joint surgery British volume. 1992;74(1):28–32. doi: 10.1302/0301-620X.74B1.1732260. [DOI] [PubMed] [Google Scholar]
- 14.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. [Google Scholar]
- 15.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]


