Abstract
Purpose
In this study, we compared the clinical efficacy of JOINS (SKI306X, SK Chemicals) with placebo on cartilage protection using magnetic resonance imaging (MRI).
Materials and Methods
Sixty-nine patients were randomized to the JOINS group (200 mg, three times daily for 1 year; n=33) or the placebo group (n=36). Changes in cartilage volume and thickness were measured using MRI. Changes in the delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) index, subchondral bone marrow abnormality scores, and clinical scores including knee pain visual analog scale (VAS) score and Korean Western Ontario and McMaster Universities Osteoarthritis Index (K-WOMAC) were also evaluated.
Results
Changes in cartilage thickness and volume and subarticular bone marrow abnormality scores were not different between groups. Changes in the dGEMRIC index in the lateral tibial plateau were greater in the JOINS group than in the placebo group (19.64±114.33 msec vs. −57.77±123.30 msec; p=0.011). Significantly greater changes in VAS were observed in the JOINS group than in the placebo group (−26.00±12.25 vs. −12.47±21.54; p=0.002) and K-WOMAC (−15.42 ± 7.73 vs. −8.15±13.71; p=0.003).
Conclusions
Compared with placebo, JOINS had superior clinical efficacy in regard to cartilage protection.
Keywords: Knee, Osteoarthritis, SKI306X, Cartilage
Introduction
Knee osteoarthritis (OA) is one of the most common joint diseases. The function of the articular cartilage is to facilitate movement by providing a soft, smooth surface for the weight bearing portion of the joint, and to protect portions of the joint under concentrated load against damage. OA is a disease in which the joint cartilage playing these important roles wears down, and the underlying bone is also damaged, resulting in deformation as the bone regenerates and occurrence of a number of symptoms1–4).
Conservative treatment options for OA consist of non-pharmacological treatment, such as exercise, weight loss, and lifestyle education and pharmacological treatment using acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and analgesics5–7). Although NSAIDs and analgesics have established efficacy in knee OA, several adverse effects have been reported, such as gastrointestinal ulcer and hemorrhage and cardiovascular, hepatic, and renal toxicity8–11). Glucosamine and chondroitin sulfate are widely used as a supplement for pain relief and cartilage protection, but their efficacy remains unclear12–15).
Herbal anti-arthritic medicines have been widely used as a treatment for OA in Eastern countries. Although the mechanism of these treatments has not been fully evaluated, there are several studies demonstrating supportive evidence concerning their efficacy16–19). JOINS tablet (SKI306X, SK Chemicals, Seongnam, Korea) is one of such herbal anti-arthritic drugs. The tablet is made with powdered extract of herbs, Clematis mandshurica, Trichosanthes kirilowii, and Prunella vulgaris mixed at 1:2:1 weight ratio in an alcoholic aqueous solution, followed by fractionating with water-saturated butanol and complete removal of residual solvents. In vitro studies showed that JOINS can inhibit the degradation of articular tissue caused by free radicals that are produced as a result of ischemic reperfusion associated with pressure change in the articular cavity20–23).
This clinical study was designed to evaluate the effect of JOINS on cartilage protection using magnetic resonance imaging (MRI) as well as its safety. Given that there is no drug currently available on the market for cartilage protection and that research on the efficacy of cartilage protection using MRI is very rare, this study would provide clinically valuable information. In this study, we compared the clinical efficacy of JOINS (200 mg, three times per day) orally administered for one year versus placebo in terms of cartilage protection using MRI. We hypothesized that the JOINS group would show more effective cartilage protection than the placebo group after 1 year of treatment.
Materials and Methods
1. Patients
We conducted a single-center double-blinded randomized controlled trial at Seoul National University from August 4, 2010 to September 25, 2013. A minimum of 30 subjects per group was considered necessary for this pilot study. To allow for a 20% drop-out rate, we sought to enroll 38 subjects per group. Regardless of gender, patients aged 45–79 years and diagnosed with primary knee OA in the medial tibiofemoral compartment based on the American College Rheumatology Criteria were enrolled in the study. The Kellgren-Lawrence grade of the knee OA was 2 or 3 with the narrowest point of the medial joint space width at least 2 mm. Exclusion criteria were rheumatoid arthritis or inflammatory arthritis, valgus knee deformity, history of any surgery on the same knee, secondary knee OA, hypersensitivity to JOINS or acetaminophen, history of venous thromboembolism or high risk of venous thromboembolism, severe renal impairment with creatinine clearance <30 mL/min (calculated using the Crockroft-Gault formula), and receipt of intra-articular hyaluronate injection within months or corticosteroids within 3 months before the screening visit. A computer-generated permuted block randomization table was prepared by the statistician and given to the study’s coordinator in a series of sealed envelopes. After study participants provided informed consent, patients were randomized to the JOINS group or the placebo group. After a 2-week washout period, patients were administered the investigational drug three times daily for 1 year. The placebo tablet had the same size and shape as JOINS and was manufactured by SK Chemicals. In this study, Tylenol ER 650 mg was provided as the rescue medication and allowed to be used up to 4,000 mg per day. However, to evaluate symptoms including pain, the subject was requested not to use it within 48 hours before visit to the clinic. The study protocol was approved by the Seoul National University Hospital Institutional Review Board (Protocol No. 06-2010-1140). This study was registered in advance with the Clinical Research Information Service, which is one of the primary registration systems listed on the World Health Organization (WHO) International Clinical Trials Registry Platform (Protocol No. NCT01293955).
2. MRI and Radiographic Evaluation
The MRI scans were obtained using a 3.0 T MRI unit (Trio TIM; Siemens, Erlangen, Germany) with an 8-channel transmit-receive knee coil 90 min after intravenous injection of a double dose (0.4 mL/kg) of gadopentetate dimeglumine (Magnevist; Schering AG, Berlin, Germany). For cartilage volume and thickness measurements, the fat-saturated proton density-weighted three-dimensional (3D) sampling perfection with application-optimized contrasts using variable flip-angle evolution fast spin echo (FSE) sequences were obtained in the same plane using the following parameters: repetition time/echo time (TR/TE), 2,000/50; flip angle, 120°; filed of view (FOV), 136×170; slice thickness, 0.6 mm; and acquisition matrix, 320×256. The volume and thickness of the femoral condyle, medial and lateral tibial plateaus, and patella were measured using a semi-automatic method. First, the segmentation of the radial scan method was used and then the radiology staff performed supplemented image segmentation. T1 maps were generated in a 3 mm thick sagittal slice on each condyle, using a dual flip angle 3D gradient-echo sequence with volumetric interpolated breath hold examination using the following parameters: TR/TE, 15.0/4.9 ms; flip angles, 5° and 26°; bandwidth, 199 Hz/pixel; FOV, 140×140 mm; slice thickness, 4.0 mm; number of sections, 20; and matrix, 256×128. Region of interest for the delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) index was examined in the central parts of the medial and lateral femoral condyles and tibial plateaus. In addition, sagittal two-dimensional proton density- and coronal T2-weighted fat-suppressed FSE sequences were acquired to assess the subchondral bone marrow abnormality scores for the entire knee using the Whole-Organ Magnetic Resonance Imaging Score (WORMS)24). All MRI analyses were performed by one musculoskeletal radiologist (CJY). MRI measurements were performed pretreatment and at 1-year follow-up.
3. Clinical and Safety Evaluation
Clinical scores, including the knee pain visual analog scale (VAS) score and Korean Western Ontario and McMaster Universities Osteoarthritis Index (K-WOMAC) were measured pretreatment and at 1 year follow-up. The safety evaluation of this clinical study was continued for 2 years with regard to the occurrence of adverse events, clinical laboratory tests, vital signs, and electrocardiography.
4. Statistical Analysis
Differences between the two groups were analyzed with the Student t-test for continuous variables and Pearson chi-square test or Fisher exact test for categorical variables. The mean changes in each group were analyzed with the paired t-test. Statistical analyses were performed with the IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). p-values <0.05 were considered statistically significant.
Results
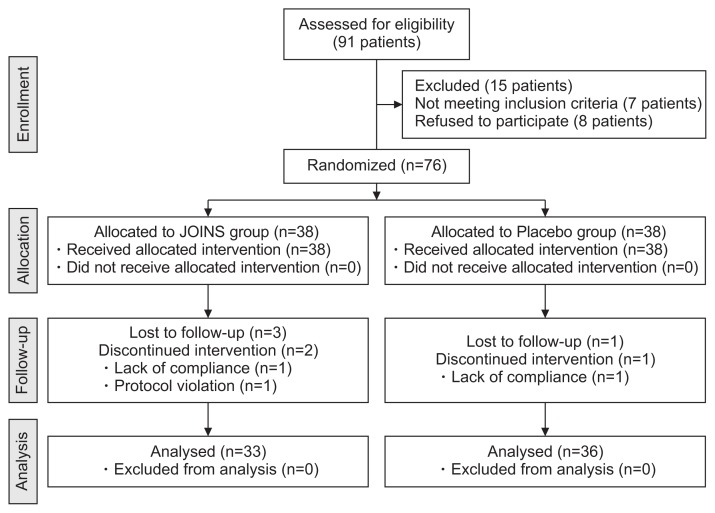
Five patients in the JOINS group and 2 patients in the placebo group were not available for follow-up at 1-year after treatment; thus 33 patients in the JOINS group and 36 patients in the placebo group were analyzed (Fig. 1). No differences in demographics were observed between the two groups (Table 1).
Fig. 1.
Flow diagram of the present study based on Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Table 1.
Comparison of Demographics and Clinical Status between Two Groups
| Characteristic | JOINS (n=33) | Placebo (n=36) | p-value |
|---|---|---|---|
| Gender | 0.431a) | ||
| Male | 2 (6.06) | 5 (13.89) | |
| Female | 31 (93.94) | 31 (86.11) | |
| Age (yr) | 60.15±6.48 | 60.03±6.03 | 0.934b) |
| Height (cm) | 156.91±5.43 | 157.44±6.56 | 0.928b) |
| Weight (kg) | 63.12±9.84 | 63.42±7.80 | 0.888b) |
| Body mass index (kg/m2) | 25.55±3.08 | 25.58±2.74 | 0.961b) |
| Duration of degenerative osteoarthritis (yr) | 4.94±3.00 | 4.33±3.22 | 0.207b) |
| Kellgren-Lawrence grade | 0.721c) | ||
| Grade 2 | 17 (51.52) | 17 (47.22) | |
| Grade 3 | 16 (48.48) | 19 (52.78) |
Values are presented as mean±standard deviation or number (%).
Fisher exact test.
Student t-test.
Pearson chi-square test.
1. MRI and Radiographic Results
Changes in the cartilage volume and thickness were not significantly different between groups (Table 2). The change in the dGEMRIC index in the lateral tibial plateau on T1 mapping was higher in the JOINS group than in the placebo group (19.64±114.33 msec vs. −57.77±123.30 msec, p=0.011) (Table 3). However, other changes were not significantly different between groups. The subarticular bone marrow abnormality scores for the entire knee were not significantly different between groups (Table 4).
Table 2.
Comparison of Cartilage Volume and Thickness between Two Groups at 1-Year Follow-up
| Variable | Volume (mm3) | Thickness (mm) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| JOINS | Placebo | p-valuea) | JOINS | Placebo | p-valuea) | |
| Femur | ||||||
| Baseline | 5,590.76±1,227.80 | 5,355.09±733.25 | 0.518 | 1.10±0.13 | 1.06±0.11 | 0.178 |
| 1-year | 4,503.90±1,128.83 | 4,297.98±809.56 | 0.647 | 0.98±0.13 | 0.95±0.12 | 0.443 |
| Difference | −1,086.86±542.08 | −1,057.10±499.67 | 0.975 | −0.11±0.06 | −0.10±0.08 | 0.366 |
| p-valueb) | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Medial tibia | ||||||
| Baseline | 1,556.57±277.08 | 1,608.17±279.44 | 0.450 | 1.44±0.29 | 1.42±0.22 | 0.695 |
| 1-year | 1,216.09±210.72 | 1,239.50±236.64 | 0.720 | 1.30±0.24 | 1.26±0.18 | 0.451 |
| Difference | −340.48±197.84 | −368.68±184.98 | 0.486 | −0.14±0.11 | −0.15±0.11 | 0.506 |
| p-valueb) | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Lateral tibia | ||||||
| Baseline | 1,500.36±290.54 | 1,958.67±2,412.66 | 0.261 | 1.45±0.28 | 1.39±0.27 | 0.402 |
| 1-year | 1,191.90±214.08 | 1,220.34±230.54 | 0.665 | 1.27±0.21 | 1.26±0.26 | 0.940 |
| Difference | −308.46±130.89 | −738.33±2,383.28 | 0.656 | −0.18±0.13 | −0.13±0.14 | 0.056 |
| p-valueb) | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Patella | ||||||
| Baseline | 1,835.58±392.57 | 1,854.35±313.32 | 0.852 | 1.85±0.32 | 1.77±0.36 | 0.353 |
| 1-year | 1,387.40±298.37 | 1,407.50±249.55 | 0.767 | 1.63±0.25 | 1.56±0.31 | 0.284 |
| Difference | −448.18±267.83 | −446.85±203.32 | 0.842 | −0.22±0.16 | −0.22±0.18 | 0.817 |
| p-valueb) | <0.001 | <0.001 | <0.001 | <0.001 | ||
Values are presented as mean±standard deviation.
Student t-test.
Paired t-test.
Table 3.
Comparison of Delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage Index between Two Groups at 1-Year Follow-up
| Variable | T1 mapping | ||
|---|---|---|---|
|
| |||
| JOINS | Placebo | p-valuea) | |
| Medial femur (msec) | |||
| Baseline | 565.09±155.44 | 576.62±105.02 | 0.816 |
| 1-year | 558.65±134.75 | 536.32±158.85 | 0.691 |
| Difference | −6.44±93.95 | −53.03±163.96 | 0.390 |
| p-valueb) | 0.787 | 0.286 | |
| Medial tibia (msec) | |||
| Baseline | 529.04±125.43 | 477.37±115.60 | 0.253 |
| 1-year | 535.88±110.47 | 474.35±159.83 | 0.238 |
| Difference | 6.85±88.78 | 6.52±134.63 | 0.993 |
| p-valueb) | 0.762 | 0.869 | |
| Lateral femur (msec) | |||
| Baseline | 619.60±123.09 | 602.58±137.54 | 0.599 |
| 1-year | 619.76±150.48 | 584.07±101.49 | 0.268 |
| Difference | 0.16±116.73 | −20.44±130.21 | 0.504 |
| p-valueb) | 0.993 | 0.373 | |
| Lateral tibia (msec) | |||
| Baseline | 635.66±154.73 | 666.61±126.79 | 0.379 |
| 1-year | 654.45±156.76 | 603.91±149.61 | 0.188 |
| Difference | 19.64±114.33 | −57.77±123.30 | 0.011 |
| p-valueb) | 0.346 | 0.011 | |
Values are presented as mean±standard deviation.
Student t-test.
Paired t-test.
Table 4.
Comparison of Subarticular Bone Marrow Abnormality Scores for Entire Knee between Two Groups at 1-Year Follow-up
| Variable | JOINS | Placebo | p-value |
|---|---|---|---|
| Baseline (point) | 5.12±2.58 | 4.18±2.29 | |
| 1-year (point) | 5.00±2.73 | 4.47±2.84 | |
| Difference (point) | −0.12±0.74 | 0.29±1.29 | 0.111a) |
| p-valueb) | 0.353 | 0.193 |
Values are presented as mean±standard deviation.
Student t-test.
Paired t-test.
2. Clinical and Safety Results
VAS and K-WOMAC scores were significantly improved in both groups. However, the JOINS group showed significantly greater changes than the placebo group in VAS (−26.00±12.25 vs. −12.47±21.54; p=0.002) and K-WOMAC (−15.42±7.73 vs. −8.15±13.71; p=0.003) (Table 5). The safety profile was not different between groups (Table 6).
Table 5.
Comparison of VAS Score and K-WOMAC Score between Two Groups at 1-Year Follow-up
| Variable | VAS | K-WOMAC | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| JOINS | Placebo | p-valuea) | JOINS | Placebo | p-valuea) | |
| Baseline (point) | 51.39± 9.27 | 53.67±7.73 | 0.114 | 39.58±10.27 | 40.03±10.44 | 0.856 |
| 1-year (point) | 25.39±12.06 | 40.82±20.65 | 0.000 | 24.15±7.51 | 31.85±12.90 | 0.004 |
| Difference (point) | −26.00±12.25 | −12.47±21.54 | 0.002 | −15.42±7.73 | −8.15±13.71 | 0.003 |
| p-valueb) | <0.001 | 0.001 | <0.001 | <0.001 | ||
Values are presented as mean±standard deviation.
VAS: visual analog scale, K-WOMAC: Korean Western Ontario and McMaster Universities Osteoarthritis Index.
Student t-test.
Paired t-test.
Table 6.
Safety Profile at 2-Year Follow-up
| Variable | JOINS (n=38) | Placebo (n=38) | p-value |
|---|---|---|---|
| Adverse events | 10 (26.32) | 12 (31.58) | 0.613a) |
| Adverse drug reaction | 3 (7.89) | 1 (2.63) | 0.614b) |
| Serious adverse events | 0 (0.00) | 1 (2.63) | 1.000b) |
| Adverse drug reaction details | |||
| Dyspepsia | 2 (5.26) | 0 (0.00) | |
| Upper abdominal pain | 1 (2.63) | 0 (0.00) | |
| Tongue edema | 0 (0.00) | 0 (0.00) | |
| Pneumonia | 0 (0.00) | 1 (2.63) | |
Pearson chi-square test.
Fisher exact test.
Discussion
We analyzed the efficacy of the orally administered JOINS for cartilage protection compared with placebo. The most important findings of this study include that although the changes in cartilage thickness and volume were not different between the two groups, the change in the dGEMRIC index was significantly greater in the lateral tibial plateau in the JOINS group. Therefore, this study shows that JOINS has the potential for superior clinical efficacy compared with placebo in regard to cartilage protection.
JOINS is a drug prepared from the ethanol extract of 3 oriental herbs—Clematis mandshurica, Trichosanthes kirilowii, and Prunella vulgaris that have been widely and traditionally used to decrease pain and improve functional capacity in patients with OA. JOINS is known to have several functions including anti-inflammation, immunomodulation, and activation of blood microcirculation20–23,25).
Additionally, several in vitro studies haven shown JOINS has a cartilage protection effect. The first study was performed by Choi et al.21) using rabbits. In the study, JOINS inhibited proteoglycan degradation in cartilage explant culture, and prophylactic administration of JOINS significantly protected the knee joint of rabbits from OA-like changes in a collagenase-induced OA model. Kim et al.23) also performed a study using rabbit cartilage explant culture and reported that JOINS inhibited matrix degradation by down-regulating matrix metalloproteinase (MMP) expression and inhibiting MMP activity during the collagen breakdown process. Hartog et al.22) performed a study using bovine cartilage explants and human peripheral blood mononuclear cells and reported that SKI306X inhibited IL-1β-induced proteoglycan release and nitric oxide production by cartilage, indicating cartilage protective activity. Recently, Choi et al.20) performed a study using human OA chondrocytes and cartilage explants and reported that JOINS inhibited IL-1β-induced glycosaminoglycan (GAG) release from cartilage explants and inhibited IL-1β-induced MMP gene expression. However, all published studies were in vitro, demonstrating the cartilage protection effect of JOINS using chondrocytes from human, bovine, and rabbit articular cartilage. A clinical study using quantitative MRI has not been previously reported.
This study evaluated the effect of JOINS on cartilage protection using MRI. Although the changes in cartilage volume and thickness were not different between the treatment and placebo groups, the change of the dGEMRIC index in the lateral tibial plateau was significantly greater in the JOINS group. Considering that knee OA primarily develops in the medial compartment, it is unclear whether the improved dGEMRIC index in the lateral tibial plateau is clinically relevant to the treatment of OA. However, Wildi et al.26) reported similar findings in a study evaluating the efficacy of chondroitin sulfate for cartilage protection using MRI. In the study, the chondroitin treatment group showed significantly less cartilage volume loss in the lateral compartment than the placebo group at 1-year follow-up. Improved cartilage preservation in the lateral compartment may be explained by the fact that, in general, cartilage damage is less severe in the lateral compartment than in the medial compartment; thus, the lateral compartment showed a greater response to cartilage protection agents. Additionally, these agents in the damaged medial compartment might require more time to demonstrate a treatment effect.
In terms of safety, JOINS was equivalent to placebo regarding adverse event occurrence, and this can be considered remarkable given that the most commonly used medications for knee OA are NSAIDs, which cause some well-defined side effects such as gastrointestinal ulcer and hemorrhage and cardiovascular, hepatic, and renal toxicity8–11).
There are several limitations of this study. First, the sample size is relatively small owing to the fact that this is a pilot study. Second, inter-rater reliability could not be tested because only one rater had conducted MRI measurements of all subjects. Third, the 1-year follow-up may be inadequate to detect a cartilage protection effect of JOINS using MRI. Although subarticular bone marrow abnormality scores for the entire knee were not different between the two groups at the 1-year follow-up, some patients who agreed to a 2-year follow-up showed changes of the subarticular bone marrow abnormality scores for the entire knee that were significantly different between groups. In the placebo group, subarticular bone marrow abnormality scores for the entire knee significantly increased, whereas scores were not changed in the JOINS group (Table 7). A long-term follow-up may be necessary to fully elucidate the cartilage protection effect with MRI. Fourth, although the dGEMRIC index was significantly higher in the lateral tibial plateau in the JOINS group, changes in cartilage thickness and volume were not different between the two groups. dGEMRIC is a technique to evaluate cartilage GAG content. Since dGEMRIC can identify GAG loss in early stage of cartilage disease with a higher sensitivity27), we think that it is more useful in assessing changes in cartilage than measurement of volume or thickness of cartilage.
Table 7.
Comparison of Subarticular Bone Marrow Abnormality Scores for Entire Knee between Two Groups at 2-Year Follow-up
| Variable | JOINS (n=21) | Placebo (n=10) | p-value |
|---|---|---|---|
| Baseline (point) | 5.38±2.58 | 3.70±2.31 | |
| 2-year (point) | 5.05±2.60 | 4.50±2.55 | |
| Difference (point) | −0.33±0.97 | 0.80±0.79 | 0.003a) |
| p-valueb) | 0.129 | 0.010 |
Values are presented as mean±standard deviation.
Student t-test.
Paired t-test.
Conclusions
The changes in cartilage volume and thickness were not different between the two groups at 1-year follow-up. However, the change of the dGEMRIC index in the lateral tibial plateau was significantly greater in the JOINS group than in the placebo group. Therefore, our results show potential clinical efficacy of JOINS superior to placebo in regard to cartilage protection.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Source of funding: This study was supported by research fund from SK Chemical.
References
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Howell DS, Woessner JF, Jr, Jimenez S, Seda H, Schumacher HR., Jr A view on the pathogenesis of osteoarthritis. Bull Rheum Dis. 1978–1979;29:996–1001. [PubMed] [Google Scholar]
- 3.Radin EL. Who gets osteoarthritis and why? An update. J Rheumatol. 2005;32:1136–8. [PubMed] [Google Scholar]
- 4.Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. CurrOpin Rheumatol. 2006;18:147–56. doi: 10.1097/01.bor.0000209426.84775.f8. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. 2013;5:30–8. doi: 10.4103/0975-7406.106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruyère O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, Hochberg MC, Kanis JA, Kvien TK, Martel-Pelletier J, Rizzoli R, Silverman S, Reginster JY. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2014;44:253–63. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Lane NE, Thompson JM. Management of osteoarthritis in the primary-care setting: an evidence-based approach to treatment. Am J Med. 1997;103(6A):25S–30S. doi: 10.1016/S0002-9343(97)90005-X. [DOI] [PubMed] [Google Scholar]
- 8.Bakowsky VS, Hanly JG. Complications of nonsteroidal antiiflammatory drug gastropathy and use of gastric cytoprotection: experience at a tertiary care health center. J Rheumatol. 1999;26:1557–63. [PubMed] [Google Scholar]
- 9.Fries JF. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol Suppl. 1991;28:6–10. [PubMed] [Google Scholar]
- 10.Murray MD, Brater DC. Adverse effects of nonsteroidal anti-inflammatory drugs on renal function. Ann Intern Med. 1990;112:559–60. doi: 10.7326/0003-4819-112-8-559. [DOI] [PubMed] [Google Scholar]
- 11.Roth SH. NSAID gastropathy: a new understanding. Arch Intern Med. 1996;156:1623–8. doi: 10.1001/archinte.1996.00440140039003. [DOI] [PubMed] [Google Scholar]
- 12.Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging. 2007;24:573–80. doi: 10.2165/00002512-200724070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Nutritional supplements for knee osteoarthritis: still no resolution. N Engl J Med. 2006;354:858–60. doi: 10.1056/NEJMe058324. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30:357–63. doi: 10.1007/s00296-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 15.Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ, Reichenbach S, Trelle S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blumle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: osteoarthritis. Phytother Res. 2009;23:1497–515. doi: 10.1002/ptr.3007. [DOI] [PubMed] [Google Scholar]
- 17.Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blümle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part 2: rheumatoid arthritis. Phytother Res. 2009;23:1647–62. doi: 10.1002/ptr.3006. [DOI] [PubMed] [Google Scholar]
- 18.Chrubasik JE, Roufogalis BD, Chrubasik S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother Res. 2007;21:675–83. doi: 10.1002/ptr.2142. [DOI] [PubMed] [Google Scholar]
- 19.Ha CW, Park YB, Min BW, Han SB, Lee JH, Won YY, Park YS. Prospective, randomized, double-blinded, double-dummy and multicenter phase IV clinical study comparing the efficacy and safety of PG201 (Layla) and SKI306X in patients with osteoarthritis. J Ethnopharmacol. 2016;181:1–7. doi: 10.1016/j.jep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Choi CH, Kim TH, Sung YK, Choi CB, Na YI, Yoo H, Jun JB. SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes. Korean J Intern Med. 2014;29:647–55. doi: 10.3904/kjim.2014.29.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JH, Choi JH, Kim DY, Yoon JH, Youn HY, Yi JB, Rhee HI, Ryu KH, Jung K, Han CK, Kwak WJ, Cho YB. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthritis Cartilage. 2002;10:471–8. doi: 10.1053/joca.2002.0526. [DOI] [PubMed] [Google Scholar]
- 22.Hartog A, Hougee S, Faber J, Sanders A, Zuurman C, Smit HF, van der Kraan PM, Hoijer MA, Garssen J. The multicomponent phytopharmaceutical SKI306X inhibits in vitro cartilage degradation and the production of inflammatory mediators. Phytomedicine. 2008;15:313–20. doi: 10.1016/j.phymed.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Ryu KH, Jung KW, Han CK, Kwak WJ, Cho YB. SKI306X suppresses cartilage destruction and inhibits the production of matrix metalloproteinase in rabbit joint cartilage explant culture. J Pharmacol Sci. 2005;98:298–306. doi: 10.1254/jphs.FPJ04058X. [DOI] [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Jung YB, Roh KJ, Jung JA, Jung K, Yoo H, Cho YB, Kwak WJ, Kim DK, Kim KH, Han CK. Effect of SKI 306X, a new herbal anti-arthritic agent, in patients with osteoarthritis of the knee: a double-blind placebo controlled study. Am J Chin Med. 2001;29:485–91. doi: 10.1142/S0192415X01000502. [DOI] [PubMed] [Google Scholar]
- 26.Wildi LM, Raynauld JP, Martel-Pelletier J, Beaulieu A, Bessette L, Morin F, Abram F, Dorais M, Pelletier JP. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011;70:982–9. doi: 10.1136/ard.2010.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488–92. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]