Summary
In this perspective piece, Drs. Justice and Braithwaite consider what has been learned and is being studied about aging with HIV in resource rich settings. The authors argue that while there is much that will be different about aging with HIV in other parts of the globe, there are common themes and approaches to care. These include the observation that most patients have more than one health condition, and the need to assess individual risk, prioritize care, and consider the total burden of disease when considering further testing and treatment.
Background
There are many differences among those aging with HIV infection in North America/Europe and Africa, even among individuals of African ancestry. In North America/Europe the current wave of individuals aging with HIV is characterized by those, now on combination antiretroviral therapy (cART), previously exposed to mono and dual therapy. As a result, levels of antiretroviral resistance may be changing as more antiretroviral naïve individuals initiate cART; in Africa prior exposure is less common and levels of resistance may be lower.
Infected populations also differ. In North America/Europe, the burden of HIV falls disproportionally among racial and sexual minorities[2] in whom aging, in general, has been poorly characterized. This “special population” of middle aged, predominantly male, aging individuals often continues to use alcohol, tobacco, and psychoactive medications (including prescription opioids)[3]. This group is also more likely to have a history of intravenous drug use, attendant HCV infection, and depression[4]. Until fairly recently in Africa, short life expectancy precluded substantial numbers of aging individuals even in the general population. Those now aging with HIV infection are similar to the general population and much more commonly female[5, 6]. In Africa, even more than in North America/Europe, alcohol (including home brew) is a more dominant problem than abuse of opioids or injection drugs[7, 8].
Importantly, in North America/Europe and Africa, the age distribution of those “aging” with HIV infection remains largely within middle age (45–65 years)[2, 5, 6]. Because we have limited information concerning what aging beyond middle age “should” look like for Africans and for racial and sexual minorities in North America/Europe, we need to understand aging more generally in these populations, as well as how HIV alters this trajectory.
Given the special issues surrounding aging with HIV, three questions come to mind: 1) what can be learned from research among those aging without infection, 2) what can be learned from research among those aging with HIV–but in a different context, and 3) what must be studied in the African context. We address these questions iteratively, as we consider the adaptation of three central geriatric concepts to those aging with HIV infection: multimorbidity, frailty, and the importance of personalized care.
Multimorbidity
Multimorbidity is defined as the presence of more than one life threatening chronic health condition [9, 10]. Mr. Smith is aging with multimorbidity; he has chronic HIV and Hepatitis C infections, hazardous alcohol consumption, is becoming obese, smokes, and has hypertension. HIV, HCV, obesity, and hazardous alcohol consumption all contribute to his likely liver fibrosis (evidenced by increased alanine and aspartate transaminases) and increasing risk for liver cancer[11]. His smoking, obesity, elevated lipids, lack of exercise, and hypertension certainly increase his risk of cardiovascular disease, renal disease, and stroke. His erectile dysfunction suggests the presence of microvascular disease.
While multimorbidity is not exclusively seen among older individuals, it is more common with increasing age. Among those aging without HIV Infection in North America/Europe, multimorbidity is the norm[4, 12, 13]. Further, among those with suppressed HIV-1 RNA, HIV has ceased to be the “dominant” comorbidity (i.e., that which eclipses management of all other comorbidities)[14] but rather is a key element in the overall milieu of multimorbidity. Multimorbidity introduces additional sources of risk, competing demands for care, added complexity, and polypharmacy[15].
There are two major sources of multimorbidity, HIV associated non AIDS conditions (HANA Conditions) and true comorbid (or HIV independent) disease. As the name “Human Immunodeficiency Virus” implies, we initially conceived of HIV as a disease of the immune system. But HIV is a systemic disease akin to diabetes, rheumatoid arthritis, or multiple myeloma, with direct and indirect effects on every major organ system (immune, blood, heart, lung, liver, kidney, muscle, bone, and both central and peripheral nerve)[16–18]. Now that we have therapies capable of maintaining a partially effective immune system, more people are experiencing long term manifestations of HIV infection, many of which interact with comorbidities of aging and substance use. HANA conditions are also seen among individuals without HIV infection, are more common among older individuals, and are associated with HIV infection even after adjustment for age and other known risk factors. The SMART trial suggested that uncontrolled viral replication is associated with greater risk of many of these conditions than is long term exposure to antiretroviral therapy[19]. Whether HANA conditions are causally related to HIV, long term sequela of HIV such as microbial translocation and chronic inflammation, or consequences of antiretroviral treatment likely depend on the condition in question. For example, increased risk of osteoporotic bone fractures among men with HIV appears to be closely tied to body mass index and proton pump inhibitor use[20]. In contrast, increased risk of myocardial infarction appears to remain even after adjustment for established risk factors[21].
Nevetheless, there is little data supporting the claim of “premature” versus “accentuated” aging[22]. Once you control for differences in the distribution of ages among uninfected versus HIV infection populations, and consider only actual clinical events rather than biomarkers, we do not see substantial differences in the age at which events occur[20–23]. While the risk of having the condition is increased among those with HIV compared to uninfected individuals, clinical events typically occur within 5 years of the ages observed among uninfected individuals.
As among those aging without HIV infection, many conditions are developing among patients aging with HIV infection due to behavioral, environmental, and genetic risk factors. While these may not be causally associated with HIV or its treatment, they contribute to multimorbidity, may be exacerbated by HIV or its treatment, and likely influence what are the most optimal therapeutic choices.
The frequency of multimorbidity will grow as the population with HIV ages. The dominant profile of multimorbidity among middle aged individuals aging with HIV in North America/Europe features chronic viral hepatitis, liver cirrhosis, hypertension, depression, cardiovascular disease, diabetes, chronic obstructive lung disease, osteoporosis, and renal disease[4, 20, 24–26]. Lung cancer, hepatocellular cancer, anal cancer, and colon cancer are also commonly observed[26]. Some of these conditions also progress more rapidly among those with HIV infection, especially if HIV RNA is not suppressed[27–29].
The dominant profile of HANA and comorbid conditions experienced by those aging with HIV in Africa remains to be characterized and may vary from those in North America/Europe. In Africa, malnutrition, tuberculosis, multidrug resistant tuberculosis, malaria, lung disease from smoke inhalation, and schistosomiasis will be more common. Of note, cardiovascular disease in part from untreated hypertension is likely important on all three continents[30].
A first step in addressing the needs of those aging with HIV in Africa will be a careful characterization of patterns of multimorbidity as is already underway within North America/Europe. While the particular conditions in North America/Europe and Africa will vary, the implications of multimorbidity are the same and include frailty and the need to tailor care to individual risk.
Frailty Among those Aging with HIV
Another important concept in geriatrics is that of frailty[31]. The concept of frailty is that the whole is greater than the sum of the parts in health and disease. Specifically, multiple organ system injuries conspire to deplete reserve, both of the individual organ system and for integrated functioning of the organism, whether functioning is measured in terms of cognition, exercise tolerance, strength, or health related quality of life. Nevertheless, reductions in functional reserve may occur above and beyond organ injury due to age-related physiological changes that may also contribute to frailty. Among those aging with HIV, chronic inflammation is thought to play an even greater role than it does among those aging without HIV infection[17, 32, 33].
Of note, organ systems within the human body do not function in a vacuum. Renal injury increases risk of cardiovascular disease[34]. Cognitive function may more often be a sign of systemic disease rather than direct neurologic injury and inflammation and fibrosis are universal processes.
Once an individual has lost reserve from injury or from age-related functional decline, they are less able to absorb additional injury from any source and are at increased risk from any acute insult including surgery, hospitalization, additional medication, or physical injury. Frailty is an attempt to quantify the extent of loss of functional reserve overall rather than simply counting disease processes.
While the geriatric community agrees on the importance of frailty[35–40], there is much debate concerning its measurement. Fried and colleagues have developed a frailty phenotype incorporating physical functioning, symptoms of fatigue, weight loss, and depression which has been shown predictive of mortality and hospitalization among those aging without HIV infection[35, 41]. However, this phenotype remains relatively uncommon among those aging with HIV infection[42, 43]. Others have emphasized components of frailty such as sarcopenia, functional performance, or markers of chronic inflammation[39, 44]. It is likely that the particular components of frailty most operative for middle aged individuals with HIV infection may differ from those among HIV uninfected individuals of more advanced age. They may also differ by geographic region and among social and ethnic groups.
Personalized Care
As can be seen from the case of Mr. Smith, multimorbidity and frailty call into question the “one size fits all” approach to care suggested by care guidelines. The geriatric literature has described this problem among those aging without HIV infection[15, 45]. One study applied guidelines for 10 common chronic diseases to a closed panel of 2,500 primary care patients who were age, sex, and disease prevalence matched to the US population[46]. They then estimated the time it would take to provide guideline driven care to these patients assuming that there was no active disease (3.5 hours a day) or some active disease (10.6 hours a day). These estimates did not allow for new problems or new patients to the practice. Another study considered guideline driven care for a hypothetical 79 year old woman with COPD, type 2 diabetes, osteoporosis, hypertension, and osteoarthritis[47]. If all relevant guidelines had been followed, the patient would be prescribed 12 medications (costing her $406/month). In the case of Mr. Smith, we are considering adding to his current regimen of 3 antiretrovirals and Gabepentin, 6 additional medications and considering a course of treatment for his chronic HCV infection. Certainly Mr. Smith faces a burden of polypharmacy, potential drug toxicities, and drug drug interactions that meets or exceeds those of the 79 year old, HIV uninfected, woman discussed in this study.
Importantly, HIV and its treatment impact the harm-to-benefit profile of care (both screening and treatment) of non-AIDS conditions through (1) direct and indirect biological effects of HIV (HANA conditions); (2) treatment toxicity; (3) increased susceptibility to injury from treatment (frailty) and 4) decreased time to benefit from preventative interventions (decrease life expectancy compared to uninfected individuals)[48].
An intervention that may be beneficial in someone with HIV might have no benefit or actually be harmful if that person has HIV and multimorbidity[48]. For example, frequent screening for lung cancer has the potential to be beneficial among HIV-infected smokers because HIV magnifies the risk of lung cancer from smoking, and therefore increases the potential benefit from lung cancer screening. However, if the false positive rate is also increased among those with HIV infection, say from prior lung infections, screening may cause more harm than benefit because of more numerous complications from downstream diagnostic procedures.
Even without multimorbidity, an intervention that may be beneficial in someone without HIV has the potential to be harmful with HIV. For example, an individual with HIV and a short life expectancy would be unlikely to benefit from colorectal cancer screening because they might be more susceptible to the immediate harms from colonoscopy (perforation), but may not live long enough to experience the benefits of avoiding a future cancer[49].
Finally, we must consider opportunity cost[50]. Providers who are focused on a long list of screening and counseling tasks may not be able to respond to the patient’s greatest priority or the problems most immediately threatening functional status, quality of life, or mortality. For Mr. Smith, the most important additional interventions to prevent further morbidity and prolong his survival are likely getting him to stop drinking, treating his hypertension, and (possibly) treating his HCV infection. In contrast, Mr. Smith is likely most interested in addressing his erectile dysfunction. What is the best course of action?
An essential step toward personalized health care is the accurate assessment of individual risk[9, 51–55]. Because multimorbidity is the norm in aging and in HIV infection, and resulting levels of frailty vary widely, the range and characterization of risk encompassed among those aging with HIV is broad. While some groups approach “normal” life expectancy, most groups experience substantial morbidity and mortality associated with chronic HIV infection[56–58]. As a result guideline driven care, focused on optimizing the “average” outcome, likely misses the mark—suggesting too much screening and treatment for some and not enough for others.
Tailoring care to individual risk involves a systematic consideration of whether to adapt the timing, intensity, and appropriateness of interventions in light of an individualized assessment. One must use validated prediction tools to estimate absolute net benefit (not relative benefit) accounting for treatment risks and costs and for the time required to benefit from the treatment[51]. This approach requires two essential inputs: accurate estimation of risk and accurate estimation of the likely risk reduction associated with the intervention.
Clinical risk indices based upon routinely collected clinical data can enhance provider assessment of risk and have come into wider use in recent years among those aging without HIV infection[59]. The Veterans Aging Cohort Risk Index (VACS Index) incorporates nine clinical biomarkers and age to generate a summary risk score that can be translated into absolute risk of all cause mortality among those with HIV infection (Figure 1). Scores vary from 0 to 140 and strongly differentiate risk of all cause mortality[60–63], hospitalization and medical intensive care unit admission[64], and functional performance[65]. Scores change dramatically over the first 12 months of cART[61–63] and when antiretroviral treatment is interrupted [66]. These changes are greater than those seen for an index restricted to age, CD4 cell count and HIV-1 RNA.
Fig. 1.
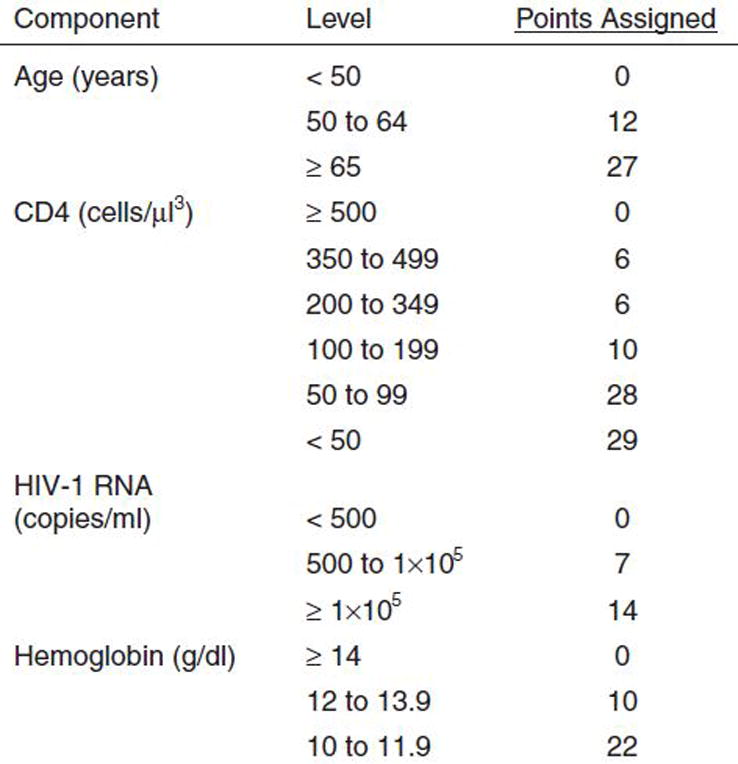
The Veterans Aging Cohort Study Risk Index.
After 12 months on cART, the median observed VACS Index score is 16 among those who have suppressed HIV-1 RNA and a good CD4 response (>100 cell improvement). In contrast, among those who fail to show a good response, scores are much higher (41 median). Consider Mr. Smith. His overall VACS Index score is 39 and his expected 5 year mortality is 18%. Thus, he is likely to live another 7 years and would benefit from colon cancer screening. If we could normalize his FIB-4 (composite marker of age, AST, ALT, and platelets) through alcohol cessation we might lower his score by 6 points and improve his 5 year mortality to 14% (a 22% reduction in risk). If we successfully treat his HCV infection, we can further reduce his score to 28 and his 5 year mortality to 12% (risk reduction of 33%). Normalization of his FIB-4 may also reduce his risk of hepatocellular cancer which is currently four times higher than that for HIV infected individuals with a normal FIB 4[11]. We do not yet know how much added protection he would get from better blood pressure control or from smoking cessation but this will be the subject of future studies. Eventually, it would be possible to rank potential interventions like these in relation to their likely benefit and the time frame required before benefits outweigh harms so that providers, patients, and policy makers can chose the most appropriate combination of treatments for individual patients.
Ultimately, only a randomized trail can prove that screening and treatment guided by a risk assessment tool like the VACS Index improves outcomes or decreases costs. Note that the VACS Index does not include all common sources of risk and might be improved by the addition of D-dimer or sCD14[67]. Similarly, a comprehensive measure of sarcopenia or functional performance might be useful. However, prior experience with prognostic indices suggest that, once an index achieves a comparable level of predictive accuracy (C statistics between 0.75 and 0.82 for all cause mortality), additional predictive variables often fail to enhance sensitivity or specificity and may only serve to complicate its application[68, 69]. This is because the variables that are already in the model often covary with omitted variables. Additional markers should only be included if they result in improved risk classification[69–71]. The VACS Index is currently as discriminating of all cause mortality among subjects from North America/Europe as the Framingham Heart Index is of myocardial infarction. In a recently published meta-analysis of prognostic inidices[59] for older adults, performance of validated indices was consistent with that established for the VACS Index. The VACS Index is likely ready for clinical application within resource rich settings.
What of a risk Index for patients aging with HIV in Africa? The need to prioritize care and identify that which is most likely to benefit a given patient is even more acute in this setting due to constrained health systems, fewer available tests, less available treatment, and limited human resources to deliver the care. Most of the variables included in the VACS Index are not routinely monitored in Africa.
What would it take to develop a useful index in Africa? A critical step would be careful ascertainment of mortality events and standardized collection of biomarkers that are routinely used in clinical management. While CD4 count is likely essential, lymphocyte count might offer some insight. Hematocrit might be more consistently measured than hemoglobin. Red Cell Distribution Width might offer a reflection of inflammation[72].
Our experience suggests that, if you have to choose, aspartate transaminase provides a better indication of mortality risk than alanine transaminase. Given the prevalence and impact of tuberculosis and multidrug resistant TB[73], these would likely be important variables to consider. Malaria and schistosomiasis are common and likely complicate outcomes. Body Mass Index (BMI) as a measure of wasting is likely an essential measure. Weight gain after cART initiation might provide an important indication of treatment response[74, 75]. Functional performance and biometrics might be used instead of some laboratory markers. Because untreated hypertension is common[76, 77], blood pressure should be evaluated as a candidate variable in the index. Other measures of malnutrition and poverty might also improve predictive accuracy. We would welcome the development and validation of such an index.
There are established frameworks for tailoring care in the context of chronic disease and multimorbidity[47, 49, 78–83] once individualized risk is characterized. These frameworks provide a roadmap for tailoring clinical guidelines and quality benchmarks, incentives, and subgroup analyses in future studies on the journey away from “one size fits all” towards more patient-centered care. They will be improved if comparative effectiveness research provides even more detailed information for tailoring care in the future.
One framework (the “Payoff Time”[49, 84]) is notable because it is prescriptive and quantitative and encompasses all major pathways(Figure 2). The Payoff Time can be used whenever an intervention would have harms that occur soon after the intervention is initiated, but has benefits that accumulate more slowly over time, and it is readily applicable to individuals with HIV. For example, the payoff time framework may be used to tailor colorectal cancer screening decisions, lung cancer screening decisions, or decisions regarding vascular surgery[85, 86].
Fig. 2.
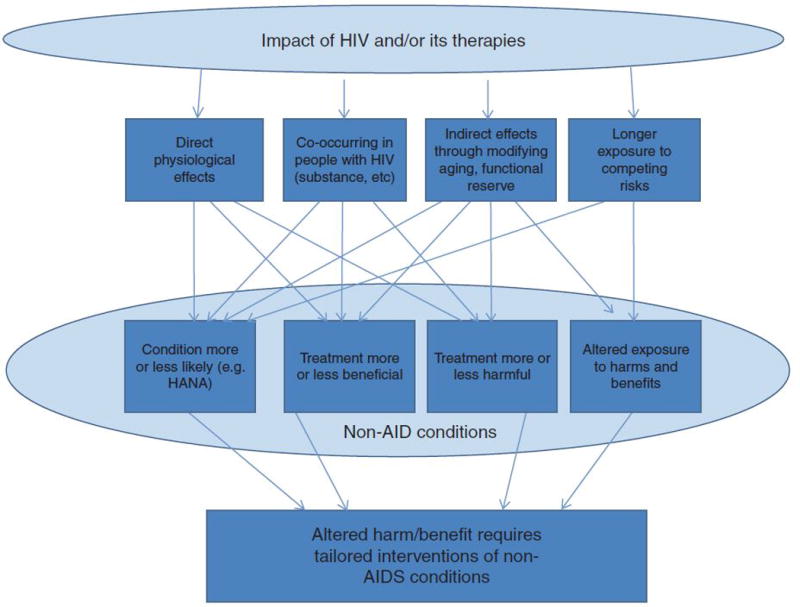
Multifaceted influence of HIV status on harms and benefits of screening and treatment for non-AIDS conditions.
It is important to note that the goal of tailoring care for individuals with HIV is not just a concern for resource-rich environments, but is a global concern, and that the same frameworks are applicable. For example, while communicable disease in sub-Saharan Africa receives much attention, a remarkably large portion of the preventable mortality burden in sub-Saharan Africa is due to preventable cardiovascular disease, in particular from untreated hypertension and smoking[76, 77]. As cART becomes increasingly utilized in Africa and as individuals with HIV live longer, many will start to die of preventable cardiovascular disease from smoking and uncontrolled hypertension. Frameworks to tailor care can be used to address important questions such as “Should individuals with HIV have different screening strategies for smoking cessation compared to the general population?” Analogous tailoring strategies can also apply to other goals of care, such as preventing and treating tuberculosis and malaria.
In conclusion, there are vital differences between the wave of individuals aging with HIV infection in North America/Europe and that beginning in Africa. Nevertheless, there is much that can be learned from our joint experiences to optimize the process and outcomes of aging with HIV infection. Specifically we need to focus on means of measuring cumulative disease burden from all sources of disease (HIV and non HIV), identifying and ranking modifiable risk factors, and intervening on factors most likely to substantively improve outcomes for the individual. In both North America/Europe and in Africa, we need to insure that our health care resources are used to do as much good, and as little harm, as possible.
Biography
Mr. Smith
Mr. Smith lives in a major metropolitan area of the United States. He is 52 year old, African American, and male with HIV infection and has been on combination antiretroviral therapy (cART) for 10 years. He started on dual therapy with stavudine and didanosine prior to switching to a triple drug regimen and has been on his second cART regimen with an undetectable HIV-1 RNA for 5 years. He has Hepatitis C infection that has not been treated.
In the United States, Department of Health and Human Services (DHHS) guidelines recommend the following regular screening evaluations for Mr. Smith: adherence, HIV risk of transmission behaviors, HIV 1 RNA, CD4 count, electrolytes, glucose, a complete blood count, alanine transaminase, aspartate transaminase, Total Bilirubin, fasting lipids, urinalysis, and anal cancer screening. If we add in the United States Preventative Task Force A or B Level Recommendations we would also screen for tobacco, alcohol, depression, diet, obesity, aspirin (if at risk of cardiovascular disease), colon cancer, blood pressure, and, if the blood pressure is greater than 135/80, diabetes.
Mr. Smith has one partner, does not use condoms due to erectile dysfunction, has an undetectable viral load and good CD4 cell count, but has low level abnormalities in creatinine, alanine transaminase, aspartate transaminase, and lipids. He has a longstanding peripheral neuropathy that developed on stavudine for which he takes Gabapentin. He smokes a pack of cigarettes a day, drinks a 6 pack of beer most Saturdays, feels blue but not suicidal, has been gaining weight, doesn’t exercise or take aspirin, has never been screened for colon cancer, and has untreated hypertension. If we address his tobacco addiction, depression, cardiovascular risk, and erectile dysfunction with medications we might add six drugs putting him on a total of 10 drugs before addressing his C infection. Among aging individuals without HIV infection the risk of adverse drug effects including drug toxicity, drug-drug interactions, and nonadherence increases with each additional drug beyond five medications. In the United States, we need to prioritize care to insure we do the most good and least harm, yet among myriad options, we have little guidance concerning prioritization.
In Africa and other resource-limited settings, Mr. Smith would likely have started on cART, rather than having had prior exposure to mono or dual therapy, and have been on treatment for a shorter period. Although his burden of comorbid disease would likely be as great, the particular conditions might differ and he would have fewer treatment options. Instead, the need for prioritization in Africa concerns which interventions should be targeted for investment to yield the greatest health benefit. In both the United States and Africa, we do not know which interventions are most likely to benefit patients with multimorbidity aging on cART or whether some treatments might actually result in net harm due to polypharmacy. Nor do we have an evidence base on which to tailor treatment choices based on individual or more homogeneous subgroup risk estimates.
Reference List
- 1.Too much medication? Yonkers NY: Consumer Reports. 2012:1–5. [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Reports 2007. 2009 [Google Scholar]
- 3.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, et al. Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110(3):208–220. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negin J, Mills EJ, Albone R. Continued neglect of ageing of HIV epidemic at UN meeting. Lancet. 2011;378(9793):768. doi: 10.1016/S0140-6736(11)61373-1. [DOI] [PubMed] [Google Scholar]
- 6.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88(11):847–853. doi: 10.2471/BLT.10.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer DN, Njeri R, Justice AC, Odero WW, Tierney WM. Alcohol abuse among patients with and without HIV infection attending public clinics in western Kenya. East Afr Med J. 2004;81(11):594–598. [PubMed] [Google Scholar]
- 8.Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS Behav. 2010;14(3):669–678. doi: 10.1007/s10461-009-9647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinetti ME, McAvay GJ, Chang SS, Newman AB, Fitzpatrick AL, Fried TR, et al. Contribution of Multiple Chronic Conditions to Universal Health Outcomes. J Am Geriatr Soc. 2011 doi: 10.1111/j.1532-5415.2011.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinetti ME, McAvay GJ, Fried TR, Allore HG, Salmon JC, Foody JM, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. 2008;56(8):1409–1416. doi: 10.1111/j.1532-5415.2008.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park LS, Tate JP, Justice AC, Lo RV, III, Lim JK, Brau N, et al. FIB-4 Index Is Associated with Hepatocellular Carcinoma Risk in HIV-Infected Patients. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2512–2517. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV Infection, Immune Suppression, and Uncontrolled Viremia Are Associated With Increased Multimorbidity Among Aging Injection Drug Users. Clin Infect Dis. 2011;53(12):1256–1264. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 14.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 15.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116(3):179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 17.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17(4):118–123. [PubMed] [Google Scholar]
- 18.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7(2):69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 19.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiberg MS, McGinnis K, Butt AA, Goetz M, Brown S, Oursler KA, et al. HIV is associated with clinically confirmed myocardial infarction after adjustment for smoking and other risk factors [Abstract] 18th Conference on Retroviruses and Opportunistic Infections. 2011 [Google Scholar]
- 22.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153(7):452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sico J, Chang C, Freiberg M, Hylek E, Butt A, Gibert C, et al. HIV Infection and the Risk of Ischemic Stroke in the VACS VC [Abstract] SGIM 2010 Poster. 2010 [Google Scholar]
- 24.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 25.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(Suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg M, Lau B, D’Souza G, Engels E, Gill J, Goedert J, et al. Trends in cumulative incidence of cancer among HIV-infected patients in North America [Abstract] 17th Conference on Retroviruses and Opportunistic Infections. 2010 [Google Scholar]
- 27.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating Liver Fibrosis Progression and the Impact of Antiretroviral Therapy in HIV and Hepatitis C Coinfection Using a Noninvasive Marker. J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35(1):182–189. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 29.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 30.Sliwa K, Stewart S, Gersh BJ. Hypertension: a global perspective. Circulation. 2011;123(24):2892–2896. doi: 10.1161/CIRCULATIONAHA.110.992362. [DOI] [PubMed] [Google Scholar]
- 31.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuller LH, Tracy R, Belloso W, De WS, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La RA, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121(5):651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 36.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Kuh D. A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. 2007;62(7):717–721. doi: 10.1093/gerona/62.7.717. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 39.Roubenoff R, Harris TB. Failure to thrive, sacropenia and functional decline in the elderly. Clin Geriatr Med. 1997;13(4):613–622. [PubMed] [Google Scholar]
- 40.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005;40(1–2):81–87. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 42.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62(11):1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 43.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50(3):299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickering G. Frail elderly, nutritional status and drugs. Archives of Gerontology & Geriatrics. 2004 Apr;38(2):174–80. doi: 10.1016/j.archger.2003.09.004. [Review] [34 refs] [DOI] [PubMed] [Google Scholar]
- 45.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 46.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 48.Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite RS, Justice AC. Cancer Screening in Patients Infected with HIV. Curr HIV AIDS Rep. 2011;8(3):142–152. doi: 10.1007/s11904-011-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braithwaite RS, Fiellin D, Justice AC. The payoff time: a flexible framework to help clinicians decide when patients with comorbid disease are not likely to benefit from practice guidelines. Med Care. 2009;47(6):610–617. doi: 10.1097/MLR.0b013e31819748d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redelmeirer DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 51.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154(9):627–634. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 52.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kent DM, Hayward R. Treating unrelated disorders in patients with chronic disease. N Engl J Med. 1998;339(13):926–928. doi: 10.1056/NEJM199809243391318. [DOI] [PubMed] [Google Scholar]
- 56.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49(10):1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogg RS, Heath KV, Yip B, Craib KJP, O’Shaughnessy MV, Schechter MT. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 58.May MT, Ingle SM. Life expectancy of HIV-positive adults: a review. Sex Health. 2011;8(4):526–533. doi: 10.1071/SH11046. [DOI] [PubMed] [Google Scholar]
- 59.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tate J, Justice AC, Hughes M, Bonnet F, Reiss P, Mocroft A, et al. An internationally validated mortality risk index for HIV infected individuals on antiretroviral therapy: performance by region, gender, risk group, and level of viral suppression. 2011 [Google Scholar]
- 61.Tate JP, Justice AC, for the VACS Project Team Change in a prognostic index for survival in HIV infection after one year on cART by level of adherence [Abstract] 48th Annual Meeting of the Infectious Disease Society of America. 2010 [Google Scholar]
- 62.Tate JP, Hughes MD, Justice AC, for the VACS Project Team Do risk factors for mortality change with time on antiretroviral therapy? [Abstract] 48th Annual Meeting of the Infectious Disease Society of America. 2010 [Google Scholar]
- 63.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. Performancy of the refined VACS Risk Index during the first 12 months of antiretroviral therapy among US and European subjects [Abstract] 15th International Workshop on HIV Observational Databases. 2011:13. [Google Scholar]
- 64.Akgun KM, Pisani MA, Fried TR, Butt AA, Gibert CL, McGinnis K, et al. Risk Factors for Medical Intensive Care Unit Admission in HIV Infected Veterans [Abstract] American Throacic Society. 2010 [Google Scholar]
- 65.Erlandson KM, Allshouse AA, Jankowski C. Prospective comparison of three functional assessments with the Veteran’s Aging Cohort Study index in virologically suppressed HIV-infected adults [Abstract] 2nd International Workshop on HIV and Aging. 2011 [Google Scholar]
- 66.Kirkwood K, Kyriakides K, Brown S, Justice AC, Holodniy M, Tate J, et al. The VACS Risk Index is Highly Correlated with and Predictive of Mortality Events in the OPTIMA Study [Abstract] JSM. 2011 [Google Scholar]
- 67.Justice AC, Freiberg MS, Tracy R, Tate J, Goetz M, Butt AA, et al. Does an Index Composed of Clinical Data Reflect Effects of Inflammation, Coagulation, and Mnoocyte Activation on Mortality Among those Aging with HIV? Clinical Infectious Diseases. 2012 doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 69.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 70.Cook NR. Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al. Statistics in Medicine (DOI: 10.1002/sim.2929) Stat Med. 2008;27(2):191–195. doi: 10.1002/sim.2987. [DOI] [PubMed] [Google Scholar]
- 71.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;20(7):115. 928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 72.Lappe JM, Horne BD, Shah SH, May HT, Muhlestein JB, Lappe DL, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011;412(23–24):2094–2099. doi: 10.1016/j.cca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 73.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 74.Koethe JR, Lukusa A, Giganti MJ, Chi BH, Nyirenda CK, Limbada MI, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2010;53(4):507–513. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011;53(9):952–960. doi: 10.1093/cid/cir606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.BeLue R, Okoror TA, Iwelunmor J, Taylor KD, Degboe AN, Agyemang C, et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health. 2009;5:10. doi: 10.1186/1744-8603-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spencer J, Phillips E, Ogedegbe G. Knowledge, attitudes, beliefs, and blood pressure control in a community-based sample in Ghana. Ethn Dis. 2005;15(4):748–752. [PubMed] [Google Scholar]
- 78.Boyd CM, Weiss CO, Halter J, Han KC, Ershler WB, Fried LP. Framework for evaluating disease severity measures in older adults with comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):286–295. doi: 10.1093/gerona/62.3.286. [DOI] [PubMed] [Google Scholar]
- 79.Walter LC, Davidowitz NP, Heineken PA, Covinsky KE. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291(20):2466–2470. doi: 10.1001/jama.291.20.2466. [DOI] [PubMed] [Google Scholar]
- 80.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 81.Braithwaite RS. Can life expectancy and QALYs be improved by a framework for deciding whether to apply clinical guidelines to patients with severe comorbid disease? Med Decis Making. 2011;31(4):582–595. doi: 10.1177/0272989X10386117. [DOI] [PubMed] [Google Scholar]
- 82.Braithwaite RS, Roberts MS, Chang CC, Goetz MB, Gibert CL, Rodriguez-Barradas MC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148(3):178–185. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364(26):2478–2481. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 84.Braithwaite RS, Concato J, Chang CC, Roberts MS, Justice AC. A framework for tailoring clinical guidelines to comorbidity at the point of care. Arch Intern Med. 2007;167(21):2361–2365. doi: 10.1001/archinte.167.21.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuo TH, Braithwaite RS, Chang CC. Applying the payoff time framework to carotid disease management [Abstract] Medical Decision Making. 2011 doi: 10.1177/0272989X13491462. [DOI] [PubMed] [Google Scholar]
- 86.Gross CP, Soulos PR, Ross JS, Cramer LD, Guerrero C, Tinetti ME, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441–1449. doi: 10.1007/s11606-011-1816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


