Abstract
Background
Children who sustain traumatic brain injury (TBI) are at risk for developing hypopituitarism, of which growth hormone deficiency (GHD) is the most common manifestation.
Objective
Determine the prevalence of GHD and associated features following TBI among children and adolescents.
Study design
32 children and adolescents were recruited from a pediatric TBI clinic. Subjects were diagnosed with GHD based on insufficient growth hormone release during both spontaneous overnight testing and following arginine/glucagon administration.
Results
GHD was diagnosed in 5/32 subjects(16%). Subjects with GHD exhibited more rapid weight gain following injury than non-GHD subjects, and had lower levels of free thyroxine and FSH. Males with GHD had lower testosterone levels.
Conclusions
GHD following TBI is common in children and adolescents, underscoring the importance of assessing for GHD, including evaluating height and weight velocities after TBI. Children and adolescents with GHD may further exhibit absence or intermediate function for other pituitary hormones.
Introduction
Traumatic brain injury (TBI) affects a growing number of pediatric patients, with an incidence of at least 180–250 per 100,000 children each year and with infants/toddlers and adolescents being the most commonly affected.1, 2 Abnormalities of pituitary function have been recognized for many years in adult survivors of TBI3–7, but have only recently been reported in children and adolescents in case-reports and case-series.8–10
Among abnormalities in pituitary function after TBI in children, growth hormone deficiency (GHD) appears the most common.8–10 GHD may affect not only linear growth but also has been implicated in lipid abnormalities and body composition changes in children and adolescents,11–14 and associated with cognitive impairments and poor recovery from TBI.15–19 Knowledge of the associated features of GHD after TBI in children and adolescents may improve the ability for treating physicians to recognize and refer patients suspected of having pituitary abnormalities, especially GHD.
To date, there have been no prospective studies that we are aware of that assess for GHD in children and adolescents who have sustained a TBI. Therefore, our goal was to determine the prevalence of GHD and its associated clinical factors among a group of children and adolescents following TBI.
Methods
This study was approved by the Institutional Review Board of the University of Virginia. All children and adolescents aged 8–21 evaluated at Kluge Children’s Rehabilitation Center (KCRC) brain injury clinic from 11/21/2007–6/1/2009 who had a history of moderate-to-severe TBI were offered the opportunity to participate in the study by the attending physician, if they met the predetermined criteria. Moderate-to-severe TBI was defined as a Glascow Coma Scale (GCS) ≤13 or in the absence of a GCS score by a history suggestive that the GCS was in the target range combined with abnormal findings on brain MRI related to the injury. Inclusion criteria also included freedom from medications or endocrine treatments that would affect GH and IGF1 levels.
After appropriate consent was obtained, pre-injury height, weight, and BMI were determined by record review and/or parental history. Puberty stage was assessed and recorded as described by Tanner. The study participants were admitted to the UVA GCRC for overnight blood sampling for GH concentrations during which an IV catheter was placed and blood samples were drawn every 20min from 20:00–08:00. In the morning laboratory studies were obtained, including IGF-1, IGFBP-3, cortisol, insulin, free-T4, TSH, estradiol, DHEA-S, testosterone, prolactin, FSH, and LH. An arginine/glucagon GH stimulation test was then administered, using 0.5g/kg of 10% arginine hydrochloride IV (maximum dose 20g) and 0.02mg/kg of glucagon SC. GHD was diagnosed based on the presence of both a peak serum GH level on overnight testing of <5ng/mL20, 21 and a peak GH level following arginine/glucagon of <7ng/mL for subjects <18y or <5ng/mL for subjects ≥18y.3, 22, 23 Subjects who did not secrete GH above these levels but had exhibited spontaneous GH release above these cut-off limits ≤1hr prior to the arginine/glucagon stimulation test were considered to have adequate GH reserve and were not classified as GHD. The cut-off level for overnight testing was chosen based on results from overnight sampling in healthy children,20, 21 while the provocative testing cut-off was based on recommendations of international endocrine societies, adjusting for alterations in GH measurement using monoclonal antibody techniques.22, 23 The cut-off for adolescents ≥18y was based on standards for diagnosing adult GHD.3
Laboratory samples were stored at −80° C and tested at the Core Laboratory of the UVa General Clinical Research Center using two-site chemiluminescence immunoassays on an Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA), according to the manufacturer’s specifications. For GH, the assay range is 0.05–40ng/mL. Intra-/inter-assay coefficients of variation for samples tested in this laboratory are as follows: growth hormone 3.3%/6.4%, IGF-1 2.8%/5.6%, free T4 4.7%/7.1%, TSH 3.9%/7.6%, LH 4.2%/6.2%, FSH 4.5%/6.8%, testosterone 6.8%/9.6%, estradiol 7.0%/5.4%, prolactin 2.1%/5.8%, DHEAS 6.0%/7.4%.
All analyses used SAS® Version 9.1 (SAS Institute Inc, Cary, NC). Standardized measures were computed with respect to gender and age in the form of z-scores. BMI z-scores were calculated from CDC reference values, and z-scores for IGF-1 were computed based on age-based references for the Immulite. Subjects found to be GHD were compared to non-GHD subjects with respect to a number of factors. Comparisons based on continuous outcomes were made using the Mann-Whitney test with corresponding exact p-values; rates of categorical outcomes were compared via Fisher’s exact test. Spearman correlations were computed to assess associations between GH levels and BMI z-score as well as injury severity (as indexed by GCS).
Results
Among the families of patients from TBI clinic who were eligible to enroll in the study, 32/52 (62%) elected to participate. Enrollees did not differ from those who declined to participate in the following characteristics: proportion male, age at injury, age at prospective enrollment, GCS (data not shown).
Among participants, 6/32 subjects failed to have an overnight GH level above 5ng/mL and 10/32 did not increase GH levels above 7ng/mL (subjects <18y) or 5ng/mL (subjects ≥18y) in response to provocative stimuli. GHD was diagnosed in 5/32 subjects (16%) who failed both testing modalities, while an additional 19% of subjects (6/32) exhibited insufficient GH secretion during one of the two tests.
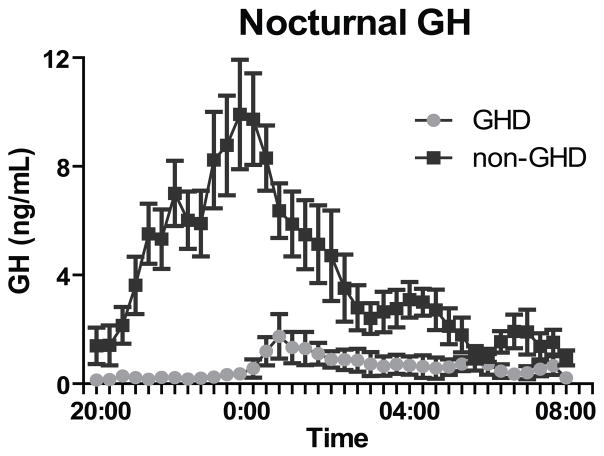
Subjects with GHD had lower spontaneous GH release overnight (p<0.001)(Figure 1), lower integrated overnight GH release (area-under-the-curve, AUC) (p<0.001), lower peak GH on provocative testing (p<0.001), and lower GH AUC during provocative testing (p<0.001)(Table 1). There was no significant difference in IGF-1 z-score between the two groups; 3/5 subjects with GHD and 3/27 subjects without GHD had IGF-1 z-scores >2 standard deviations below the mean for age (NS)(Table 1).
Figure 1.
Overnight growth hormone secretion following TBI for subjects with GHD vs. non-GHD. Average growth hormone values on every 20 min sampling.
Table 1.
Characteristics and laboratory values for subjects with and without GHD
| GHD | Non-GHD | |
|---|---|---|
| n | 5 | 27 |
| Subject characteristics: Mean (SD)# | ||
| Male (Freqency (percent)) | 4 (80) | 16 (59) |
| Age at injury (years) | 17.5 (1.9)* | 11.8 (5.3) |
| Age at testing (years) | 18.2 (2.0) | 15.2 (3.7) |
| Interval between injury and testing (yrs) | 0.7 (0.6)* | 3.4 (3.8) |
| Clinical characteristics: Mean (SD)# | ||
| Tanner stage 3–5 | 5 (100) | 23 (85) |
| Initial GCS (Median (Range)) | 3.0 (3–7) | 5.5 (3–15) |
| BMI z-score at overnight testing | 1.4 (1.8) | 0.5 (1.1) |
| Weight gain, injury to simulation testing (kg/yr) | 25.2 (22.1)* | −0.5 (19.1) |
| Clinical characteristics: Mean (SD)# | ||
| IGF-1 | 167.2 (55.8) | 219.4 (114.3) |
| IGF-1 Z-score | −2.05 (0.53) | −1.42 (0.32) |
| IGF-1 low (Freqency (percent)) | 3 (60) | 9 (33) |
| Free T4 | 0.92 (0.45)* | 1.10 (0.03) |
| TSH | 1.53 (0.53) | 1.52 (0.21) |
| Cortisol (8 am) | 5.82 (1.67) | 10.05 (0.97) |
| LH (Tanner 3–5) | 2.98 (8.4) | 3.11 (0.48) |
| FSH (Tanner 3–5) | 2.12 (0.42)* | 4.65 (0.60) |
| Testosterone (males Tanner 3–5) | 313 (43.6)* | 477 (34.6) |
| Insulin | 12.6 (10.0) | 6.9 (6.1) |
| Prolactin | 21.0 (10.0) | 16.9 (12.8) |
| Growth hormone testing | ||
| Overnight GH testing: | ||
| Peak | 2.1 (1.7)*** | 19.0 (9.9) |
| Mean | 0.6 (0.5)*** | 4.0 (2.3) |
| Provocative GH testing: | ||
| Peak | 2.3 (2.1)*** | 13.6 (10.8) |
| Mean | 0.5 (0.3)*** | 4.1 (3.3) |
Unless otherwise noted
p<0.05
p<0.01
p<0.001
Characteristics and additional laboratory values of these subjects compared to non-GHD subjects are shown in Table 1. In addition to these characteristics, one subject (with GHD) had diabetes insipidus. Compared to non-GHD subjects, those with GHD were older at time of injury and had a shorter interval between injury and testing. Regarding other pituitary hormones, subjects with GHD had lower free-T4 values than those without GHD (p<0.05), though only one of these subjects had a frankly-low free-T4 (data not shown). There was no difference in TSH concentration between the groups. Morning cortisol levels for subjects with GHD was 5.8+/−1.7 compared to 10.1+/−1.0 for those without GHD (NS); morning cortisol was <5ug/dL in 3/5 subjects with GHD compared to 3/27 without GHD (NS)(data not shown). Among subjects at Tanner 3–5, FSH levels were lower in those with GHD (p<0.05) and males with GHD had a lower testosterone level (p<0.05). There were no differences in LH or estradiol concentrations (females Tanner 3–5: GHD 49.0, n=1; non GHD 50.5+/−19.5, n=11; p=1.0).
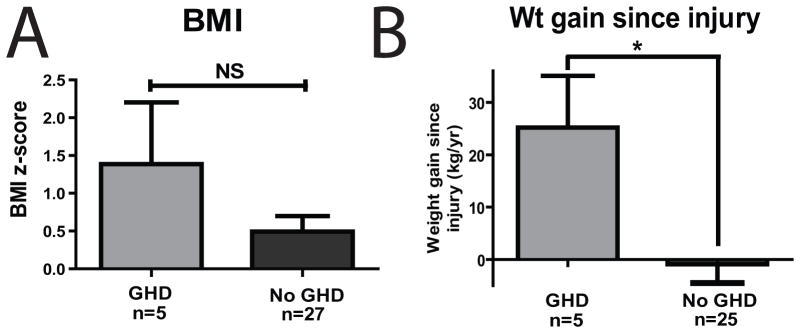
Although not statistically significant (p=0.0833), subjects with GHD had a higher mean BMI z-score (Table 1 and Figure 2A). Subjects with GHD were more likely to gain weight excessively in the interval between injury and GH stimulation testing (Table 1 and Figure 2B, p=0.02). Given that all of the subjects in the GHD group were ≥15y and thus would not have the same growth potential as younger subjects, weight gain was compared between those with GHD and those without GHD who were ≥15y. Weight gain was still significantly greater for those with GHD (p=0.02), with older non-GHD individuals gaining virtually no weight (mean=0.8 kg/y).
Figure 2.
Body weight and GHD. A. BMI z-score was not different between GHD and non-GHD subjects (p>0.05); B. Subjects with GHD were more likely to gain weight between injury and time of stimulation testing.
Regarding severity of injury, there was no significant difference in GCS following injury between subjects with or without GHD (Table 1).
Discussion
Traumatic brain injury is a serious cause of morbidity and mortality in children and adolescents and is increasingly recognized as a cause of pituitary dysfunction in this population. Previous prospective investigations in adults and retrospective investigations in children and adolescents have identified GHD as the most common anterior pituitary deficit following TBI.3, 6, 8–10, 17 GHD is an important issue for pediatric health care providers to recognize in TBI patients because of its potential effects on recovery from TBI, future growth, attainment of adult body composition,12–14 neurocognitive function15, 17, 18 and quality of life15—deficits that may improve following treatment with human GH.24 Thus far, case reports and case series have suggested that GHD after TBI in children and adolescents may be under-recognized.8–10
In the current study of 32 children and adolescents with a history of moderate to severe TBI, 15% (5/32) of subjects exhibited clear GHD, including low GH response to stimulation testing and a low spontaneous peak GH values during overnight testing. An additional 19% of subjects (6/32) had an abnormal response in one or the other of these tests, such that of 34% (11/32) of children and adolescents exhibited some laboratory abnormalities suggestive of diminished GH function. The subjects who exhibited an abnormal response on only one of the two tests may represent individuals at a less-severe place on the spectrum of GHD or may merely reflect the imperfect nature of these tests. These prevalence rates in the current study compare to a rate of 42% of GHD following TBI in the largest cross-sectional study following TBI in children, which used provocative stimulation on 26 children and adolescents as their sole assessment of GH sufficiency.9. Among adults who have suffered TBI, 15–28% are reported to have GHD.3, 15
The most notable effect of GH action in children relates to linear growth. Height velocity was difficult to evaluate in our cohort, due to poor records regarding pre-injury heights and due to many subjects being past the age of expected epiphyseal fusion. All of our subjects with GHD were >15.5y at the time of injury, and would not have been expected to have much growth potential. Nevertheless, height velocity remains an important parameter to follow prospectively in younger children after TBI, as it may be the first indication of GHD.
Our subjects with clear GHD exhibited more rapid weight gain in the interval between injury and testing. Indeed, while only 2 of the GHD subjects were overweight at the time of injury, as a group they gained an average of 25kg/yr following their TBI. These observations are consistent with data from studies in adults3, 15 and with our understanding of the effects of GHD on body composition and weight gain.13, 25 In addition, rapid weight gain has been noted after injury to the hypothalamus.26 Obesity is associated with lower GH secretion and may make the definition of true GHD more difficult.20
Subjects with GHD had a significantly lower interval between injury and testing compared to non-GHD subjects. Prior studies in adults have shown stable levels of GHD among subjects tested at 3mo and 6–9mo after injury.6, 15 All of our subjects but one with GHD were tested at least 6 months following injury.
Subjects with GHD tended to have low levels of IGF-1 (z-score −2.05+/−0.53), though these levels were not significantly lower than those in subjects without GHD (z-score −1.42+/−0.32). This finding is in keeping with data in adults suggesting that IGF-1 levels are not a reliable way to identify GHD following TBI, in that only 17–30% of adult GHD subjects had a low IGF-1 level.3, 19
Though our study was designed to detect differences in GH and not other pituitary hormones, we did test the integrity of other hormone axes. Among subjects at Tanner 3–5 development, GHD individuals had lower FSH levels (but similar LH levels) compared to non-GHD subjects and males with GHD had lower testosterone levels. Though our subjects with GHD did not have pathologically low testosterone levels, the decrease in testosterone may reflect a decrease in gonadotropin-driven testosterone production. These data are in keeping with prior reports in adults demonstrating gonadotropin deficiency as the second-most common pituitary defect following TBI.3, 15, 17
Our subjects with GHD had lower free T4 levels but similar TSH levels as those without GHD, though only one of the subjects with GHD had a fT4 value below the normal range. The lower levels of fT4 may imply a decrease in TSH secretion to drive thyroxine production. Similarly, GHD subjects tended toward lower morning cortisol values, with 3/5 subjects with GHD having a cortisol <5ug/dL compared to 3/27 subjects without GHD. These findings serve as a reminder that patients who had TBI are at risk for multiple pituitary deficiencies besides GHD and that treating physicians should have a high index of suspicion for the presence of pituitary deficiencies for months-years post-injury.15
There has been much debate regarding the mechanism determining hypopituitarism in subjects with TBI. Injury to the hypothalamus and/or pituitary has been commonly thought to be due to shearing forces and hemorrhage. Initial GCS continues to be commonly used to determine the severity of TBI. There have been conflicting reports in adults regarding the ability of initial GCS to predict the risk of later pituitary deficiency, with studies reporting positive correlation27, 28 or no relationship.3, 17, 29 We did not find a difference in GCS between those with and without GHD and would submit that close follow for signs of pituitary deficiency—with particular attention to growth and adolescent development—is important after any TBI, regardless of its severity. Children with suspected pituitary findings, including suspected GHD, should be referred for further evaluation.
In conclusion, our results support recent observations that pituitary dysfunction—and particularly growth hormone deficiency—after TBI is more common in children and adolescents than previously concluded. Given this high prevalence, pediatricians who care for children after TBI should monitor for growth and excessive weight gain following TBI, and have a low threshold to refer for suspected GHD or other pituitary abnormalities.30 Our results emphasize the need for larger, prospective studies in this population. A better understanding of the prevalence of GHD and other pituitary abnormalities after childhood TBI is likely to improve the medical management of these children and result in improved recovery from TBI.
Acknowledgments
Funding:
Commonwealth Neurotrauma Initiative Trust Fund, administered by VA Dept. of Rehabilitative Services, Grant #07-302.
General Clinical Research Center grant M01 RR00847 (to UVA)
NIH HD060739-01 (MDD)
We would like to thank Jodi Darring for her helpful assistance with data management and subject follow-up.
References
- 1.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005 May-Jun;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Agha A, Rogers B, Sherlock M, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004 Oct;89(10):4929–4936. doi: 10.1210/jc.2004-0511. [DOI] [PubMed] [Google Scholar]
- 4.Agha A, Sherlock M, Phillips J, Tormey W, Thompson CJ. The natural history of post-traumatic neurohypophysial dysfunction. Eur J Endocrinol. 2005 Mar;152(3):371–377. doi: 10.1530/eje.1.01861. [DOI] [PubMed] [Google Scholar]
- 5.Aimaretti G, Ambrosio MR, Di Somma C, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) 2004 Sep;61(3):320–326. doi: 10.1111/j.1365-2265.2004.02094.x. [DOI] [PubMed] [Google Scholar]
- 6.Aimaretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab. 2005 Nov;90(11):6085–6092. doi: 10.1210/jc.2005-0504. [DOI] [PubMed] [Google Scholar]
- 7.Leal-Cerro A, Flores JM, Rincon M, et al. Prevalence of hypopituitarism and growth hormone deficiency in adults long-term after severe traumatic brain injury. Clin Endocrinol (Oxf) 2005 May;62(5):525–532. doi: 10.1111/j.1365-2265.2005.02250.x. [DOI] [PubMed] [Google Scholar]
- 8.Einaudi S, Matarazzo P, Peretta P, et al. Hypothalamo-hypophysial dysfunction after traumatic brain injury in children and adolescents: a preliminary retrospective and prospective study. J Pediatr Endocrinol Metab. 2006 May;19(5):691–703. doi: 10.1515/jpem.2006.19.5.691. [DOI] [PubMed] [Google Scholar]
- 9.Niederland T, Makovi H, Gal V, Andreka B, Abraham CS, Kovacs J. Abnormalities of pituitary function after traumatic brain injury in children. J Neurotrauma. 2007 Jan;24(1):119–127. doi: 10.1089/neu.2005.369ER. [DOI] [PubMed] [Google Scholar]
- 10.Poomthavorn P, Maixner W, Zacharin M. Pituitary function in paediatric survivors of severe traumatic brain injury. Arch Dis Child. 2008 Feb;93(2):133–137. doi: 10.1136/adc.2007.121137. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson H, Barreto ES, Salvatori R, et al. Metabolic effects of growth hormone (GH) replacement in children and adolescents with severe isolated GH deficiency due to a GHRH receptor mutation. Clin Endocrinol (Oxf) 2007 Apr;66(4):466–474. doi: 10.1111/j.1365-2265.2007.02753.x. [DOI] [PubMed] [Google Scholar]
- 12.Hulthen L, Bengtsson BA, Sunnerhagen KS, Hallberg L, Grimby G, Johannsson G. GH is needed for the maturation of muscle mass and strength in adolescents. J Clin Endocrinol Metab. 2001 Oct;86(10):4765–4770. doi: 10.1210/jcem.86.10.7897. [DOI] [PubMed] [Google Scholar]
- 13.Roemmich JN, Huerta MG, Sundaresan SM, Rogol AD. Alterations in body composition and fat distribution in growth hormone-deficient prepubertal children during growth hormone therapy. Metabolism. 2001 May;50(5):537–547. doi: 10.1053/meta.2001.22510. [DOI] [PubMed] [Google Scholar]
- 14.Boot AM, Engels MA, Boerma GJ, Krenning EP, De Muinck Keizer-Schrama SM. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997 Aug;82(8):2423–2428. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- 15.Bavisetty S, Bavisetty S, McArthur DL, et al. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery. 2008 May;62(5):1080–1093. doi: 10.1227/01.neu.0000325870.60129.6a. discussion 1093–1084. [DOI] [PubMed] [Google Scholar]
- 16.Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008 Jul;79(7):753–759. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- 17.Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007 Nov;24(11):1687–1697. doi: 10.1089/neu.2007.0343. [DOI] [PubMed] [Google Scholar]
- 18.Kelly DF, McArthur DL, Levin H, et al. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J Neurotrauma. 2006 Jun;23(6):928–942. doi: 10.1089/neu.2006.23.928. [DOI] [PubMed] [Google Scholar]
- 19.Popovic V, Pekic S, Pavlovic D, et al. Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. J Endocrinol Invest. 2004 Dec;27(11):1048–1054. doi: 10.1007/BF03345308. [DOI] [PubMed] [Google Scholar]
- 20.Roemmich JN, Clark PA, Weltman A, Veldhuis JD, Rogol AD. Pubertal alterations in growth and body composition: IX. Altered spontaneous secretion and metabolic clearance of growth hormone in overweight youth. Metabolism. 2005 Oct;54(10):1374–1383. doi: 10.1016/j.metabol.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Rose SR, Municchi G, Barnes KM, Cutler GB., Jr Overnight growth hormone concentrations are usually normal in pubertal children with idiopathic short stature--a Clinical Research Center study. J Clin Endocrinol Metab. 1996 Mar;81(3):1063–1068. doi: 10.1210/jcem.81.3.8772577. [DOI] [PubMed] [Google Scholar]
- 22.Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000 Nov;85(11):3990–3993. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 23.Juul A, Bernasconi S, Clayton PE, Kiess W, DeMuinck-Keizer Schrama S. European audit of current practice in diagnosis and treatment of childhood growth hormone deficiency. Horm Res. 2002;58(5):233–241. doi: 10.1159/000066265. [DOI] [PubMed] [Google Scholar]
- 24.Springer J, Chollet A. A traumatic car crash. Lancet. 2001 Jun 9;357(9271):1848. doi: 10.1016/S0140-6736(00)04953-9. [DOI] [PubMed] [Google Scholar]
- 25.Monson JP. Long-term experience with GH replacement therapy: efficacy and safety. Eur J Endocrinol. 2003 Apr;148(Suppl 2):S9–14. doi: 10.1530/eje.0.148s009. [DOI] [PubMed] [Google Scholar]
- 26.Lustig RH. Hypothalamic obesity: causes, consequences, treatment. Pediatr Endocrinol Rev. 2008 Dec;6(2):220–227. [PubMed] [Google Scholar]
- 27.Lee SC, Zasler ND, Kreutzer JS. Male pituitary-gonadal dysfunction following severe traumatic brain injury. Brain Inj. 1994 Aug-Sep;8(6):571–577. doi: 10.3109/02699059409151009. [DOI] [PubMed] [Google Scholar]
- 28.Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006 Jun;91(6):2105–2111. doi: 10.1210/jc.2005-2476. [DOI] [PubMed] [Google Scholar]
- 29.Benvenga S, Campenni A, Ruggeri RM, Trimarchi F. Clinical review 113: Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000 Apr;85(4):1353–1361. doi: 10.1210/jcem.85.4.6506. [DOI] [PubMed] [Google Scholar]
- 30.Moon RJ, Wilson P, Kirkham FJ, Davies JH. Growth monitoring following traumatic brain injury. Arch Dis Child. 2009 Sep;94(9):699–701. doi: 10.1136/adc.2008.145235. [DOI] [PubMed] [Google Scholar]