Abstract
Pediatric obesity is highly prevalent in developed countries globally (and worsening in developing countries) and threatens to shorten the lifespan of the current generation. At highest risk for weight-related comorbidities including Type 2 diabetes mellitus, non-alcoholic fatty liver disease and dyslipidemia is a sub-set of children with severe obesity, often defined as a body mass index (BMI) percentile ≥99th percentile for age and sex. The pathophysiology of severe obesity in childhood is complex, resulting from the dynamic interplay of a myriad of individual and societal factors including genetic predisposition and health behaviors contributing to energy imbalance. Approximately 4–6% of children have severe obesity, representing a common scenario encountered by providers, and intervention is critical to halt ongoing weight gain and, when possible, reverse the trend. Clinical approaches promoting behavioral weight loss may result in modest, albeit clinically significant, reductions in BMI; however, such changes are often difficult to maintain long-term. Data regarding the impact of targeted pharmacotherapy including agents such as orlistat are limited in the pediatric population and again only suggest modest results. However, increasing evidence suggest that surgical treatment, as an adjunct to ongoing lifestyle changes, may be a promising option in carefully-screened adolescents with severe obesity and weight-related comorbidities who are motivated to adhere to the long-term treatment needs.
Introduction
Although recent data suggest that the alarming increase in prevalence rates of obesity among children and adolescent in the United States (US) observed over the past three decades may have plateaued,1 this trend has not reversed in all age groups, and a significant number of youth remain at risk of immediate and long-term complications secondary to excess weight gain. In fact, current estimates using 2011–2012 National Health and Nutrition Examination Survey (NHANES) data suggest that 31.8% and 17.2% of US children 2 to 19 years of age are overweight (BMI ≥85th percentile for age and sex) and obese (BMI ≥95th percentile), respectively.1 Among the approximately one out of six US children currently affected by obesity, an increasing number would be considered to have severe obesity and at highest-risk of weight related comorbidities. However, overcoming the inertia of the numerous factors within the current obesogenic environment2 that influence individual health behaviors and energy homeostasis, and subsequently promote weight gain, is frequently challenging. As outlined below, the optimal approach to severe obesity in children and adolescents involves family-based lifestyle interventions implemented under the guidance of a multidisciplinary treatment team, and in refractory cases among older adolescents, a critical eye towards surgery as a potential adjuvant therapy to ongoing behavioral weight loss.
Prevalence of severe obesity and associated risks in childhood and adolescence
A BMI ≥99th percentile for age and sex corresponds to the most common definition of severe obesity in the pediatric population3 and was proposed by an Expert Committee in 2007 to identify children at highest cardiometabolic risk and trigger more intensive therapeutic interventions.4 However, given concerns regarding the statistical performance of BMI percentile (or even BMI Z-scores) at values >99th percentile, a recent American Heart Association Scientific Statement proposes that severe obesity in children ≥2 years of age be defined as a BMI ≥120% of the age and sex-appropriate 95th percentile or an absolute BMI ≥35 kg/m2 (the cut-off of severe [Class 2] obesity in adults), whichever is lower. The term “severe” obesity is currently recommended as the standard nomenclature to describe very high BMI values as opposed to “extreme” or “morbid.”3 “Severe” accurately reflects the potentially grave impact of the condition on the individual’s health in both the short and long-term while minimizing stigma that may be associated with other terms.
Severe obesity in childhood is unfortunately not uncommon, with estimated prevalence rates ranging from 1.6–11.9% among US children, depending on the specific study population and definition applied.5–9 Thus, pediatric health care providers, regardless of practice setting or specialty will frequently care for youth with severe obesity. Moreover, no age group remains unaffected. Approximately 2% of preschoolers currently meet criteria for severe obesity6, 10 and prevalence rates rise to over 6% among adolescents ages 12–19 years of age.6 Although sex-specific differences in rates of severe pediatric obesity are not clearly apparent, racial/ethnic disparities do exist, with higher prevalence among Hispanic and non-Hispanic black children.6 Prevalence rates of severe obesity rise further in adulthood, currently affecting approximately 14.5% of US adults.1 It bears noting that children with less-severe obesity (BMI 95–98th percentile) have a 37–51% chance of developing severe obesity in adulthood,11 underscoring the importance of optimizing obesity treatment efforts to prevent continued progression among all children with obesity even if their BMI values are below the 99th percentile.
Although childhood obesity is associated with significant long-term sequelae including increased risk of Type 2 diabetes (T2DM), cardiovascular disease (CVD) and premature death in adulthood,4,12 numerous immediate cardiometabolic health risks are already apparent among youth with obesity and these risks appear to be increased in the setting of severe obesity.3 In fact, compared with children with milder forms of obesity, those with severe obesity demonstrate higher rates and increased severity of multiple metabolic disturbances and weight-related comorbidities including hypertension, dyslipidemia, insulin resistance/glucose intolerance/T2DM, non-alcoholic fatty liver disease (NAFLD), obstructive sleep apnea syndrome (OSAS), inflammation, and vascular dysfunction.3 Rates of musculoskeletal disorders including tibia vara (Blount disease)13 and slipped capital femoral epiphysis14 are also increased in severe pediatric obesity. Disordered eating patterns (binge-eating and loss-of control behaviors) appear to be common (8–18%) in children with obesity;15–17 however, it is unclear if such behaviors are specifically related to the degree of adiposity and corresponding prevalence rates in normal weight children are not well-established.3 Although it is also unclear if the prevalence of clinically significant depression is increased in youth with severe obesity,18, 19 quality of life may be severely reduced in affected children and adolescents, similar in extent to those diagnosed with cancer, and is inversely related to BMI.20 Given the high burden of these comorbidities, the clinical care of children and adolescents with severe obesity requires ongoing surveillance for the development and treatment of weight-related medical and mental health disorders.
Etiology
The imbalance in energy homeostasis leading to excess weight gain results from complex interactions among individual behaviors, environmental factors and genetic susceptibility.21 In fact, twin studies suggest a heritability of 40–80% for adiposity and BMI,22–24 underscoring the central role of genetic contributions to the weight gain trajectory of an individual child in the setting of an obesogenic environment. Although syndromes associated with severe obesity and hyperphagia resulting from single gene mutations (e.g. congenital leptin deficiency) or defects in specific chromosomal regions (e.g. Prader-Willi syndrome) are well-described,25, 26 such conditions are rare.27 Moreover, such syndromic etiologies are generally associated with neurodevelopmental abnormalities or specific phenotypic features. However, one monogenic cause of early-onset obesity, mutations in the gene encoding for the melanocortin 4 receptor (MC4R), may be present in 2–6% of patients with severe obesity and is not characterized by the short stature and neurocognitive disabilities that hallmark many other obesity-related syndromes.27–29 The MC4R gene is located on chromosome 18 and encodes for a hypothalamic 7-transmembrane G-coupled receptor that integrates central nervous system (CNS) input to signal satiety.30 Deficiency of MC4R is transmitted in a co-dominant manner, with homozygotes more severely affected that heterozygotes, and results in hyperphagia.28 Although genome wide association studies (GWAS) have identified polymorphisms in over 50 other genetic loci that contribute the heritability of body weight and less severe obesity phenotypes, the inheritance of any one of these common polymorphisms alone accounts for only small variations in body weight.21, 31 However, when genetic mutations and epigenetic changes (environmentally-induced and potentially heritable alterations which impact gene expression and function without modifying the DNA sequence itself), are present in multiple at-risk loci simultaneously in a given individual, they likely collectively contribute to alterations in the energy intake and expenditure that contribute to significant weight gain.21
The functional significance of many of the genetic loci associated with body weight underscore the importance of the intricate CNS pathways involved in the regulation of appetite and energy expenditure; damage to these hypothalamic areas as a result of tumors, surgery, radiation or trauma can result in the development of severe and intractable obesity.32 Children and adolescent with such “hypothalamic obesity” manifest severe hyperphagia in addition to metabolic disturbances that contribute to ongoing weight gain, even in the setting of aggressive caloric restriction.32 Endocrine disorders such as hypercortisolemia (Cushing’s syndrome), hypothyroidism, or growth hormone deficiency can also adversely impact energy homeostasis and contribute to the development of obesity. However, such “classic” endocrine etiologies of obesity are characterized by poor linear growth, in contrast to the slightly increased stature and accelerated growth velocity generally observed in most children with obesity.26
It is importance to emphasize that, with the exception of MC4R defects, clinically identifiable syndromic or endocrinologic causes of obesity are rare in children, even among those seeking treatment at specialized centers, and account for less than 1% of cases of pediatric obesity.27 However, it is essential for the medical team to appreciate the complexities of the neuroendocrine regulation of appetite and energy homeostasis and how alterations in these pathways may influence behavior. In some cases, highlighting our growing but still limited understanding in this field helps explain to the patient and/or his/her family the observed differences in the suspectability of many children to weight gain when exposed to an obesogenic environment as well as challenges in initiating and sustaining the lifestyle modifications which remain the mainstay of treatment.
Initial assessment
The initial assessment of a child with severe obesity includes a focus on establishing rapport with the family and child, evaluating for underlying etiologies contributing to excess weight gain, carefully screening for the presence of weight-related comorbidities, and partnering with the family and child to develop and initiate an appropriate treatment plan. For many patients and their families, discussions regarding weight, dietary patterns and other behaviors are sensitive topics, and providers may find it beneficial to acknowledge such as part of initial interactions. In addition, the thoughtful selection of terms used to describe weight during patient interactions; use of open-ended questions assessing the parents’ and child’s level of concern about weight followed by neutral questions regarding timing of weight gain (e.g. early onset, or association with initiation of certain medications including second-generation antipsychotics), current health behaviors (including sleep duration and quality), dietary patterns (including loss of control eating), and previous attempts at weight loss; reflective listening; and engaging the child or adolescent as developmentally appropriate may assist the provider establishing rapport. Providers should also consider how the structure of their own practice environments (e.g. triage practices or appropriately-sized furniture and equipment) may contribute to rapport-building with families and patients with severe obesity.
As outlined previously, identifiable endocrinologic and syndromic etiologies are rare causes of severe weight gain in childhood.27 To assist in identifying such conditions, providers should obtain a thorough developmental history, review growth charts to assess linear growth, and conduct a careful physical assessment to identify dysmorphic features or clinical signs of specific endocrinopathies. However, current guidelines for the treatment of pediatric obesity recommend against universal screening for genetic and endocrine etiologies for children and adolescents with obesity, unless specific findings suggestive of such underlying conditions are identified.4, 33 For patients with significant neurodevelopmental abnormalities, particularly in the setting of dysmorphic physical features or short stature, referral to a geneticist for additional evaluation and testing may be appropriate.33 Given the common feature of impaired height velocity in children with endocrine causes of obesity, the routine laboratory evaluation for underlying endocrine disorders (including hypothyroidism) is not recommended unless a reduced growth velocity, assessed in light of pubertal status and genetic potential, is present.33 However, the absence of easily identifiable clinical characteristics (besides obesity) in children and adolescents with MC4R deficiency represents a diagnostic challenge for health care providers. Genetic testing for MC4R mutations is commercially available but not inexpensive. As a result, Clinical Practice Guidelines from the Endocrine Society propose discussing the availability of MC4R genetic testing with parents in the clinical setting of severe weight gain from early infancy in children who are above the 97th percentile for weight by 3 years of age.33 The level of parental and patient concern regarding weight gain may vary significantly case-to-case, with some not identifying the severe obesity as a concern and still others convinced that there is an underlying disorder contributing to weight gain despite clinical evidence to the contrary. In the later scenarios, it may be beneficial for the provider to inquire what specific conditions the parent or patient are concerned about in order to address these concerns directly.
Although underlying disorders leading to excess weight gain are unlikely to be identified, a careful and comprehensive assessment for the presence of weight-related comorbidities will frequently uncover such conditions in youth with severe obesity. Consequently, a complete review of systems should be conducted and include an assessment for severe or recurrent headaches (suggestive of pseudotumor cerebri or OSAS); snoring, apnea or daytime somnolence (OSAS); exercise intolerance; blurry vision, polyuria, or and polydipsia (T2DM); abdominal pain (NAFLD, gastroesophageal reflux or gallbladder disease); joint pain (slipped capital femoral epiphysis [SCFE], tibia vara, or premature osteoarthritis); menstrual irregularities [especially if persistent 2 years beyond menarache], hirsutism, and acne (polycystic ovary syndrome [PCOS]); and depression or anxiety.4 The physical examination should specifically include the careful measurement and interpretation of blood pressure (BP), including the use of an appropriately sized cuff. Blood pressure values should be compared with normative values for age, sex and height;34 abnormal values should be confirmed via auscultation and then reassessed as part of close follow-up. Systolic and/or diastolic BP values ≥95th percentile on at least three occasions are consistent with hypertension and warrant further evaluation and treatment.34 Blood pressure values ≥90th percentile but <95th percentile (as well as any value ≥120/80 mmHg) are consistent with “prehypertension” and should continue to be monitored closely.34 Of note, BP abnormalities may also be a subtle indicator of the presence sleep disordered breathing (SDB), including OSAS,35 and should prompt further testing for SDB if present in the setting of severe obesity. For pediatric patients with severe obesity, physical examination should additionally include assessments for papilledema via fundoscopic exam (pseudotumor cerebri); acanthosis (insulin resistance); hepatomegaly (NAFLD); gait disturbances or joint problems (tibia vara or SCFE); pubertal development; and excessive acne or hirsutism (PCOS).4 Unfortunately, the early stages of many common weight-related comorbities such dyslipidemia, NAFLD, and T2DM may not be identified on history and physical exam alone and routine laboratory screening for such disorders is recommended. Although published guidelines vary slightly on the frequency and specific tests that are recommended, laboratory screening including a fasting lipid panel and fasting glucose or hemoglobin A1C should be conducted at least every 2–3 years in all children and adolescents with severe obesity starting at 10 years of age or the onset of puberty, whichever is earlier.4, 33, 36 Although the Endocrine Society and Barlow Expert Panel Guidelines recommend obtaining serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) to screen for NAFLD,4, 33 guidelines endorsed by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association concluded that a formal recommendation for laboratory screening for NAFLD in obese children could not be made given due to a paucity of evidence.37 When identified, lifestyle modification and weight loss remain the cornerstones of treatment for each of these co-morbidities; however, additional disease-specific treatments may also be warranted depending on the specific disorder and its severity. For some families and children, the identification of one or more of these comorbidities may prompt increased motivation for lifestyle changes. Providers should also partner with families and patients to proactively identify how the presence of such comorbidities may impact the child or adolescent’s specific approach to lifestyle modifications, particularly physical activity.
Clinical Treatment of Severe Obesity in Children and Adolescents
Lifestyle modifications to reduce energy intake and increase energy expenditure and behavioral therapy to promote sustained lifestyle changes remain the cornerstones of the treatment for youth with severe obesity (Table 1). In 2007, the Barlow Expert Committee recommended a staged approach (Stages 1–4) to the treatment of pediatric obesity.4 Stage 1 treatment is designed to be implemented in the primary care provider’s office and focuses on developing or re-enforcing basic healthy lifestyle habits for the entire family. Stage 2 treatment, also designed to be implemented in the primary care setting, consists of more structured weight management approaches including the development of an eating plan in consultation with a dietitian that reduces the consumption of calorie-dense, nutrient-poor foods and beverages and controls caloric intake through portion control; limiting sedentary behaviors such as television and screen time to ≤1 hour per day; increasing physical activity to at least 60 minutes a day; incorporating self-monitoring strategies; planned re-enforcement for achieving targeted behaviors; and follow-up at least once a month. The efficacy of these approaches in the short term is shown in in-patient controlled trials that demonstrate the potential benefits in weight reduction.38 As part of Stage 1 and 2 treatment approaches, providers should encourage family-wide change, avoiding the identification of the child or adolescent as the only family member targeted for behavior modification; identify and set specific and measurable behavioral goals based on collaborative input from caregivers and patients that are consistent with their readiness to change; and anticipate and address potential barriers to specific behavior change that may arise from psychosocial factors and medical comorbidities. A non-judgemental assessment of social structure, family dynamics, and the home environment often reveals important information than can direct the health care provider in assisting families in the development and implementation of more effective behavior changes. Parents may also benefit from guidance regarding appropriate limit setting, while avoiding overly restrictive practices. It is often important for providers, as part of goal-setting, to review that successful behavioral weight loss generally requires changes in dietary habits (which can be challenging for many patients and families) and that increased physical activity alone has not been associated with significant reductions in weight.39 However, physical activity levels likely play an important role in weight maintenance and a comprehensive approach that includes both dietary modification and increased physical activity appear to be associated with improved clinical responses.39 In a clinical setting, such behavioral counseling can be time intensive and will likely need to be implemented over multiple visits.
Table 1.
Summary of Foci for Initial Assessment and Treatment for Severe Obesity in Childhood assessment & treatment of obesity.
| Assessment | |
|---|---|
| Medical History |
|
| Lifestyle/behavior |
|
| Physical exam |
|
| Laboratory studies |
|
| Treatment: | Barlow Report Recommendations:
|
Developmental delay may suggest a syndromic or genetic cause of weight gain.
Growth abnormalities including short stature and/or decreased growth velocity may suggest an underlying endocrinologic or syndromic etiology of obesity; early onset (< age 3 years) severe obesity may suggest monogenic cause of obesity (e.g., melacortin 4 receptor [MC4R] mutations, leptin deficiency).
Most children and adolescents with severe obesity will requires at least Stage 3 care.
Most children and adolescents with severe obesity will require Stage 3 treatment consisting of a comprehensive multidisciplinary intervention that includes a structured behavioral modification program with frequent (ideally weekly) follow-up that is delivered by an integrated care team including a behavioral specialist (e.g., psychologist, social worker or other mental health care provider), registered dietitian, and exercise specialist (e.g., physical therapist or exercise physiologist). Although such programs are frequently available at specialized pediatric obesity treatment centers; primary care providers should familiarize themselves with what specific resources are available within their local practice areas. Intensive lifestyle modifications in the pediatric population are associated with small to modest reduction in BMI;39, 40 however, several factors appear to be associated with improved response to Stage 3 interventions. In a meta-analysis of 23 randomized control trials (RCT) by McGovern and colleagues, combined lifestyle modifications interventions for children and adolescents were associated with small to modest reductions in BMI, with the largest effects being observed when parents where included in the intervention.39 In a meta-analysis of 20 RTCs investigating the efficacy of comprehensive behavioral family lifestyle interventions on pediatric obesity by Janicke et al, increased treatment duration, session frequency and contact hours in addition to individual and in-person formats were associated with improved outcomes. Although relatively few lifestyle modification interventions have specifically targeted youth with severe obesity; available results demonstrate reductions in percent overweight or BMI, blood pressure, and insulin resistance in children and adolescents with severe obesity after participation in 6 months of lifestyle modification.41, 42 However, such programs are often characterized by high attrition rates43 and weight regain over time.41, 42 The results of a study by Danielsson and colleagues44 evaluating the efficacy of intensive lifestyle modification in a cohort of 643 Swiss children and adolescents may yield important insights regarding the treatment of severe pediatric obesity via lifestyle modification. Specifically, the authors found that age and degrees of obesity (i.e., moderate obesity [ BMI Z-score 1.5 to <3.5] versus severe obesity [BMI Z-score ≥3.5]) were important predictors of patient’s clinical response, with younger (6–9 years of age) patients demonstrating greater improvements in BMI Z-scores than older (14–16 years of age) adolescents and superior responses in less severely obese youth. Nevertheless, it should be noted that even though younger children demonstrated greater improvements in BMI Z-scores, the children’s BMI status remained in the obese category after 3 years of follow, demonstrating the long-term time frame likely required for normalizing BMI in the setting of severe obesity.44 Such findings further underscore the importance of early identification and treatment initiation among children with severe obesity—as well as the primary role of prevention and interventions for less severe weight gain.
However, despite the challenges of behavior change, it is important for treating clinicians to not lose faith in the process. Some patients and families may undergo several false starts before making durable lifestyle changes. Moreover, data suggests that insulin resistance can improved without appreciable change in BMI, potentially due to favorable changes in body composition with increases in lean body mass and reductions in fat mass.45 Furthermore, without intervention, many children and adolescents with obesity will experience continued weight gain. Thus, preventing further increases in BMI may represent an improvement in many children, particularly younger children who still have linear growth ahead of them. Finally, intensive family-based lifestyle modification remains an essential foundational component of more intensive Stage 4 approaches including pharmacotherapy and weight loss surgery (WLS).
The behavioral treatment of severe obesity may also involve additional approaches that have had variable success, including residential treatment centers,46 meal replacements47 and very low calorie diets.48 These offer potential benefits to cases that have been refractive to the approaches described above; however, additional well-designed studies with results supporting the role of each of these treatment modalities in the pediatric population are required before their routine use can be recommended.
Pharmacotherapy
Although guidelines support considering the role of pharmacotherapy in adolescents with severe obesity with weight-related comorbidities who have not experienced sufficient improvements with intensive lifestyle modification alone,33 limited options are available and are associated with only modest reductions in BMI.3 The Food and Drug Administration (FDA) has approved two medications for use in weight loss treatment in adolescents: orlistat (Xenical) and sibutramine (Meridia); however, sibutramine was removed from the US market due to concerns of potential increased risk for cardiovascular side effects.49 Newer weight loss medications (lorcaserin [Belviq] and combination phentermine/topiramate [Qsymia]) have been approved by the FDA for use in adults with obesity in conjunction with lifestyle modification but have not been approved for use in children or adolescents.
The only currently FDA weight loss medication for use in adolescents ≥12 years of age is orlistat, a lipase inhibitor that blocks fat absorption. Orlistat is dosed at 120 mg orally three times a day. In clinical trials involving adolescents with obesity, orlistat was associated with mean BMI reductions of 0.7–0.85 kg/m2 compared with placebo.39, 50, 51 The tolerability of orlistat may also be limited by oily spotting and fecal urgency leading to soiling,50 adverse effects particularly troubling in many adolescents. Given the risk of malabsorption of fat-soluble vitamins with orlistat use, patients should be prescribed a multivitamin supplement containing fat-soluble vitamins. Orlistat is also available over-the-counter (OTC) at a dose of 60 mg (Alli); however, use of the OTC formulation is not approved in individuals younger than 18 years of age.
Metformin is a biguanide insulin sensitizing agent that is FDA-approved for the treatment of T2DM in children ≥10 years of age. Although not FDA-approved for weight loss, a growing number of studies have investigated the potential role of metformin in treating non-diabetic children and adolescents with obesity. A recent meta-analysis suggested a modest 6-month reduction in BMI (−1.38 [95% CI: −1.93 to −0.82] kg/m2) compared with control groups among obese youth less than 18 years of age; however, statistically significant sustained reductions in BMI at 1 year were not apparent.52 Importantly, the Diabetes Prevention Program demonstrated significant reductions in the incidence in diabetes among high-risk adults with glucose intolerance with metformin (although less robust than lifestyle modification with a goal of 7% weight loss and at least 150 minutes of exercise);53 however, similar large prospective studies have not been performed in children and adolescents. There is also significant interest in the potential role of metformin therapy in the prevention and treatment of second-generation antipsychotic-induced weight gain. Although metformin may be associated with modest reductions in BMI in these settings, limited data are available from well-designed trials in children and adolescents on anti-psychotic weight gain to support its widespread use in this scenario.54, 55 Overall, metformin has a strong safety profile, although gastrointestinal symptoms including bloating, nausea, crampy abdominal pain, and loose stools are not uncommon, occurring in ~ 25% of patients.52 Despite being associated with modest weight loss over the short-term, the durability of the effects of metformin on weight are unclear. As a result, the authors recommend against its routine use for pediatric obesity except for cases of pre-diabetes (glucose-intolerance), PCOS or, of course T2DM, for which it is the first line therapy.36
The impact of several additional pharmacologic agents have been evaluated in the context of refractory weight gain associated with hypothalamic obesity. Octreotide, a somatostatin analog, suppresses insulin hypersecretion, a mechanism proposed to contribute to weight gain following hypothalamic injury via disinhibited vagal nerve stimulation of the pancreatic β-cell;32 and, octreotide has been associated with stabilization of weight gain and mild reductions in BMI among a small cohort of adolescents with severe hypothalamic obesity.56 Glucagon-like peptide-1 (GLP-1) receptor agonists (e.g.,exenatide and liraglutide) are approved for the treatment of treatment of T2DM in adults and their use are associated with modest reductions in body weight.57 In light of the centrally mediated effects of GLP-1 on satiety and appetite suppression, this class of medications may represent a treatment option for patients with hypothalamic obesity; and, results in adults with hypothalamic obesity are promising.58 However, at this time, the use of octreotide or GLP-1 agonists in children or adolescents with hypothalamic obesity should be considered investigational.
The potential role of GLP-1 mimetics as weight loss medications in adults without T2DM or hypothalamic obesity are also being pursued.59 Administration of the GLP-1 agonist, exenatide, was associated with significant reductions in BMI (−1.13 kg/m2) after 3 months compared with placebo in a randomized control trial of 26 adolescents with severe obesity.3 However, current GLP-1 agonists must be administered as a subcutaneous injection; may be associated with nausea and vomiting, and rarely pancreatitis; and are not currently FDA-approved for use in pediatric patients or as weight loss medication in patients without diabetes.
Although expert panels recognize the potential role of pharmacotherapy (in conjunction with ongoing lifestyle changes) in select pediatric patients with significant obesity and comorbidities, such groups suggest that the use of weight loss medications should only be offered by clinicians who are experienced in their use and aware of potential adverse effects,33 and ideally in the setting of a specialized pediatric weight management centers with comprehensive services (Stage 4 – Tertiary Care).4
Weight Loss Surgery
Extensive data in adults with obesity suggest that weight loss surgery (WLS) is associated significant reductions in BMI and resolution or improvements of many weight-related comorbidities.60, 61 As a result of the significant prevalence rate of severe comorbidities and substantial long-term health risks associated with severe obesity in childhood, WLS has emerged as an important consideration for adolescents with severe obesity and serious weight-related comorbidities who have experienced insufficient responses with other treatment approaches. In fact, WLS surgery in adolescents has progressed from being a rarity in 1997 (51 cases in the U.S. per year)62 to an increasingly common component of a comprehensive treatment plan (in conjunction with ongoing lifestyle changes) for carefully-selected adolescents with severe obesity. Multiple factors must be balanced when considering the role of WLS in a specific adolescent patient including the severity of current weight-related comorbidities and the longer-term risk of worsening obesity; the physical and emotional maturity of the adolescent; the short-term and long-term safety of the specific surgical procedure employed, the experience of the surgeon and the multidisciplinary team/program; and the ethical implications of performing a life-altering procedure on a minor. In addition to the usual surgical risks (e.g., wound infections and other complications), risks of WLS in the pediatric population include potential adverse effects on linear growth and development related to malabsorption and nutritional deficiencies.63 Another important safety consideration is the experience level of the surgeon, with a lower rate of surgical complications among surgeons who perform these procedures regularly.62 Moreover, it is strongly recommended that adolescent WLS only be performed in a center with a comprehensive care team including pediatric specialists, dietitians, and mental health professionals with specific expertise in the assessment, pre- and post-operative care of adolescents undergoing WLS.4, 33, 64–66 In light of these considerations and growing experience and data in the pediatric population, the criteria for considering WLS in adolescents is evolving; Table 2 outlines an approach consistent with recent recommendations from the American Society for Metabolic and Bariatric Surgery (ASMBS) and International Pediatric Endosurgery Group (IPEG)64–66 which addresses the severity of obesity using BMI thresholds, the number and severity weight-related comorbidities, current physical maturity, and ability to adhere to treatment recommendations before and after the surgery In summary, current recommendations for adolescents with severe obesity who may benefit from WLS include a BMI ≥35 kg/m2 with at least one severe comorbidity (i.e., T2DM, moderate to severe OSAS, pseudotumor cerebri, or severe NAFLD) or a BMI ≥40 kg/m2 with other weight-related comorbidities who have experienced insufficient weight loss despite participation in at least 6 months of intensive, medically-supervised, family-based behavioral weight management. When these criteria are followed, adolescent WLS is associated with significant weight loss and improvements/resolution of associated comorbidities, with the results varying according to the type of surgery pursued. The different WLS procedures offered and their relative advantages and disadvantages have been reviewed in depth previously.63
Table 2. Recommended criteria for consideration of weight loss surgery in adolescents with severe obesity.
Adapted from Hsai et al. Arch Ped Adol Med 2012;166(8):757–766, Ibele et al Surg Clin North Am 2011; 91(6):1339–1351; and Pratt et al. Obesity 2009;17:901–910
| BMI >35 kg/m2 with severe comorbidities: | BMI >40 kg/m2 with at least one milder comorbidity: |
|---|---|
|
|
| Additional requirements for adolescents: | |
| |
The Roux-en-Y gastric bypass (RYGB), a combined restrictive and malabsorptive procedure in which a small gastric pouch is created and then anastomosed distal to the proximal intestine, is associated with the most robust and long-term data in the adolescent population. In a recent meta-analysis, RYGB in adolescents with severe obesity with associated with mean reductions in BMI of 17.2 (95% CI: −20.1 to −14.3) kg/m2 at 12 months after surgery,67 with durable weight loss persisting at 5–9 years post-surgery.68 Moreover, comorbidity resolution rates are estimated at 67–100% in adolescents undergoing RYGB.67 Although no deaths have been reported in the perioperative period in adolescents undergoing RYBG, a few patients undergoing RYGB as adolescents have died between 9 months to 6 years after the procedures; however, these deaths were thought to be unrelated to the surgery itself.3 Post-surgical complications following RYGB include bleeding, anastomatic strictures/leaks, wound infections, small bowel obstructions, hernias, venous thromboembolism, and nutritional deficiencies.3, 67 Although the exact prevalence of post-operative complications following RYBG in adolescents is unclear given variations in reporting among study groups,67 complication and reoperation rates in adolescents following RYGB appear to be lower than their adult counterparts.3
The adjustable gastric band (AGB) is a purely restrictive procedure that uses an implantable device to create a small gastric pouch in the proximal stomach. Studies involving AGB in the adolescent population are associated with mean reductions in BMI at 12-months of 10.4 (95% CI: −11.8 to −19.1) kg/m2.67 Although post-operative complications following AGB in adolescents are reported less frequently than with RYGB,67 the device is still not FDA-approved for use in patients <18 years of age. Moreover, long-term follow-up data in adults indicate that one-third of patients develop erosions at the device site and approximately 50% of adults ultimately required surgical removal of the AGB,69 suggesting the long-term durability of the AGB in adolescents may not be ideal.3
With vertical sleeve gastrectomy (VSG), 85–90% of the stomach is removed leaving a small gastric pouch; this WLS surgery approach does not involve placement of foreign body or a substantial malabsorption component and is being formed with increasing frequency among adolescents with severe obesity.70 Studies involving VSG among adolescents included in a meta-analysis demonstrate mean 12-month BMI reductions of 14.52 (95% CI: −17.33 to −11.73) kg/m267 and subsequent studies investigating the role of VSG in the treatment of severe adolescent obesity demonstrate similar results.71, 72
In summary, WLS, in conjunction with ongoing lifestyle modification, is associated with significant and sustained reductions in BMI and improvements in comorbidities among adolescents with severe obesity; however, adolescents undergoing WLS require close ongoing medical evaluation. Following bariatric surgery, patients are at risk for dehydration, protein deficiency and deficiencies of water soluble vitamins, requiring post-operative guidance and follow-up.63 Moreover, as emphasized by Black and colleagues, ongoing studies are needed to more clearly define the long-term implications of WLS in this population.67 To this end, several centers performing weight adolescent WLS have developed an ongoing collaboration (TEEN-Longitudinal Assessment of Bariatric Surgery [TEEN-LABS]) to better characterize long-term outcomes and complications.70
Special Considerations: State intervention in severe life-threatening childhood obesity
The successful treatment of severe obesity in childhood is resource intensive, requiring sustained lifestyle modifications, frequent follow-up with the health care team, and on-going adherence by the child’s family and, in older children and adolescents, the patient’s themselves. As outlined previously, sustained behavior change is challenging and behavioral weight loss interventions are often characterized by periods of relapse. As a result, when a family with a child or adolescent with severe appears to be unwilling to engage in a treatment it is important for the care team to carefully explore the parent’s understanding of the problem and recommended treatment plan; assess for specific psychosocial factors that may represent substantial barriers to treatment adherence; and offer to connect the family with resources to assist in addressing such barriers. Rarely, parental failure to seek medical care or to provide appropriate treatments for a child with severe obesity and serious weight-related comorbidities may place the affected youth at risk of serious imminent harm, including death. Some experts have proposed whether such circumstances constitute medical neglect and warrant involvement of social service agencies, with the ultimate potential of removing the child from the home.73 In such unusual circumstances, this weighty and controversial decision regarding state intervention can be guided by criteria proposed by Varness and colleagues.74
Conclusions
As a result of its strong associations with current and future comorbidities, severe obesity in childhood represents a serious medical condition requiring aggressive multidisciplinary treatment. The mainstay of treatment for severe pediatric and adolescent obesity remains intensive, family-based, lifestyle modification and behavioral therapy. Adherence with such interventions is associated with modest reductions in BMI and may result in significant improvements in cardiometabolic risk factors. However, treatment adherence is frequently challenging and associated improvements may not be sustained over time. Consequently, in some children and adolescent with severe obesity, lifestyle modification alone may not result in sufficient weight loss to successfully ameliorate serious-weight related comorbidities. In such cases, increasing evidence supports the potential role of weight loss surgery, in conjunction with ongoing lifestyle modification, in appropriately-selected adolescents with severe obesity and weight-related comorbidities. In light of the morbidity associated with severe obesity even in childhood and the challenges regarding effective and sustainable treatments once the condition is established, early identification of and intervention in children and adolescents with less severe obesity may represent important approaches in ultimately addressing the serious disorder.
Figure 1.
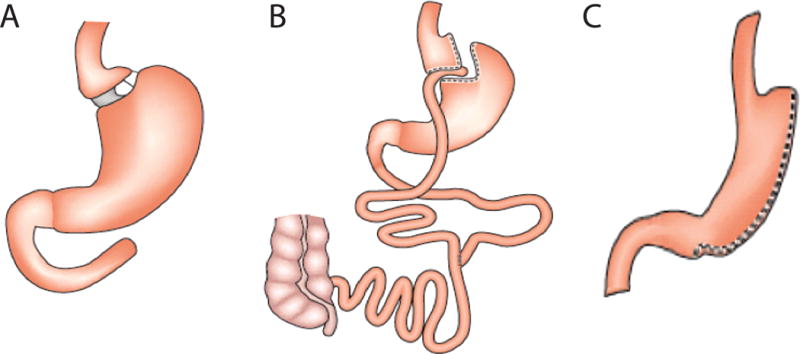
Types of bariatric surgery procedures. A. An adjustable gastric band is an implantable device that creates a small gastric pouch by restricting food entry into the distal stomach. B. A roux-en-y procedure creates a small gastric pouch that is then then anastomosed to the proximal intestine, creating restrictive and malabsorptive limitations on ingested calories. C. A vertical sleeve gastrectomy removes 85–90% of the stomach, leaving a small gastric pouch. Adapted from reference61, used by permission.
Acknowledgments
Sources of support: NIH K23HD053742 (EPW) and K08HD060739 (MDD).
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. Jama-Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier KI, Krajicek MJ. Obesogenic environment: A concept analysis and pediatric perspective. Journal for Specialists in Pediatric Nursing. 2013;18(3):202–210. doi: 10.1111/jspn.12027. [DOI] [PubMed] [Google Scholar]
- 3.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe Obesity in Children and Adolescents: Identification, Associated Health Risks, and Treatment Approaches A Scientific Statement From the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 4.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 5.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and Trends of Severe Obesity Among US Children and Adolescents. Academic Pediatrics. 2009;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999–2012. Jama Pediatrics. 2014;168(6):561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. International Journal of Pediatric Obesity. 2011;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. American Journal of Clinical Nutrition. 2009;90(5):1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 9.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of Extreme Obesity in a Multiethnic Cohort of Children and Adolescents. Journal of Pediatrics. 2010;157(1):26–U61. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L, Blanck HM, Sherry B, Dalenius K, Grummer-Strawn LM. Trends in the prevalence of extreme obesity among US preschool-aged children living in low-income families, 1998–2010. JAMA. 2012;308(24):2563–2565. doi: 10.1001/jama.2012.108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67(6):1111–1118. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery CO, Young KL, Austen M, Jo C-H, Blasier RD, Ilyas M. Increased Risk of Blount Disease in Obese Children and Adolescents With Vitamin D Deficiency. Journal of Pediatric Orthopaedics. 2010;30(8):879–882. doi: 10.1097/BPO.0b013e3181f5a0b3. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia NN, Pirpiris M, Otsuka NY. Body mass index in patients with slipped capital femoral epiphysis. Journal of Pediatric Orthopaedics. 2006;26(2):197–199. doi: 10.1097/01.bpo.0000218526.36362.3f. [DOI] [PubMed] [Google Scholar]
- 15.Goossens L, Braet C, Decaluwe V. Loss of control over eating in obese youngsters. Behaviour Research and Therapy. 2007;45(1):1–9. doi: 10.1016/j.brat.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Eddy KT, Tanofsky-Kraff M, Thompson-Brenner H, Herzog DB, Brown TA, Ludwig DS. Eating disorder pathology among overweight treatment-seeking youth: Clinical correlates and cross-sectional risk modeling. Behaviour Research and Therapy. 2007;45(10):2360–2371. doi: 10.1016/j.brat.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: impact on weight change in a randomized controlled trial of family-based treatment. International Journal of Obesity. 2010;34(7):1143–1148. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117(4):1155–1161. doi: 10.1542/peds.2005-1141. [DOI] [PubMed] [Google Scholar]
- 19.Goodman E, Must A. Depressive Symptoms in Severely Obese Compared With Normal Weight Adolescents: Results From a Community-Based Longitudinal Study. Journal of Adolescent Health. 2011;49(1):64–69. doi: 10.1016/j.jadohealth.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. Jama-Journal of the American Medical Association. 2003;289(14):1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 21.Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011;69(1):41–49. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stunkard AJ, Foch TT, Hrubec Z. A TWIN STUDY OF HUMAN OBESITY. Jama-Journal of the American Medical Association. 1986;256(1):51–54. [PubMed] [Google Scholar]
- 23.Turula M, Kaprio J, Rissanen A, Koskenvuo M. BODY-WEIGHT IN THE FINNISH TWIN COHORT. Diabetes Research and Clinical Practice. 1990;10:S33–S36. doi: 10.1016/0168-8227(90)90137-i. [DOI] [PubMed] [Google Scholar]
- 24.Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. American Journal of Clinical Nutrition. 2008;87(2):398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- 25.Farooqi IS, O’Rahilly S. Genetics of obesity in humans. Endocrine Reviews. 2006;27(7):710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 26.Lustig RH. Pediatric endocrine disorders of energy balance. Reviews in Endocrine & Metabolic Disorders. 2005;6(4):245–260. doi: 10.1007/s11154-005-6183-1. [DOI] [PubMed] [Google Scholar]
- 27.Reinehr T, Hinney A, De Sousa G, Austrup F, Hebebrand J, Andler W. Definable somatic disorders in overweight children and adolescents. Journal of Pediatrics. 2007;150(6):618–622. doi: 10.1016/j.jpeds.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 28.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 29.Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang SM, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: Prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. Journal of Clinical Endocrinology & Metabolism. 2006;91(5):1811–1818. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- 30.van der Klaauw AA, von dem Hagen EA, Keogh JM, Henning E, O’Rahilly S, Lawrence AD, et al. Obesity-associated melanocortin-4 receptor mutations are associated with changes in the brain response to food cues. J Clin Endocrinol Metab. 2014;99(10):E2 101–106. doi: 10.1210/jc.2014-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2012.08.018. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Lustig RH. Hypothalamic obesity: causes, consequences, treatment. Pediatric endocrinology reviews: PER. 2008;6(2):220–227. [PubMed] [Google Scholar]
- 33.August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, et al. Prevention and Treatment of Pediatric Obesity: An Endocrine Society Clinical Practice Guideline Based on Expert Opinion. Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 35.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. doi: 10.1016/j.smrv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Amer Diabet A. Standards of Medical Care in Diabetes-2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 37.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association (vol 107, pg 811, 2012) American Journal of Gastroenterology. 2012;107(10):1598–1598. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 38.Garrow JS, Webster JD, Pearson M, Pacy PJ, Harpin G. Inpatient-outpatient randomized comparison of Cambridge diet versus milk diet in 17 obese women over 24 weeks. Int J Obes. 1989;13(4):521–529. [PubMed] [Google Scholar]
- 39.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Treatment of Pediatric Obesity: A Systematic Review and Meta-Analysis of Randomized Trials. Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 40.Janicke DM, Steele RG, Gayes LA, Lim CS, Clifford LM, Schneider EM, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. Journal of pediatric psychology. 2014;39(8):809–825. doi: 10.1093/jpepsy/jsu023. [DOI] [PubMed] [Google Scholar]
- 41.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr. 2011;158(4):624–627. doi: 10.1016/j.jpeds.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 42.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30(3):318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- 44.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of Severely Obese Children and Adolescents to Behavioral Treatment. Archives of Pediatrics & Adolescent Medicine. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 45.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298(4):E824–831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 46.Deforche B, De Bourdeaudhuij I, Debode P, Vinaimont F, Hills AP, Verstraete S, et al. Changes in fat mass, fat-free mass and aerobic fitness in severely obese children and adolescents following a residential treatment programme. Eur J Pediatr. 2003;162(9):616–622. doi: 10.1007/s00431-003-1247-2. [DOI] [PubMed] [Google Scholar]
- 47.Keogh JB, Clifton PM. The role of meal replacements in obesity treatment. Obes Rev. 2005;6(3):229–234. doi: 10.1111/j.1467-789X.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 48.Sothern, Udall JN, Suskind RM, Vargas A, Blecker U. Weight loss and growth velocity in obese children after very low calorie diet, exercise, and behavior modification. Acta Paediatr. 2000;89(9):1036–1043. doi: 10.1080/713794562. [DOI] [PubMed] [Google Scholar]
- 49.Lenders CM, Gorman K, Lim-Miller A, Puklin S, Pratt J. Practical approaches to the treatment of severe pediatric obesity. Pediatr Clin North Am. 2011;58(6):1425–1438. x–xi. doi: 10.1016/j.pcl.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Viner RM, Hsia Y, Tomsic T, Wong ICK. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obesity Reviews. 2010;11(8):593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 51.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of Weight Management Interventions in Children: A Targeted Systematic Review for the USPSTF. Pediatrics. 2010;125(2):E396–E418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 52.McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic Review of the Benefits and Risks of Metformin in Treating Obesity in Children Aged 18 Years and Younger. Jama Pediatrics. 2014;168(2):178–184. doi: 10.1001/jamapediatrics.2013.4200. [DOI] [PubMed] [Google Scholar]
- 53.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Björkhem-Bergman L, Asplund AB, Lindh JD. Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol. 2011;25(3):299–305. doi: 10.1177/0269881109353461. [DOI] [PubMed] [Google Scholar]
- 55.Newall H, Myles N, Ward PB, Samaras K, Shiers D, Curtis J. Efficacy of metformin for prevention of weight gain in psychiatric populations: a review. Int Clin Psychopharmacol. 2012;27(2):69–75. doi: 10.1097/YIC.0b013e32834d0a5b. [DOI] [PubMed] [Google Scholar]
- 56.Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 57.Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. doi: 10.1155/2012/672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zoicas F, Droste M, Mayr B, Buchfelder M, Schöfl C. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol. 2013;168(5):699–706. doi: 10.1530/EJE-12-0997. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Tong Y, Su N, Li Y, Tang L, Huang L, et al. Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: A systematic review and meta-analysis of randomized controlled trials. J Diabetes. 2014 doi: 10.1111/1753-0407.12198. [DOI] [PubMed] [Google Scholar]
- 60.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. The Cochrane database of systematic reviews. 2014;8:CD003641–CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubino F, R’bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6(2):102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schilling PL, Davis MM, Albanese CT, Dutta S, Morton J. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008;206(1):1–12. doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 63.Hsia DS, Fallon SC, Brandt ML. Adolescent bariatric surgery. Arch Pediatr Adolesc Med. 2012;166(8):757–766. doi: 10.1001/archpediatrics.2012.1011. [DOI] [PubMed] [Google Scholar]
- 64.Ipeg. IPEG Guidelines for Surgical Treatment of Extremely Obese Adolescents. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2009;19:XIV–XVI. doi: 10.1089/lap.2009.9997. [DOI] [PubMed] [Google Scholar]
- 65.Pratt JSA, Lenders CM, Dionne EA, Hoppin AG, Hsu GLK, Inge TH, et al. Best Practice Updates for Pediatric/Adolescent Weight Loss Surgery. Obesity. 2009;17(5):901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines. Surgery for Obesity and Related Diseases. 2012;8(1):1–7. doi: 10.1016/j.soard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obesity Reviews. 2013;14(8):634–644. doi: 10.1111/obr.12037. [DOI] [PubMed] [Google Scholar]
- 68.Nijhawan S, Martinez T, Wittgrove AC. Laparoscopic gastric bypass for the adolescent patient: long-term results. Obes Surg. 2012;22(9):1445–1449. doi: 10.1007/s11695-012-0670-8. [DOI] [PubMed] [Google Scholar]
- 69.Himpens J, Cadière GB, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–807. doi: 10.1001/archsurg.2011.45. [DOI] [PubMed] [Google Scholar]
- 70.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Sabah SK, Almazeedi SM, Dashti SA, Al-Mulla AY, Ali DA, Jumaa TH. The Efficacy of Laparoscopic Sleeve Gastrectomy in Treating Adolescent Obesity. Obes Surg. 2014 doi: 10.1007/s11695-014-1340-9. [DOI] [PubMed] [Google Scholar]
- 72.Nocca D, Nedelcu M, Nedelcu A, Noel P, Leger P, Skalli M, et al. Laparoscopic sleeve gastrectomy for late adolescent population. Obes Surg. 2014;24(6):861–865. doi: 10.1007/s11695-014-1200-7. [DOI] [PubMed] [Google Scholar]
- 73.Murtagh L, Ludwig DS. State intervention in life-threatening childhood obesity. JAMA. 2011;306(2):206–207. doi: 10.1001/jama.2011.903. [DOI] [PubMed] [Google Scholar]
- 74.Varness T, Allen DB, Carrel AL, Fost N. Childhood obesity and medical neglect. Pediatrics. 2009;123(1):399–406. doi: 10.1542/peds.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]


