Abstract
Background
Four epidemic waves of human infection with H7N9 have been recorded in China up to 1 June 2016, including in Jiangsu Province. However, few studies have investigated the differences in patients' characteristics among the four epidemic waves, and the analyses of factors associated with fatal infection lacked statistical power in previous studies due to limited sample size.
Methods
All laboratory‐confirmed A(H7N9) patients in Jiangsu province were analysed. Patients' characteristics were compared across four waves and between survivors and those who died. Multivariate analyses were used to identify independent predictors of death.
Results
Significant differences were found in the lengths of several time intervals (from onset of disease to laboratory confirmation, to onset of ARDS and respiratory failure, and to death) and in the development of heart failure. The proportions of overweight patients and rural patients increased significantly across the four waves. Administration of glucocorticoids and double‐dose neuraminidase inhibitors became the norm. Predictors of death included complications such as ARDS, heart failure and septic shock, administration of glucocorticoids, and disease duration.
Conclusion
Characteristics of H7N9 patients and clinical treatment options changed over time. Particular complications and the use of particular treatment, along with disease duration, could help clinicians predict the outcome of H7N9 infections.
Keywords: Avian influenza, fatal infection, H7N9, predictor, risk factor
1. INTRODUCTION
Since 2013 when the A(H7N9) virus emerged,1 a total of 793 laboratory‐confirmed human cases have been reported globally up to 19 July 2016. There have been 319 reported deaths, a fatality rate of 40%.2 The novel virus was initially identified in the Yangtze River delta situated in Eastern China and a majority of cases were reported from this district,3 which comprises Jiangsu, Zhejiang and Shanghai Provinces/Municipalities. The virus has raised severe concerns due to its ability of binding to receptors in the upper respiratory tract4 and the high death rate in human infections. Several published studies have presented preliminary analyses of the risk factors or predictors for fatal human infections with H7N9.5, 6, 7 However, statistical analyses, especially multivariate analyses, were underpowered in most studies due to small sample size (≤40 cases) or patients' limited epidemiological/clinical information. As of 1 June 2016, the H7N9 virus has caused four epidemic waves in Jiangsu Province, resulting in a total of 103 laboratory‐confirmed H7N9 cases with 47 deaths. This study aims to comprehensively identify the epidemiological or clinical predictors of death in human infections with H7N9 using multivariate analyses, based on a relatively large sample size obtained from Jiangsu province from 2013 to 2016.
2. METHODS
2.1. Subjects
All laboratory‐confirmed human infections with H7N9 are reported through a national system for reporting of notifiable infectious diseases.8 Demographic, epidemiological and clinical information on patients infected with H7N9 was collected using standardized questionnaires by local CDC staff or trained clinical doctors in Jiangsu Province and was reported through this system. All patients infected with H7N9 as of 1 June 2016 in Jiangsu Province were included in this analysis.
2.2. Ethic statement
The National Health and Family Planning Commission ruled that the collection of data from cases of H7N9 was part of the public health investigation of an emerging outbreak, and thus, the investigation was exempt from institutional review board assessment.8 Patients' information was collected and reported to Jiangsu Provincial CDC and China CDC through a national system for reporting of notifiable infectious diseases. Jiangsu Provincial CDC is responsible for checking and monitoring the reported information and will take part in patients' investigations if necessary. The data set was not anonymized in the reporting system but was anonymized before data analysis.
2.3. Statistical analysis
Medians and interquartile ranges (IQRs) were calculated for continuous variables and absolute numbers and proportions for categorical variables. Demographic, epidemiological and clinical characteristics were compared among four observed epidemic waves (March to April in 2013, December 2013 to May 2014, October 2014 to May 2015 and December 2015 to May 2016) and between surviving and dead patients infected with H7N9. According to the Diagnosis and Treatment Guideline for human infection with H7N9 published by National Health and Family Planning Commission of China, patients with no clinical symptoms and two consecutive PCR‐negative results (at least 24 hours of sampling interval) could be discharged from hospital; thus, the endpoint of disease duration for survived patients in our study was defined as the date of hospital discharge. The Pearson chi‐squared test with continuity correction was used for comparing proportions, or Fisher's exact test was used when appropriate. Mann‐Whitney U tests (for two groups) or Kruskal‐Wallis H tests (for multiple groups) were used for comparing medians. All study variables with statistical significance in univariate analyses between patients with survival and fatal outcome were included in multivariate analyses. A step‐forward logistic model was employed to select independent variables associated with fatal infection with H7N9 virus. Receiver‐operating characteristic (ROC) analysis was used to investigate the cut‐off value of disease duration for predicting cases' poor outcomes. Statistical analyses were done in r version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and statistical significance set at P≤.05. Information on missing data is shown in Table S1. Cases with missing data on variables being analysed were excluded.
3. RESULTS
Four epidemic waves of human infections with H7N9 have been observed in Jiangsu Province, China: March to April in 2013 (wave 1), December 2013 to May 2014 (wave 2), October 2014 to May 2015 (wave 3) and December 2015 to May 2016 (wave 4) (Figure 1). The reported numbers of laboratory‐confirmed H7N9 cases were 29, 27, 22 and 25, respectively (Table 1). The median age of all cases was 54 years (IQR, 41‐68), and 69.9% were male. Epidemiological and clinical characteristics of human H7N9 cases were compared among the four waves. There were no statistically significant differences in age, gender or chronic medical conditions. Interestingly, both the proportion of overweight (from 10.7% to 40.0%, P=.029) and of rural residents (from 13.8% to 40.0%, P=.028) increased significantly across four epidemic waves. Significant differences were observed in time interval from onset of disease to laboratory confirmation, to onset of ARDS and of respiratory failure (P=.007, .017 and .004, respectively), but not in the initiation of or the time length of antivirals and glucocorticoids administration. The median number of days from onset of disease to administration of antivirals was 9 in the first wave and then decreased to 7 in the following three waves and was 7 days for both the survivors and fatal cases, with no statistically difference observed among the four waves, nor between survivors and fatal cases (P=.567, .980, respectively). The median days from onset of disease to laboratory confirmation decreased from 12 in wave 1 to 8 in waves 3 and 4. The median time intervals from onset of disease to onset of ARDS and of respiratory failure were both shortest during wave 3. Similarly, disease durations of fatal cases were also shortest during wave 3. No significant differences existed in poultry or live poultry market exposure history or in clinical complications among the four epidemic waves, except for heart failure. Half of the cases in wave 3 developed heart failure, while this proportion only ranged from 8.3% to 28.0% during the other epidemic waves (P=.023). A large majority of the cases were admitted to ICU (ranged from 74.1% to 85.0%) and treated with neuraminidase inhibitors (74.1%‐100%) and with antibiotics (95%‐100%). Notably, a significant increase in glucocorticoid administration was observed across four epidemic waves, from 61.5% to 100% (P=.010, P for trend =.001). In addition, the prescription of a higher dose of neuraminidase inhibitors (150 mg vs 75 mg) became more common (from 15.8% to 90.5%). The case fatality rate ranged from 34.5% to 61.9% across four epidemic waves, with no statistically significant difference (P=.287) (Table 1).
Figure 1.
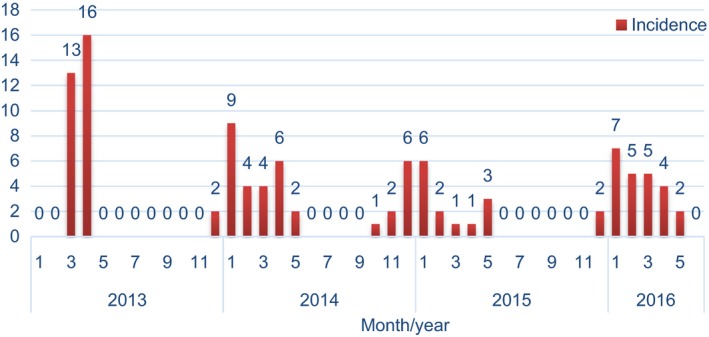
Four epidemic waves of human infections with H7N9 in Jiangsu Province, China, 2013‐2016
Table 1.
Comparisons of selected demographic, epidemiological and clinical characteristics among four epidemic waves and between survival and dead patients infected with H7N9
| Selected characteristics | Epidemic wave | P a | Clinical outcome | P b | ||||
|---|---|---|---|---|---|---|---|---|
| 1 (N=29) | 2 (N=27) | 3 (N=22) | 4 (N=25) | Survival (N=55) | Fatal (N=47) | |||
| Demographic characteristics | ||||||||
| Male, n (%) | 21 (72.4) | 20 (74.1) | 14 (63.6) | 17 (68.0) | .858 | 40 (72.7) | 31 (66.0) | .459 |
| Age, y, median (interquartile) | 54 (34‐71) | 53 (42‐66) | 57 (49.75‐69.25) | 53 (38.5‐65) | .698 | 52.00 (36.00‐60.00) | 60.00 (49.00‐73.00) | .005 |
| Overweight, n (%) | 3 (10.7) | 9 (33.3) | 6 (30.0) | 10 (40.0) | .096 | 12 (21.8) | 16 (35.6) | .128 |
| Rural residence, n (%) | 4 (13.8) | 7 (25.9) | 7 (31.8) | 10 (40.0) | .175 | 16 (29.1) | 12 (25.5) | .688 |
| Chronic medical condition | ||||||||
| Chronic pulmonary disease, n (%) | 5 (18.5) | 1 (3.7) | 4 (20.0) | 2 (8.0) | .226c | 3 (5.6) | 9 (20.0) | .028 |
| Chronic cardiovascular disease, n (%) | 7 (25.9) | 11 (40.7) | 12 (60.0) | 8 (32.0) | .102 | 14 (25.9) | 24 (53.3) | .005 |
| Chronic metabolic disease, n (%) | 3 (11.1) | 5 (18.5) | 1 (5.0) | 3 (12.0) | .573c | 6 (11.1) | 6 (13.3) | .736 |
| Chronic liver disease, n (%) | 1 (3.7) | 2 (7.4) | 2 (10.0) | 2 (8.0) | .860c | 4 (7.4) | 3 (6.7) | 1.000 |
| Any medical condition, n (%) | 14 (51.9) | 17 (63.0) | 13 (65.0) | 13 (52.0) | .693 | 29 (53.7) | 28 (62.2) | .393 |
| Selected time intervals, d | ||||||||
| Time interval from onset of symptom to first medical consultation, median (interquartile) | 2.00 (.00‐5.00) | 3.00 (1.00‐4.00) | 2.50 (.00‐5.75) | 3.00 (.00‐4.50) | .911 | 2.50 (0.75‐5.00) | 3.00 (0.00‐4.50) | .760 |
| Time interval from onset of disease to hospital admission, median (interquartile) | 5.00 (4.00‐8.50) | 5.00 (4.00‐7.00) | 5.00 (3.25‐7.75) | 5.00 (3.50‐6.50) | .850 | 5.00 (4.00‐7.00) | 5.00 (4.00‐7.00) | .759 |
| Time interval from onset of disease to laboratory confirmation, median (interquartile) | 12.00 (9.00‐15.00) | 10.00 (8.00‐12.00) | 8.00 (7.00‐12.00) | 8.00 (7.00‐11.50) | .007 | 10.00 (8.00‐12.00) | 10.00 (8.00‐13.00) | .895 |
| Time interval from onset of disease to antivirals administration, median (interquartile) | 9.00 (5.00‐14.00) | 7.00 (6.00‐9.00) | 7.00 (6.00‐9.00) | 7.00 (5.00‐8.50) | .567 | 7.00 (5.00‐10.25) | 7.00 (6.00‐9.00) | .980 |
| Time interval from onset of disease to glucocorticoids administration, median (interquartile) | 9.00 (5.00‐15.00) | 8.00 (6.00‐10.00) | 7.00 (5.25‐8.00) | 7.00 (5.00‐8.00) | .258 | 7.00 (6.00‐11.50) | 7.00 (5.00‐9.00) | .318 |
| Time length of antivirals administration, median (interquartile) | 12.00 (5.00‐15.00) | 14.00 (6.00‐23.00) | 9.50 (5.50‐13.75) | 12.00 (7.50‐14.00) | .228 | 11.50 (7.00‐14.25) | 13.00 (5.00‐19.50) | .598 |
| Time length of glucocorticoids administration, median (interquartile) | 7.00 (2.25‐15.25) | 8.50 (4.25‐12.00) | 8.50 (4.50‐18.00) | 13.25 (5.00‐20.75) | .483 | 10.00 (3.50‐16.50) | 7.00 (4.00‐18.00) | .685 |
| Time interval from onset of disease to ICU admission, median (interquartile) | 9.95 (6.46‐13.69) | 9.07 (7.59‐10.87) | 6.89 (5.60‐9.04) | 7.62 (5.77‐10.06) | .104 | 8.92 (6.69‐11.46) | 8.50 (6.04‐10.01) | .431 |
| Time interval from onset of disease to onset of ARDS, median (interquartile) | 8.50 (5.00‐14.00) | 9.50 (8.00‐11.50) | 6.00 (5.00‐8.00) | 8.00 (6.00‐8.75) | .017 | 8.00 (6.00‐11.00) | 8.00 (5.00‐10.00) | .260 |
| Time interval from onset of disease to onset of Respiratory failure, median (interquartile) | 8.00 (5.25‐14.00) | 9.00 (7.50‐10.50) | 6.00 (5.00‐8.00) | 7.00 (5.00‐8.00) | .004 | 7.50 (6.00‐10.00) | 8.00 (5.50‐10.00) | .810 |
| Disease duration, d | 31.00 (26.00‐46.00) | 21.00 (17.00‐35.00) | <.0001 | |||||
| Survival, median (interquartile) | 41.00 (26.00‐88.00) | 29.50 (26.50‐44.00) | 28.50 (21.00‐41.75) | 29.00 (26.75‐39.50) | .318 | |||
| Dead, median (interquartile) | 28.00 (20.00‐48.00) | 24.00 (19.50‐41.00) | 15.00 (12.50‐24.00) | 21.00 (13.00‐27.00) | .028 | |||
| Poultry exposure | ||||||||
| Occupational exposure, n (%) | 4 (14.8) | 2 (7.4) | 3 (15.0) | 3 (13.6) | .908 | 9 (17.6) | 3 (6.7) | .105 |
| Direct contact with poultry, n (%) | 6 (28.6) | 14 (51.9) | 8 (42.1) | 13 (59.1) | .206 | 23 (50.0) | 18 (41.9) | .441 |
| Visit to live poultry market or poultry farm, n (%) | 12 (60.0) | 20 (74.1) | 13 (68.4) | 12 (54.5) | .504 | 29 (61.7) | 28 (68.3) | .519 |
| Any poultry exposure, n (%) | 14 (51.9) | 20 (74.1) | 15 (75.0) | 17 (77.3) | .168 | 34 (66.7) | 32 (71.1) | .639 |
| Clinical outcome | ||||||||
| ARDS, n (%) | 16 (61.5) | 18 (66.7) | 16 (80.0) | 16 (66.7) | .601 | 23 (44.2) | 43 (95.6) | <.0001 |
| Respiratory failure, n (%) | 20 (76.9) | 21 (80.8) | 15 (78.9) | 15 (65.2) | .603 | 30 (57.7) | 41 (97.6) | <.0001 |
| Liver dysfunction, n (%) | 11 (45.8) | 11 (42.3) | 11 (55.0) | 5 (20.8) | .116 | 15 (30.0) | 23 (52.3) | .028 |
| Renal dysfunction, n (%) | 11 (44.0) | 7 (26.9) | 7 (35.0) | 6 (25.0) | .468 | 9 (17.6) | 22 (50.0) | .001 |
| Heart failure, n (%) | 7 (28.0) | 7 (26.9) | 10 (50.0) | 2 (8.3) | .023 | 4 (7.8) | 22 (50.0) | <.0001 |
| Septic shock, n (%) | 8 (33.3) | 7 (26.9) | 10 (50.0) | 8 (33.3) | .427 | 6 (11.8) | 27 (62.8) | <.0001 |
| Fatality, n (%) | 10 (34.5) | 13 (48.1) | 13 (61.9) | 11 (44.0) | .287 | NA | ||
| Treatment | ||||||||
| Neuraminidase inhibitors, n (%) | 20 (74.1) | 27 (100.0) | 20 (100.0) | 21 (87.5) | .006 c | 47 (87.0) | 41 (93.2) | .507 |
| 75 mg/per time, n (%) | 16 (84.2) | 9 (33.3) | 5 (25.0) | 2 (9.5) | <.0001 | 18 (39.1) | 14 (34.1) | .630 |
| 150 mg/per time, n (%) | 3 (15.8) | 18 (66.7) | 15 (75.0) | 19 (90.5) | 28 (60.9) | 27 (65.9) | ||
| Glucocorticoids, n (%) | 16 (61.5) | 20 (76.9) | 16 (80.0) | 24 (100.0) | .010 | 37 (69.8) | 39 (90.7) | .012 |
| Antibiotics, n (%) | 25 (96.2) | 27 (100.0) | 19 (95.0) | 25 (100.0) | .501c | 51 (96.2) | 45 (100.0) | .190c |
| ICU admission, n (%) | 21 (77.8) | 20 (74.1) | 17 (85.0) | 19 (79.2) | .842 | 35 (64.8) | 42 (95.5) | <.0001 |
Comparisons among four epidemic waves.
Comparisons between survival and dead patients.
Mann‐Whitney U tests (for two groups) or Kruskal‐Wallis H tests (for multiple groups) were used.
We further compared the epidemiological and clinical characteristics between all survival and fatal H7N9 cases. The median age of fatal cases was significantly greater than that of survivors (60 vs 52, P=.005). Chronic pulmonary disease (20.0% vs 5.6%, P=.028) and chronic cardiovascular disease (53.3% vs 25.9%, P=.005) were found more commonly in fatal cases than in survivors. No significant differences were observed in overweight, rural residence, chronic metabolic disease, chronic liver disease and poultry or live poultry market exposure history between fatal and surviving cases. There were no significant differences in selected time intervals between fatal and survival cases either, such as time interval from onset of symptom to first medical consultation, to laboratory confirmation or to onset of respiratory failure, the initial and time length of antivirals and glucocorticoids administration.
Disease duration was defined as the time interval from onset of disease to either hospital discharge or death. The median disease duration of fatal cases was considerably shorter than that of survivors (21 days vs 31 days, P<.0001). Fatal cases were more likely to be admitted into ICU and to have complications such as ARDS, respiratory failure, liver and renal dysfunction, heart failure and septic shock (P<.05). Administrations of antibiotics and neuraminidase inhibitors (including different doses) were not associated with fatal outcome, while glucocorticoids were administrated more frequently in fatal cases (P=.012).
All statistically significant factors found in univariate analyses aforementioned were included in a multivariate step‐forward logistic regression model to identify the independent predictors of fatal outcome of human infections with H7N9. Development of complications such as ARDS (OR=14.94, 95%CI: 1.82‐122.84), heart failure (OR=11.15, 95%CI: 0.95‐130.55) and septic shock (OR=22.97, 95%CI=1.79‐295.50) and administration of glucocorticoids (OR=34.11, 95%CI=1.62‐720.60) was found to be associated with an elevated risk of death, while prolonged disease duration was associated with a reduced risk (OR=0.91, 95%CI=0.85‐0.97). The combination of these factors could best predict death with an overall correct classification percentage of 94.4%. Furthermore, ROC analyses indicated that disease duration of 25.5 days could serve as the cut‐off value for predicting cases' poor outcomes (sensitivity=0.889, specificity=0.533).
4. DISCUSSION
H7N9 influenza virus continues to cause human infections and has resulted in four epidemic waves in Jiangsu Province, eastern China, up to June 2016. Both the proportion of overweight and of rural residents increased significantly across four waves in reported H7N9 patients. However, no significant difference in overweight between urban and rural H7N9 patients was observed (P=.937). Thus, the change in overweight might be attributed to the characteristics of subpopulations (cases were reported from different regions of Jiangsu Province across four waves) or to the overall rise of overweight rate in Jiangsu Province as a whole, rather than the involvement of more rural patients. According to the surveillance data of Jiangsu Provincial CDC, the overall obesity rate has increased from 9.5% to 14.6% from 2007 to 2013. It was reported that overweight could impair immunity against the influenza virus9 and was associated with an increased risk of severe infection.10 The increasing trend of overweight observed in the study has reminded public health experts of the importance of strengthening health education and of promoting precaution implementation in this high‐risk population. Several major cities afflicted by H7N9 in Jiangsu Province, such as Nanjing, Suzhou, Wuxi and Zhenjiang, suspended live poultry markets (LPMs) in urban areas to alleviate human infections.11 This may have boosted the live poultry trading in rural areas, leading to an increased number of rural H7N9 patients. A similar increasing trend was also observed in Zhejiang province, which was another hot spot of H7N9 epidemic in China.12
The time interval from onset of disease to laboratory confirmation of H7N9 patients has been shortened from a median of 12 days to 8 days across four epidemic waves. This might be attributed to improved laboratory capacity of municipal Centers for Disease Control and Prevention (CDC),13 avoiding repeated tests for confirmation by Provincial CDC.3 In addition, increased awareness of clinical doctors and the public might also be involved.13 A similar trend was also reported by Wu et al (2013) at the national level in China.3
It seemed that patients' disease progress was fastest during the third epidemic wave, as the time interval from onset of disease to onset of ARDS and to respiratory failure, and the disease duration of fatal cases were all shortest during this wave. The observed highest prevalence of chronic cardiovascular disease (60%) in reported H7N9 cases of this wave might be responsible. Incidence of heart failure was highest in cases of this wave as well (50%, P=.023). This indicates a critical role of cardiovascular function in the prognosis of human infections with H7N9.14, 15
A large majority of the cases were treated with neuraminidase inhibitors, and the implementation of a higher dose (150 mg) became the norm (from 15.8% to 90.5%). However, the higher dose was not found to be associated with a better prognosis in this study. Double dose of oseltamivir administration was originally recommended by WHO during the 2009 influenza A(H1N1) pandemic16 and was recommended for treating severe human infections with H7N9 in the Diagnosis and Treatment Guideline published by National Health and Family Planning Commission of China since 2013. As shown in our study, more and more clinicians have adopted this recommendation, but unfortunately no evidence of benefits was found. Yang et al. (2012)17 indicated that higher doses of oseltamivir did not improve clinical outcome in patients with pneumonia caused by influenza A pandemic (H1N1) virus. Clinical trials and prospective studies also found no virological or clinical advantages with double‐dose oseltamivir compared with standard dose in patients with severe influenza.18, 19 Our study implied a similar finding of this issue in the context of H7N9 infection. Moreover, reports of neuraminidase inhibitor resistance in the H7N9 virus call for a more optimized neuraminidase inhibitor use in clinical procedures.20, 21
We found in this study that clinical complications such as ARDS, heart failure and septic shock could pose an elevated risk of death from H7N9 infection. This finding is consistent with previous studies and case reports.5, 6, 7 A longer disease duration was found to be associated with a reduced risk of death in our study. Previous studies also reported that the disease duration of fatal cases was shorter than that of patients who recovered.22, 23 Infections with several avian influenza subtypes, such as H9N2, H7N7, H7N2, H7N3, H10N7 and H6N1, are commonly regarded as self‐limiting diseases,24 and essential supportive care has been proved to be effective for recovery.25, 26 For H7N9, studies showed that viral load peaked within the first 10 days after symptom onset during the acute exudative phase,27 and individuals' anti‐H7N9 antibodies could be detected from day 21, along with a significant decline of viral load.28 This indicated that infection with H7N9 can also be considered self‐limiting, which highlighted the critical role of supportive health care in treatment. We suggest that a disease duration of 25.5 days might serve as the time indicator for patients' survival.
Interestingly, administration of glucocorticoids was associated with a significantly elevated risk of death, while its clinical implementation jumped from a proportion of 61.5% to 100% across the observed four epidemic waves in this study. Glucocorticoid is mainly used as an anti‐inflammatory agent and is also an alternative for the treatment of septic shock.29, 30, 31 The administration of glucocorticoids thus reflects disease severity to some extent. However, as observed in this study, glucocorticoids have been used more and more comprehensively for treating H7N9 patient, not only restricted to these with special need. Treating influenza with glucocorticoids remains controversial. A number of studies have reported that use of glucocorticoids increased the death risk from H1N132, 33, 34, 35 and from H5N1.36 Slower viral clearance of influenza A (H3N2) virus was observed in patients treated with systemic glucocorticoid.37 Furthermore, a systematic review and meta‐analysis using data from 19 studies of glucocorticoid treatment and human infection of influenza virus have concluded that glucocorticoids were related with mortality, nosocomial infection, longer mechanical ventilation and longer ICU stay.38 As a result, a much more rational use of glucocorticoids based on sturdy scientific evidences is urgently needed,39 for instance, assessing patients' disease progression with objective medical indicators along with the types, doses and administrative routes of glucocorticoids.
In conclusion, the differences in epidemiological and clinical characteristics across four observed epidemic waves of H7N9 in Jiangsu of China were reported in this study and predictors for fatal infection were investigated using multivariate analyses based on a relatively large sample size. These results help us better understand the evolution of H7N9 and help clinicians prejudge the prognosis of H7N9 patients. Suggestions for appropriate medication treatment such as neuraminidase inhibitors doses and glucocorticoids administration were also provided.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Keith Dear, Research Professor of Global Health, Duke Kunshan University for his help in language polishing.
Ma W, Huang H, Chen J, et al. Predictors for fatal human infections with avian H7N9 influenza, evidence from four epidemic waves in Jiangsu Province, Eastern China, 2013‐2016. Influenza Other Respi Viruses. 2017;11:418‐424. https://doi.org/10.1111/irv.12461
Funding information
The authors were supported in part by Science & Technology Demonstration Project for Emerging Infectious Diseases Control and Prevention for Emerging Infectious Diseases Control and Prevention (grant number BE2015714), Research Foundation of National Health and Family Planning Commission of China (grant number W201303), National Science and Technology Major Project: Major New Drug Development (grant number 2015ZX09101044) and National Natural Science Foundation of China Youth Science Fund Project (grant number 81501793).
Ma, Huang and Chen contributed equally to this study.
Contributor Information
Xiang Huo, Email: huox@foxmail.com.
Minghao Zhou, Email: zmh@jscdc.cn.
REFERENCES
- 1. Gao R, Cao B, Hu Y, et al. Human infection with a novel avian‐origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888‐1897. [DOI] [PubMed] [Google Scholar]
- 2. WHO . (http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_07_19_2016.pdf?ua=1). Accessed August 18, 2016.
- 3. Wu P, Peng Z, Fang VJ, et al. Human Infection with Influenza A(H7N9) virus during 3 major epidemic waves, China, 2013‐2015. Emerg Infect Dis. 2016;22:964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou J, Wang D, Gao R, et al. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499:500‐503. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Yang Z, Lu Y, Xu Q, Wang Q, Chen L. Clinical features and factors associated with outcomes of patients infected with a Novel Influenza A (H7N9) virus: a preliminary study. PLoS One. 2013;8:e73362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, Sun J, Cai J, et al. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza A (H7N9) virus in Zhejiang Province China. J Infect. 2013;67:595‐605. [DOI] [PubMed] [Google Scholar]
- 7. Shen Z, Chen Z, Li X, et al. Host immunological response and factors associated with clinical outcome in patients with the novel influenza A H7N9 infection. Clin Microbiol Infect. 2014;20:O493‐O500. [DOI] [PubMed] [Google Scholar]
- 8. Cowling BJ, Jin L, Lau EH, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population‐based study of laboratory‐confirmed cases. Lancet. 2013;382:129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paich HA, Sheridan PA, Handy J, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity. 2013;21:2377‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren YY, Yin YY, Li WQ, et al. Risk factors associated with severe manifestations of 2009 pandemic influenza A (H1N1) infection in China: a case‐control study. Virol J. 2013;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murhekar M, Arima Y, Horby P, et al. Avian influenza A(H7N9) and the closure of live bird markets. Western Pac Surveill Response J. 2013;4:4‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He F, Chen EF, Li FD, Wang XY, Wang XX, Lin JF. Human infection and environmental contamination with Avian Influenza A (H7N9) Virus in Zhejiang Province, China: risk trend across the three waves of infection. BMC Public Health. 2015;15:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y. The H7N9 influenza virus in China–changes since SARS. N Engl J Med. 2013;368:2348‐2349. [DOI] [PubMed] [Google Scholar]
- 14. Wang C, Yu H, Horby PW, et al. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis. 2014;58:1095‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He F, Zhang M, Wang X, et al. Distinct risk profiles for human infections with the Influenza A(H7N9) virus among rural and urban residents: Zhejiang Province, China, 2013. PLoS One. 2014;9:e95015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anon . WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and Other Influenza Viruses. Geneva; 2010. (WHO Guidelines Approved by the Guidelines Review Committee). [PubMed]
- 17. Yang SG, Cao B, Liang LR, et al. Antiviral therapy and outcomes of patients with pneumonia caused by influenza A pandemic (H1N1) virus. PLoS One. 2012;7:e29652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. South East Asia Infectious Disease Clinical Research N . Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ. 2013;346:f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee N, Hui DS, Zuo Z, et al. A prospective intervention study on higher‐dose oseltamivir treatment in adults hospitalized with influenza a and B infections. Clin Infect Dis. 2013;57:1511‐1519. [DOI] [PubMed] [Google Scholar]
- 20. Hai R, Schmolke M, Leyva‐Grado VH, et al. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat Commun. 2013;4:2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Y, Lu S, Song Z, et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273‐2279. [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mei Z, Lu S, Wu X, et al. Avian influenza A(H7N9) virus infections, Shanghai, China. Emerg Infect Dis. 2013;19:1179‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuen KY. Solving the mystery of H7N9 by crystal balls. Cell Res. 2013;23:1335‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzgerald DA. Human swine influenza A [H1N1]: practical advice for clinicians early in the pandemic. Paediatr Respir Rev. 2009;10:154‐158. [DOI] [PubMed] [Google Scholar]
- 26. Liong T, Lee KL, Poon YS, et al. The first novel influenza A (H1N1) fatality despite antiviral treatment and extracorporeal membrane oxygenation in Hong Kong. Hong Kong Med J. 2009;15:381‐384. [PubMed] [Google Scholar]
- 27. Yu L, Wang Z, Chen Y, et al. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis. 2013;57:1449‐1457. [DOI] [PubMed] [Google Scholar]
- 28. Lin PH, Chao TL, Kuo SW, et al. Virological, serological, and antiviral studies in an imported human case of avian influenza A(H7N9) virus in Taiwan. Clin Infect Dis. 2014;58:242‐246. [DOI] [PubMed] [Google Scholar]
- 29. Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862‐871. [DOI] [PubMed] [Google Scholar]
- 30. Keh D, Boehnke T, Weber‐Cartens S, et al. Immunologic and hemodynamic effects of “low‐dose” hydrocortisone in septic shock: a double‐blind, randomized, placebo‐controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512‐520. [DOI] [PubMed] [Google Scholar]
- 31. Boyer A, Chadda K, Salah A, Annane D. Glucocorticoid treatment in patients with septic shock: effects on vasopressor use and mortality. Int J Clin Pharmacol Ther. 2006;44:309‐318. [DOI] [PubMed] [Google Scholar]
- 32. Han K, Ma H, An X, et al. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis. 2011;53:326‐333. [DOI] [PubMed] [Google Scholar]
- 33. Xi X, Xu Y, Jiang L, et al. Hospitalized adult patients with 2009 influenza A(H1N1) in Beijing, China: risk factors for hospital mortality. BMC Infect Dis. 2010;10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chien YS, Su CP, Tsai HT, et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infect. 2010;60:168‐174. [DOI] [PubMed] [Google Scholar]
- 35. Brun‐Buisson C, Richard JC, Mercat A, Thiebaut AC, Brochard L; Group R‐SAHNvR . Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183:1200‐1206. [DOI] [PubMed] [Google Scholar]
- 36. Liem NT, Tung CV, Hien ND, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004‐2006. Clin Infect Dis. 2009;48:1639‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang JW, Fan LC, Miao XY, et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta‐analysis. Clin Microbiol Infect. 2015;21:956‐963. [DOI] [PubMed] [Google Scholar]
- 39. Carter MJ. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J Med Microbiol. 2007;56:875‐883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


