Abstract
Diabetic cardiomyopathy (DCM) is a serious complication of diabetes. Hydrogen sulphide (H2S), a newly found gaseous signalling molecule, has an important role in many regulatory functions. The purpose of this study is to investigate the effects of exogenous H2S on autophagy and its possible mechanism in DCM induced by type II diabetes (T2DCM). In this study, we found that sodium hydrosulphide (NaHS) attenuated the augment in left ventricular (LV) mass and increased LV volume, decreased reactive oxygen species (ROS) production and ameliorated H2S production in the hearts of db/db mice. NaHS facilitated autophagosome content degradation, reduced the expression of P62 (a known substrate of autophagy) and increased the expression of microtubule-associated protein 1 light chain 3 II. It also increased the expression of autophagy-related protein 7 (ATG7) and Beclin1 in db/db mouse hearts. NaHS increased the expression of Kelch-like ECH-associated protein 1 (Keap-1) and reduced the ubiquitylation level in the hearts of db/db mice. 1,4-Dithiothreitol, an inhibitor of disulphide bonds, increased the ubiquitylation level of Keap-1, suppressed the expression of Keap-1 and abolished the effects of NaHS on ubiquitin aggregate clearance and ROS production in H9C2 cells treated with high glucose and palmitate. Overall, we concluded that exogenous H2S promoted ubiquitin aggregate clearance via autophagy, which might exert its antioxidative effect in db/db mouse myocardia. Moreover, exogenous H2S increased Keap-1 expression by suppressing its ubiquitylation, which might have an important role in ubiquitin aggregate clearance via autophagy. Our findings provide new insight into the mechanisms responsible for the antioxidative effects of H2S in the context of T2DCM.
Diabetes mellitus has been firmly established as a major threat to human health due to its severe complications in the cardiovascular system.1 Diabetic cardiomyopathy (DCM) is one of the serious complications of diabetes, which greatly increases the incidence and severity of heart failure in patients with type 2 diabetes.2 Type 2 diabetes mellitus is defined as a protein-misfolding disease, which is characterized by misfolded and aggregated peptides and proteins.3 The accumulation of misfolded proteins results in a prolonged unfolded protein response, which contributes to mitochondria injury, reactive oxygen species (ROS) production and apoptosis. The prolonged unfolded protein response could also obstruct the ubiquitin–proteasome system leading to ubiquitinated proteins aggregating.4, 5
Autophagy is one of the protective factors for cell survival and is involved in eliminating damaged proteins and organelles, and autophagy has an important role in DCM.6, 7 Damaged organelles could be eliminated through autophagy, which preserves their functions and suppresses mitochondrial ROS production.8, 9 Meanwhile, the ubiquitin aggregates that can lead to apoptosis and ROS production are mainly cleared by autophagy.10, 11
Hydrogen sulphide (H2S), a newly found gaseous signalling molecule, has an important role in many regulatory functions, such as vasodilatation, antioxidation and smooth muscle relaxation.12, 13 In mammalian tissues, the biosynthesis of H2S is catalysed by the pyridoxal-5-phosphate-dependent enzymes, including cystathionine-β-synthetase, cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulphurtransferase. In the cardiovascular system, H2S synthesis is mainly catalysed by CSE. Studies also indicate that H2S could promote disulphide formation between two Kelch-like ECH-associated protein 1 (Keap-1) molecules.14 Zhou et al.15 have demonstrated that exogenous H2S could prevent the development of DCM by reducing ROS production through the Keap-1-nuclear respiratory factor 2 (Nrf2) signalling pathway. Meanwhile, some evidence has revealed that Keap-1 has a pivotal role in ubiquitin aggregate clearance via autophagy in HEK293T cells.16 Although our previous study reported that H2S could attenuate the apoptosis of DCM induced by type 1 diabetes,17 the mechanism is not very clear between H2S and DCM. Our present study reports that exogenous H2S could protect myocardiocytes by promoting autophagy in type 2 diabetes. The effect of exogenous H2S might contribute to upregulating Keap-1 expression. Keap-1 could facilitate p62-mediated ubiquitin aggregate clearance via autophagy, which could be a key pathway for the protective effects of exogenous on myocardiocytes in type 2 diabetes. Therefore, we speculated that exogenous H2S was likely to increase the expression of Keap-1 by suppressing its ubiquitylation, which contributes to ubiquitin aggregate clearance via autophagy.
Results
Exogenous H2S improved cardiac diastolic function and H2S production in db/db mice
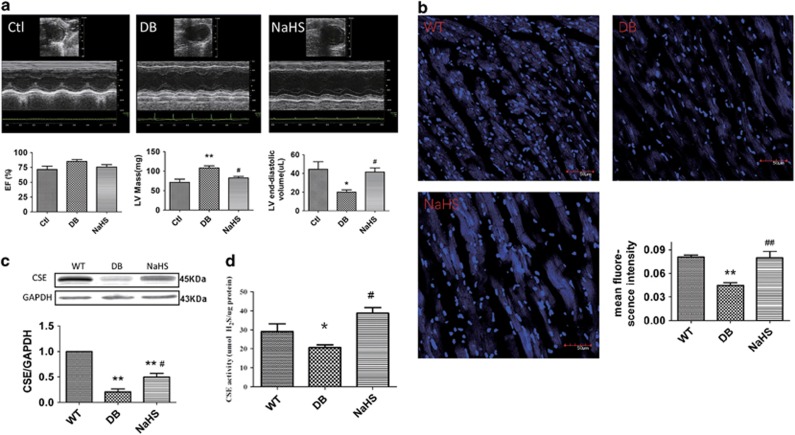
The db/db mice, leptin receptor-deficient mice, were chosen as the type 2 diabetes animal model. The blood glucose levels and serum lipids were increased, and glucose tolerance was significantly decreased in db/db mice, whereas there was no effect after sodium hydrosulphide (NaHS) injection (Supplementary Figures S1a and b). To investigate the effects of exogenous H2S on cardiac function in db/db mice, we observed the cardiac functions of mice using echocardiography. Though the ejection fraction (EF) did not change in db/db mice, the left ventricular (LV) mass was increased, and the LV volume was decreased. These alterations were ameliorated by NaHS (Figure 1a). These results suggested that db/db mice suffering from DCM could be alleviated by exogenous H2S. H2S is an important gaseous signalling molecule. Although our previous study showed that exogenous H2S improved H2S production in a type I diabetic model,18 here we reveal that H2S concentration can elicit changes in the hearts of db/db mice. The H2S probe 7-azido-4-methylcoumarin (C-7Az) was used to test the H2S levels in mouse hearts. The results showed that H2S levels were decreased in the hearts of db/db mice and those levels recovered after NaHS injection (Figure 1b). We also tested the expression and activity of CSE in the hearts of db/db mice. The results showed that the expression and activity of CSE were downregulated in db/db mice, while they were elevated with the treatment of NaHS (Figures 1c and d). Furthermore, exogenous H2S protected cardiomyocytes against apoptosis in the hearts of db/db mice (Supplementary Figure S2).
Figure 1.
Exogenous H2S could protect cardiomyocytes and improve H2S levels in type 2 diabetes. Eight- to ten-week-old db/db mice treated with or without 100 μmol/kg NaHS by intraperitoneal injection were kept on a standard chow diet for 12 weeks. (a) The cardiac function of mice was examined by heart echocardiography. (b) The content of H2S was detected by an H2S probe in mouse myocardia (blue); scale bars: 50 μm. (c) The expression of CSE in mouse hearts was detected by western blotting. (d) The activity of CSE in mouse hearts was detected using CSE detection kits. Values are presented as the mean±S.D. from n=6 replicates. *P<0.05, **P<0.01 compared with the WT group; #P<0.05, ##P<0.01 compared with the db/db group
H9C2 cells were treated with high glucose (HG) and palmitate (HG+Pal) to mimic cardiomyocytes in type 2 diabetes, and the results showed that the H2S levels and expression of CSE were downregulated in the HG+Pal group but were elevated with the treatment of NaHS (Supplementary Figures S3a and b). Meanwhile, the ratio of apoptotic cells was increased in the HG+Pal group, whereas it was decreased with the treatment of NaHS (Supplementary Figure S3c).
Exogenous H2S attenuated ROS production in cardiomyocytes
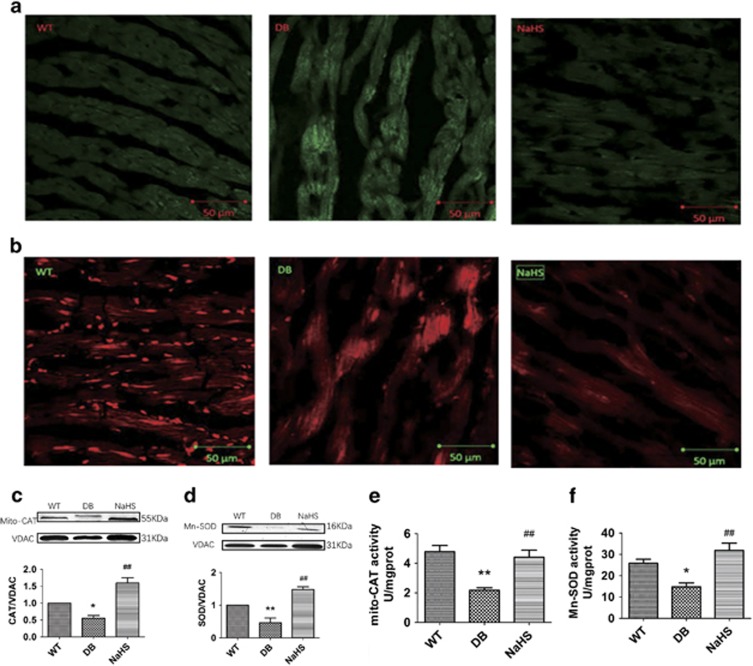
It has been reported that oxidative stress can promote DCM.19 To investigate the effect of exogenous H2S on oxidative stress, we used 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and mito-SOX staining to examine the cytosolic and mitochondrial ROS content, respectively, in mouse hearts. The results showed that the fluorescence intensity in the hearts of db/db mice was enhanced, whereas NaHS attenuated these alterations (Figures 2a and b). The expression and activity of mitochondrial catalase (Mito-CAT) and manganese-dependent superoxide dismutase (Mn-SOD) were detected to further examine the role of exogenous H2S on ROS production in the hearts of db/db mice. The results showed that the expression and activity of Mito-CAT and Mn-SOD, which were downregulated in the hearts of db/db mice, were upregulated by NaHS treatment (Figures 2c–f). Furthermore, exogenous H2S suppressed the ROS production in H9C2 cells treated with HG and palmitate (Supplementary Figure S4), which reinforced the concept that exogenous H2S attenuated oxidative stress in cardiomyocytes.
Figure 2.
Exogenous H2S attenuated ROS production in db/db mouse hearts. (a and b) Cytosolic and mitochondrial ROS production was detected by DCFH stain and Mito-SOX in mouse hearts; scale bars, 50 μm. (c and d) The expression of Mito-CAT and Mn-SOD in mouse hearts was detected by western blotting. (e and f) The activity of Mito-CAT and Mn-SOD in mouse hearts was detected using activity assay kits. Values are presented as the mean±S.D. from n=6 replicates. *P<0.05, **P<0.01 compared with the WT group; #P<0.05, ##P<0.01 compared with the db/db group
Exogenous H2S had no significant effects on Nrf2 nuclear translocation
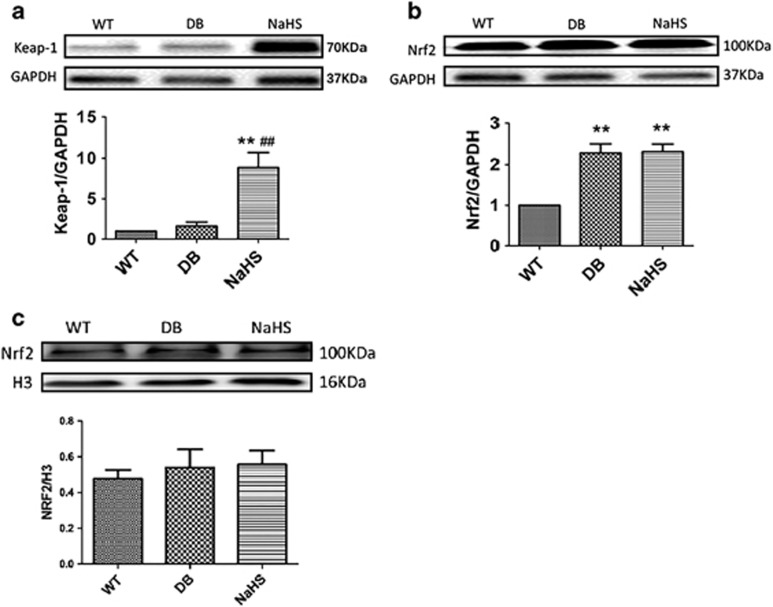
It has been proven that exogenous H2S attenuates oxidative stress through the Keap-1/Nrf2 pathway.19, 20, 21, 22 Therefore, we detected the expression of Keap-1 and Nrf2. The results showed that the expression of Keap-1 was significantly upregulated by exogenous H2S (Figure 3a), whereas the expression of Nrf2 was upregulated in the hearts of both in db/db mice and NaHS injection mice (Figure 3b). However, the expression of Nrf2 in the nucleus showed no significant change (Figure 3c). These results imply that exogenous H2S had no significant effects on the Keap-1/Nrf2 pathway and that its antioxidative effects should be attributed to another mechanism.
Figure 3.
The effects of H2S on the Keap-1/Nrf2 pathway in db/db mouse hearts. (a) The expression of Keap-1 was detected by western blotting in mouse myocardia. (b and c) The expression of Nrf2 was detected by western blotting in mouse hearts and the nucleus. Values are presented as the mean±S.D. from n=6 replicates. **P<0.01 compared with the WT group; ##P<0.01 compared with the db/db group
Exogenous H2S facilitated ubiquitin aggregate clearance via autophagy by suppressing ubiquitylation of Keap-1
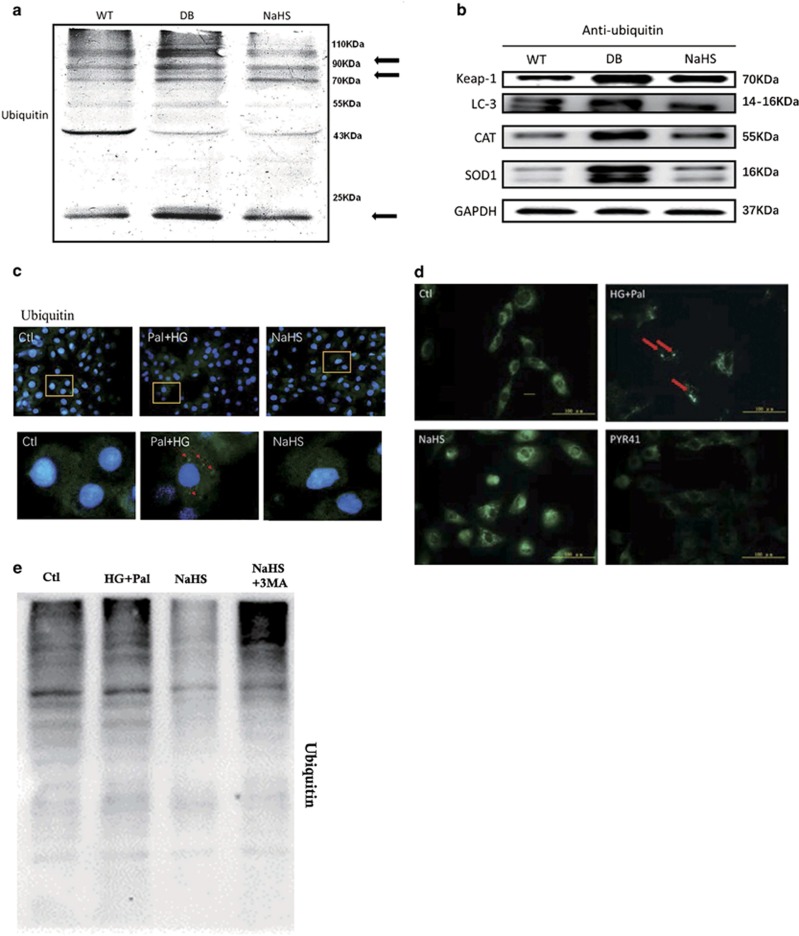
To find a new explanation for the antioxidative effect of H2S, the effect of Keap-1 on facilitating ubiquitin aggregate clearance was considered. It has been shown that Keap-1 has a pivotal role in eliminating ubiquitin aggregates.16 Therefore, we detected the ubiquitylation level in mouse hearts. The results showed that the ubiquitylation levels of 90–110, 70–90 and 10 kDa proteins were increased in the hearts of db/db mice and that this alteration was attenuated with the treatment of NaHS (Figure 4a). Meanwhile, the ubiquitylation levels of Keap-1, CAT and SOD were increased in the hearts of db/db mice, while they were attenuated following NaHS treatment (Figure 4b). This might be the reason for exogenous H2S upregulating Keap-1 expression and suppressing ROS production. To further investigate the effect of exogenous H2S on ubiquitin aggregate clearance, we observed the ubiquitylation level in H9C2 cells using an immunofluorescent assay. The results showed that the ubiquitin-positive protein aggregates accumulated in the HG+Pal group, whereas NaHS promoted ubiquitin aggregate clearance (Figure 4c, red arrows). It has been reported that ubiquitin aggregates are mainly eliminated by autophagy.23 To determine whether the ubiquitylated proteins were the main source of autophagosomes content in the cells, PYR41 (3 μM), an inhibitor of ubiquitin-activating enzyme (E1), was used to suppress the ubiquitylation. The autophagosomes aggregated in the HG+Pal group and PYR41 eliminated the autophagosome aggregates (Figure 4d, red arrows). To investigate the NaHS-facilitated ubiquitinated protein aggregate clearance via autophagy, we added 3-methyladenine (3MA) under HG+Pal+NaHS conditions and detected the ubiquitinated protein level. Our results showed that the ubiquitinated protein level was augmented in the NaHS+3MA group compared with the NaHS group and was even higher than that in the HG+Pal group (Figure 4e). These results indicate that the NaHS-facilitated ubiquitinated protein aggregate clearance occurs mainly via autophagy. The above results demonstrated that exogenous H2S could eliminate ubiquitin aggregates, which might be a new explanation for the antioxidative effect of H2S.
Figure 4.
Exogenous H2S attenuated ubiquitylation levels. (a) The expression of ubiquitous protein was analysed by western blotting in mouse myocardia. (b) The ubiquitylation level of Keap-1, SOD and CAT in mice myocardium was detected by immunoprecipitation and western blotting. H9C2 cells were treated with HG (40 mM)+palmitate (Pal, 200 μM), HG+Pal+NaHS (100 μM), HG+Pal+NaHS+3MA (2 mM, an inhibitor of autophagy) and HG+Pal+PYR41 (3 μM, an inhibitor of ubiquitin-activating enzyme (E1) for 48 h. (c) The ubiquitinated proteins in H9C2 cells were detected by an immunofluorescent assay (green); red arrows indicate ubiquitinated protein aggregates; scale bar: 100 μm. (d) The autophagosomes were detected by MDC test in H9C2 cells (green). Red arrows indicate autophagosome accumulation; scale bar: 100 μm. (e) The ubiquitinated protein level was detected by western blotting
Exogenous H2S could promote autophagy in the hearts of db/db mice and H9C2 cells
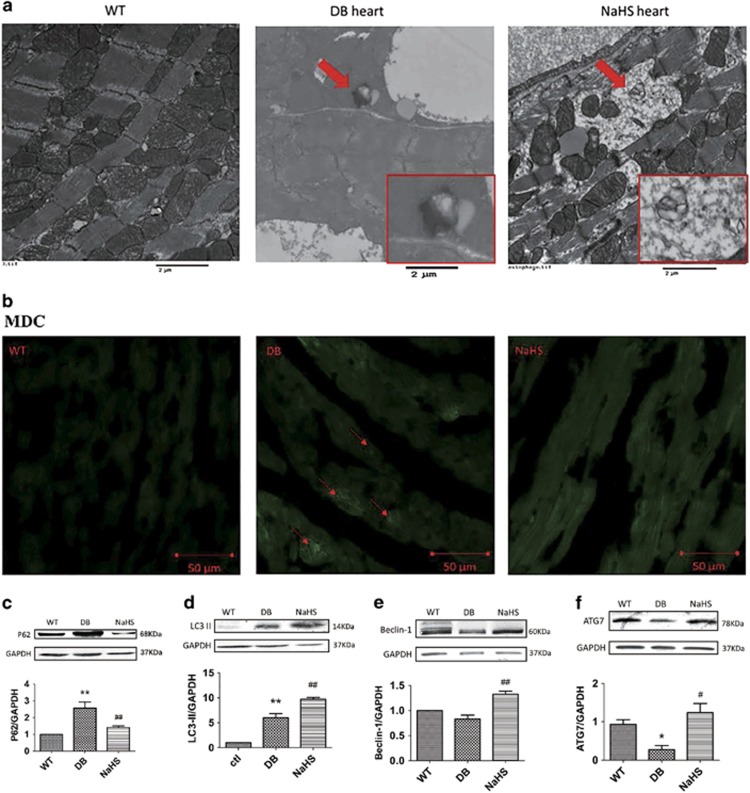
Because ubiquitin aggregates are mainly eliminated by autophagy, and some studies have shown that autophagy is interrupted in type 2 diabetes,24, 25 we examined the autophagosome content in mouse hearts using a transmission electron microscope. Autophagosomes were found in the myocardiocytes of db/db mice with or without NaHS treatment. The autophagosome contents in db/db mice were not degraded and manifested as a high-density area, whereas they were degraded following NaHS treatment, manifesting as a low-density area (Figure 5a, red arrow). The monodansylcadaverine (MDC) staining showed that autophagosomes accumulated in the hearts of db/db mice, manifesting as green patches of fluorescence (Figure 5b, red arrows), which were reduced with the treatment of NaHS, and these data reinforced the concept that exogenous H2S facilitated autophagosome content elimination. To investigate the effect of exogenous H2S on the elimination of autophagosome content, we detected two autophagosomal markers, P62 and microtubule-associated protein 1 light chain 3 II (LC3 II). The results showed that the expression of P62 and LC3 II was increased in the hearts of db/db mice, whereas exogenous H2S reduced P62 expression but increased LC3 II expression (Figures 5c and d), which was in accordance with the disruption of autophagy and lysosome bonding.26 To further investigate the effects of exogenous H2S on autophagy, we detected the expression of two upstream factors, Beclin1 and autophagy-related protein 7 (ATG7). The results demonstrated that the expression of ATG7 was decreased in the hearts of db/db mice, and the expression of both Beclin1 and ATG7 was increased with the treatment of NaHS (Figures 5e and f), which indicated that exogenous H2S promoted autophagy.
Figure 5.
Exogenous H2S could promote autophagy in the hearts of db/db mice. (a) The ultrastructure of mouse myocardia was observed using a transmission electron microscope. The red arrow indicates an autophagosome and it was amplified in the red rectangle; scale bars: 2 μm. (b) The autophagosome was detected using an MDC test in mouse myocardia (green). Red arrows indicate autophagosome accumulation. (c–f) The expression of P62, LC3 II, Beclin1 and ATG7 was detected by western blotting. Values are presented as the mean±S.D. from n=6 replicates. *P<0.05, **P<0.01 compared with the WT group; #P<0.05, ##P<0.01 compared with the db/db group
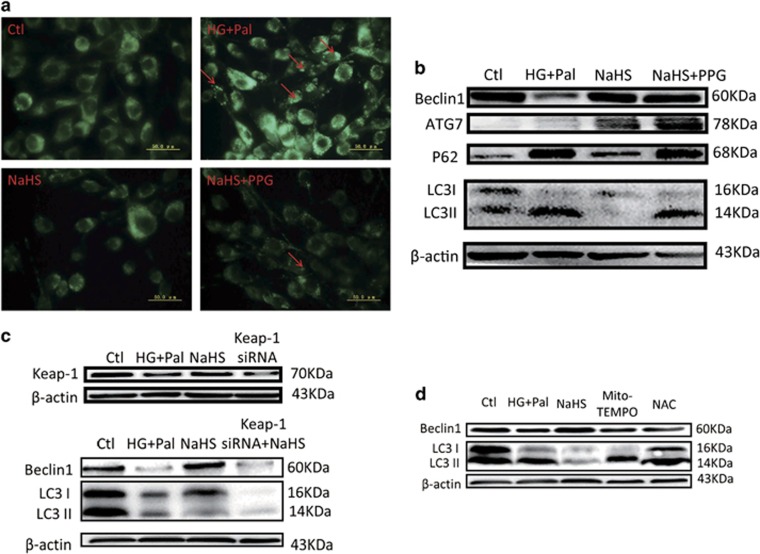
To further demonstrate the effects of exogenous H2S on autophagy, we detected the autophagosomes and the expression of P62, LC3 II, ATG7 and Beclin1 in H9C2 cells. The results showed that autophagosomes accumulated in the HG+Pal group (Figure 6a, red arrows), which was attenuated with NaHS treatment. In addition, in accordance with the in vivo data, the expression of P62 and LC3 II was increased in the HG+Pal group, while the expression of ATG7 and Beclin1 was decreased, and these alterations were ameliorated by exogenous H2S (Figure 6b). dl-propargylglycine (PPG), an inhibitor of CSE, was used to investigate whether endogenous H2S affects autophagosome clearance under the HG+Pal+NaHS conditions. Our results showed that NaHS reduced the content of the autophagosomes in H9C2 cells treated with HG and palmitate, while the addition of PPG had no influence (Figure 6a). However, the addition of PPG increased the expression of P62 and LC3 II simultaneously, while NaHS alone decreased the expression of the two proteins (Figure 6b). These results suggested that endogenous H2S may have a part role in autophagosomal clearance. To investigate whether Keap-1 is the target of NaHS, the Keap-1 short interfering RNA (siRNA) was used to downregulate the expression of Keap-1. The results indicated that Keap-1 siRNA reduced the expression of Beclin1, whereas the ratio of LC3 II to LC3 I was increased (Figure 6c). To investigate whether the antioxidative effect of exogenous H2S played a main role in autophagy, the inhibitor of mitochondrial ROS, Mito-TEMPO, and the inhibitor of total ROS, acetylcysteine (NAC), were applied in our study. The results indicated that Mito-TEMPO and NAC reduced the expression of Beclin1 and increased the expression of LC3 II (Figure 6d), which indicated that direct inhibition of ROS production had no significant effects on autophagosomal degradation. These data suggested that the antioxidative effect of exogenous H2S attributed to its promotion of autophagy, which played a crucial role in ubiquitin aggregate clearance.
Figure 6.
The effects of H2S on autophagy are attributed to Keap-1. H9C2 cells were treated with HG+Pal, HG+Pal+NaHS, HG+Pal+NaHS+PPG (10 nM, an irreversible competitive CSE inhibitor), HG+Pal+Mito-TEMPO (2 μM, an inhibitor of mitochondrial ROS), HG+Pal+NAC (100 μM, an inhibitor of ROS) and HG+Pal+NaHS+Keap-1 siRNA for 48 h. (a) The autophagosome was detected using an MDC test in H9C2 cells (green). Red arrows indicate autophagosome accumulation. (b) The expression of P62, ATG7, Beclin1 and LC3 II/I was detected by western blotting. (c) The effect of Keap-1 siRNA on autophagy was detected by western blotting. (d) The expression of Beclin1 and the ratio of LC3 II to LC3 I were detected by western blotting
Exogenous H2S-attenuated Keap-1 ubiquitylation possibly by promoting its disulphide formation
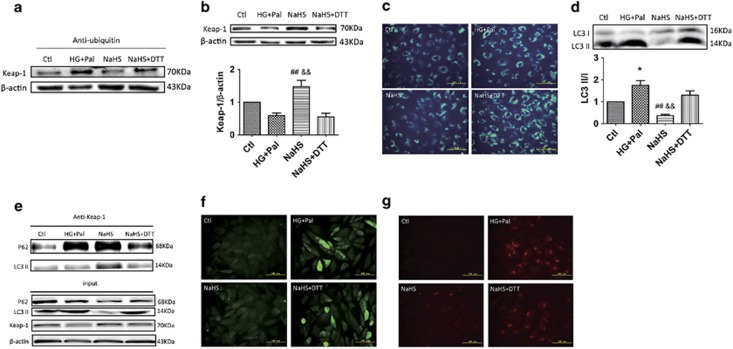
Some studies have reported that H2S could promote disulphide formation between two Keap-1 molecules.13 Therefore, 1,4-dithiothreitol (DTT) was used to inhibit disulphide formation. The results showed that the effects of exogenous H2S on Keap-1 ubiquitylation and expression were blocked with DTT treatment (Figures 7a and b). The effect of NaHS on autophagosomal elimination was also blocked with DTT treatment (Figures 7c and d). To determine whether exogenous H2S could promote Keap-1 interactions with P62 and LC3 II, Keap-1 was co-precipitated with P62 and LC3 II, respectively. We found that the interaction between Keap-1 and P62 was increased with or without NaHS treatment, but exogenous H2S also increased the interaction between Keap-1 and LC3 II. DTT blocked the effect of exogenous H2S on Keap-1 (Figure 7e). Furthermore, the treatment of DTT attenuated the antioxidative effect of H2S (Figures 7f and g), which reinforced the concept that autophagy promotion might be the reason for antioxidative effect of H2S.
Figure 7.
The effects of H2S on Keap-1 ubiquitylation might contribute to promoting disulphide formation of Keap-1. H9C2 cells were treated with HG+Pal, HG+Pal+NaHS and HG+Pal+NaHS+DTT (20 μM, an inhibitor of disulphide bonds) for 48 h. (a) The ubiquitylation level of Keap-1 in mouse myocardia was detected by immunoprecipitation and western blotting. (b) The expression of Keap-1 was detected by western blotting. (c) The autophagosome was detected using an MDC test in H9C2 cells (green); scale bar: 100 μm. (d) The ratio of LC3 II to LC3 I was detected by western blotting. (e) The interaction of Keap-1 with P62 and LC3 II was detected by immunoprecipitation and western blotting. Values are presented as the mean±S.D. from n=3 replicates. *P<0.05 compared with the Ctl group; ##P<0.01 compared with the HG+Pal group; &&P<0.01 compared with the NaHS+DTT group
Discussion
The results of the current study provided new insights into the mechanisms of type 2 DCM (T2DCM) and revealed an effective protection of exogenous H2S in the model. Our results indicated that (i) exogenous H2S could attenuate ROS production and increase H2S production in db/db mouse hearts; (ii) exogenous H2S could facilitate the clearance of ubiquitin aggregates through promoting autophagy, which contributed to its antioxidative effect; and (iii) exogenous H2S could upregulate Keap-1 expression by suppressing its ubiquitylation.
DCM greatly increases the incidence and severity of heart failure in patients with diabetes.2 It has been reported that in patients with diabetes, increased LV mass27 and LV diastolic dysfunction, which are regarded as features for DCM, could be detected.28 In our study, we found that LV mass was increased and that LV volume was decreased in 20-week-old db/db mice. Hence, db/db mice were considered to be suffering cardiomyopathy on the twentieth week. NaHS attenuated these alterations, which might provide a new way to fight against T2DCM. H2S, as a newly found gaseous signalling molecule, has an important role in many regulatory functions.29 In mammalian hearts, the biosynthesis of H2S is mainly catalysed by CSE, and our previous study demonstrated that CSE was downregulated in type I diabetic hearts.15 In the current study, we also found that H2S production was impaired in the hearts of db/db mice, which was improved by NaHS. This inferred that H2S might be an important modulator in the development of T2DCM.
Increasing evidence has shown that oxidative stress can promote the development of DCM.19 The antioxidative stress effect of H2S has an important role in the functions of H2S.30, 31, 32, 33 It has been reported that H2S could suppress ROS production34 and increase SOD activity in cardiomyocytes.35 Our study demonstrated that exogenous H2S could suppress the production of ROS in the hearts of db/db mice.
However, previous studies have demonstrated that oxidative stress can interact with autophagy, and autophagy has an important role in modulating ROS production.36, 37, 38 Autophagy is a protective factor for cell survival, which is involved in eliminating damaged proteins and organelles, and the obstruction of autophagy could result in aggregation of injured organelles and proteins, especially injured mitochondria and ubiquitinated proteins.5, 39, 40, 41 Meanwhile, growing evidence has demonstrated that the development of DCM is associated with dysregulated autophagy.42, 43 In addition, H2S has its regulative roles in the autophagy process during the development and progression of many diseases such as heart failure, hepatitis and Parkinson's disease.44, 45 However, the effect of H2S on autophagy in T2DCM is only slightly illuminated. Hence, our study focused on how H2S regulates autophagy in the hearts of db/db mice.
Autophagy is a well-coordinated, multi-step process regulated by autophagy-related gene products and proteins such as Beclin1 and P62.46 In our study, we found that the autophagosome contents were not degraded in the hearts of db/db mice. Moreover, the enhanced expression of LC3 II and P62, a substrate of the autophagy-lysosomal degradation pathway, also indicated that the degradation of autophagosome contents was impaired. The upstream autophagy-related proteins Beclin1 and ATG7 were also downregulated, which might ascribe to the failed degradation of the autophagosome contents. From the above results, the obstruction of autophagy might have a pivotal role in the development of T2DCM. In addition, exogenous H2S facilitated autophagosome content clearance, which seemed to improve the autophagy in T2DCM. The promotive effects of exogenous H2S on autophagy may be an important reason for decreased ROS production.
It has been reported that ubiquitin aggregate clearance mainly depends on autophagy and the disruption of autophagy results in ubiquitin aggregate accumulation in cells.47, 48 We found that exogenous H2S reduced the ubiquitination level in the hearts of db/db mice. PYR41, an inhibitor of ubiquitination, abolished the autophagosomes formation, which indicated that ubiquitinated proteins were the main contents of autophagosome. However, how exogenous H2S promotion of ubiquitin aggregate clearance via autophagy still needs an explanation. Recent studies have found that Keap-1 has a crucial role in eliminating ubiquitin proteins.16 So Keap-1 may be a key factor for the promotive function of exogenous H2S on ubiquitin aggregate clearance via autophagy. We found that exogenous H2S upregulated the expression of Keap-1. It has also been reported that Keap-1 regulates the translocation of Nrf2, a negative regulator of ROS production, to the nucleus.49 However, in our study, exogenous H2S had no significant effects on the translocation of Nrf2 to the nucleus. A recent study by Ji and his colleague demonstrated that H2S suppressed diabetes-accelerated atherosclerosis via Nrf2 activation by inducing Keap-1S-sulphydration.22 The different findings between our and Ji’s studies are perhaps attributed to two different kinds of cells. Or rather, in a long-term high-sugar, high-fat and low-energy environment, the translocation of Nrf2 may be repressed and this might explain why H2S upregulates cellular antioxidants in the short-term ischaemia-reperfusion heart in a Nrf2-dependent manner50, 51 but had no significant effects in our study. Meanwhile, using Mito-TEMPO and NAC alone could not reduce the expression of LC3 II, which suggested that Mito-TEMPO and NAC could not promote autophagosome clearance. Therefore, we speculated that the promotion of H2S on autophagosomal clearance was not due to its antioxidative properties and might be the mechanism for its antioxidative properties in DCM. Therefore, the protective effect of exogenous H2S on Keap-1 was focused on ubiquitin aggregate clearance via autophagy. We found that in H9C2 cells, HG and palmitate increased the interaction between Keap-1 and P62 but decreased the interaction between Keap-1 and LC3 II. This indicated that the ubiquitylation level of Keap-1 was elevated, which is tethered to P62 through molecular ubiquitin, and suppressed ubiquitin aggregate clearance via autophagy. Exogenous H2S enhanced the interaction of both Keap-1–P62 and Keap-1–LC3 II, which reinforced that exogenous H2S could facilitate ubiquitin aggregate clearance via autophagy. Meanwhile, the application of Keap-1 siRNA also repressed the effect of exogenous H2S on autophagy. From the above, the results suggested that the effect of exogenous H2S on autophagy were mainly attributed to Keap-1-mediated ubiquitin aggregate clearance.
However, it is unknown how exogenous H2S could reduce the ubiquitylation level of Keap-1, as we found. Studies have reported that H2S could promote disulphide formation between two Keap-1 molecules.22 Therefore, we detected whether exogenous H2S could suppress the ubiquitylation of Keap-1 through promoting disulphide formation of Keap-1. Therefore, DTT was used to inhibit disulphide formation. We found that DTT nearly abrogated the effect of exogenous H2S on autophagy, ROS production and Keap-1 expression. Further, DTT inhibited the interaction of Keap-1 with P62 and LC3 II, respectively. These observations suggested that exogenous H2S could facilitate ubiquitin aggregate clearance via autophagy through promoting disulphide formation of Keap-1, which might contribute to ROS scavenging. However, the details of how exogenous H2S attenuated Keap-1 ubiquitylation needs more study.
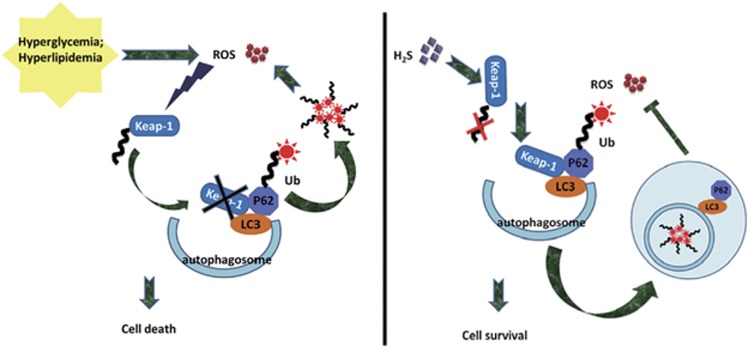
In summary, our results demonstrated that the cardiac impairment induced by type 2 diabetes might contribute to ubiquitin aggregate accumulation. Exogenous H2S could facilitate ubiquitin aggregate clearance via autophagy, which might be a new explanation for the antioxidative effect of H2S. The effect of exogenous H2S on ubiquitin aggregate clearance via autophagy might contribute to suppressing the ubiquitination of Keap-1, which upregulated the expression of Keap-1. The above evidence provides new insight into the mechanisms responsible for the antioxidative effects of H2S in the context of T2DCM (Figure 8). Upon description of the mechanism conferred by exogenous H2S protection, it is possible to provide a new avenue of therapeutic opportunities for T2DCM induced by type 2 diabetes.
Figure 8.
The protective effect of exogenous H2S on cardiomyocytes in a type 2 diabetes model. Hyperglycaemia and hyperlipidaemia induced by type 2 diabetes increase ROS production, which results in the ubiquitylation of Keap-1. As the ubiquitylation of Keap-1 was increased, the ubiquitin aggregates cannot be resolved in time. The ubiquitin aggregates accumulating in cells cause more production of ROS, which forms a vicious circle and finally induces cell death. Exogenous H2S could promote attenuated ubiquitylation of Keap-1, which has a protective effect on Keap-1. The increased expression of Keap-1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy, which suppresses the ROS production and leads to cell survival
Materials and methods
Materials
H2S donor NaHS, palmitate, MDC, CSE inhibitor PPG, autophagy inhibitor 3MA, disulphide bond inhibitor DTT, mitochondrial ROS inhibitor Mito-TEMPO, ROS inhibitor NAC, E1 inhibitor PYR41 and C-7Az were purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA). Mito-SOX, DCFH-DA and JC-1 were purchased from Invitrogen (Grand Island, NY, USA). SOD and CAT activity assay kits were purchased from Jiancheng Institute of Bioengineering, Nanjing, China. Keap-1 siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to cleaved caspase 3 (25546-1-AP), Bax (50599-2-lg), Bcl2 (12789-1-AP), SOD (10269-1-AP), CAT (21260-1-AP), Beclin1 (11306-1-AP), ATG7 (10088-2-AP), P62 (18420-1-AP), LC3 A/B (66139-1-lg), LC3 B (18725-1-AP), Keap-1 (10503-2-AP), ubiquitin (10201-2-AP), VDAC1 (55259-1-AP), GAPDH (10494-1-AP) and β-actin (66009-1-AP) were purchased from Proteintech (Rosemont, IL, USA).
Animals
Leptin receptor-deficient (db/db) mice (8–10 weeks old) and wild-type C57BL/6 mice were purchased from the Animal Model Institute of Nanjing (Nanjing, China). The animals were housed under diurnal lighting conditions and fed standard mouse chow and water throughout the study period. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the China National Institute of Health and approved by the Animal Care Committees of Harbin Medical University, China.
Experimental groups
The animal experiment was divided into three groups. Each group included 8 mice (n=8). The wild-type C57BL/6 mice (8–10 weeks old) were kept on a standard chow diet for 12 weeks as control. The db/db mice (8–10 weeks old, blood glucose concentration ⩾16.7 mM) were divided into groups treated with vehicle or NaHS by intraperitoneal injection and kept on a standard chow diet for 12 weeks. The dose of NaHS was 100 μmol/kg used as an effective dose in previous studies.52, 53
Echocardiographic analysis of cardiac function
Cardiac functions of mice were assessed using an echocardiography system (GE VIVID7 10S, St. CT., Fairfield, USA) after 12 weeks treated. Echocardiography was performed on self-breathing mice under anaesthesia (intraperitoneal injection of 1% pentobarbital sodium at 6 ml/kg body weight). The following LV parameters were measured, including LV mass, LV end-diastolic volume and EF.
Cell culture and treatment
Primary cultures of H9C2 rat cardiac myoblasts, purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China), were grown as monolayers at a density of 5 × 104 cells/cm in Dulbecco’s modified Eagle medium and incubated at 37 °C in humidified air containing 5% CO2. The medium contained 10% calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Two days after being seeded, the cultured H9C2 were randomly divided into the following seven groups: control (low glucose, 5.5 mM), HG (40 mM)+palmitate (Pal, 200 μM), HG+Pal+NaHS (100 μM), HG+Pal+NaSH+PPG (10 nM, an irreversible competitive CSE inhibitor), HG+Pal+NaSH+3MA (2 mM, an inhibitor of autophagy), HG+Pal+NaHS+DTT (20 μM, an inhibitor of disulphide bond), HG+Pal+Mito-TEMPO (2 μM, an inhibitor of mitochondrial ROS), HG+Pal+NAC (100 μM, an inhibitor of ROS) and HG+Pal+PYR41 (3 μM, an inhibitor of ubiquitin-activating enzyme (E1)). Drugs were added directly to the culture for 48 h. H9C2 cells treated with HG and palmitate classically mimic the myocardiocytes in hyperglycaemia and hyperlipidaemia.54
Detection of H2S in frozen sections of mouse heart using H2S probe C-7Az
The turn-on fluorescence response of H2S in RAECs was tested C-7Az, which has been demonstrated to selectively respond to H2S. The frozen sections of mouse hearts were incubated with 50 μM C-7Az PBS for 30 min, followed by washing with PBS. Visualization of the turn-on fluorescence response of C-7Az to H2S in the frozen sections of mouse hearts was carried out using confocal laser scanning with the excitation of a 720 nm laser. These results confirmed that excitation fluorescence imaging could be used to detect H2S through the triggered fluorescence response of C-7Az.
Mitochondrial ROS and cellular ROS level analysis
Mitochondrial ROS production was measured using Mito-SOX Red mitochondrial superoxide indicator (Invitrogen). The frozen sections of mice hearts and H9C2 cells were loaded with 5 μM Mito-SOX Red at 37 °C for 15 min. Red fluorescence was measured at 583 nm following excitation at 488 nm using a Zeiss LSM 510 inverted confocal microscope (Heidenheim, Germany). Intracellular ROS levels were examined using the DCFH-DA staining method based on the conversion of non-fluorescent DCFH-DA to the highly fluorescent DCF upon intracellular oxidation by ROS. The frozen sections of mice hearts and H9C2 cells were seeded on coverslips and incubated (45 min, 37 °C, in the dark) in serum-free media containing DCFH-DA (10 μM) in the presence of control, HG and NaHS. After incubation, the conversion of DCFH-DA to the fluorescent product DCF was measured using a spectrofluorometer with excitation at 484 nm and emission at 530 nm. Background fluorescence (conversion of DCFH-DA in the absence of cells) was corrected by the inclusion of parallel blanks.
Total protein extraction from diabetic mouse hearts and H9C2 cells and western blot analysis
Diabetic mouse hearts and H9C2 cells were homogenized in 0.5 ml of RIPA buffer before being transferred into small tubes and rotated for 1 h at 4 °C. Solubilized proteins were collected after centrifugation at 3000 × g for 30 min. The supernatant was collected and stored at −80 °C. The protein concentration of each sample was quantified using the BCA Protein Assay kit (Beyotime, Shanghai, China). Protein lysates from each group of cells and tissues via electrophoresis were separated by SDS-PAGE and electrotransferred onto a PVDF membrane (Millipore). Polyacrylamide gels (12%) were used for protein testing. The nonspecific proteins on membranes were blocked with 5% non-fat dried milk for 2 h at room temperature. Membranes were incubated with 2 μg/ml primary antibodies overnight at 4 °C. Membranes were washed and then incubated with anti-mouse/anti-rabbit IgG antibody at a 1:5000 dilution for 1 h at room temperature. The specific complex was visualized using ECL plus western blot detection system. The relative intensities of protein bands were quantified using a Bio-Rad Chemi EQ densitometer and Bio-Rad Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
MDC assay for visualization of autophagic vacuoles
The frozen sections of mouse hearts and H9C2 cells were incubated with 50 μM MDC in PBS at 37 °C for 30 min. Autophagic vacuoles were analysed using confocal laser scanning and fluorescence microscopy (Olympus, XSZ-D2, Tokyo, Japan).
Immunofluorescence assays
For immunofluorescent staining with anti-ubiquitin antibody, the H9C2 cells were fixed in 4% paraformaldehyde for 30 min and then permeabilized with 0.5% Triton X-100 for 30 min. Coverslips were blocked with goat serum and incubated for 1 h at 37 °C. Cells were incubated with anti-ubiquitin antibody at 4 °C overnight and washed three times with PBS, followed by incubation for 1 h with anti-rabbit IgG. Analysis and photomicrography were carried out with fluorescence microscopy.
Immunoprecipitation
Next, 1 ml samples (2 μg/μl) were incubated with IgG conjugated agarose beads (50 μl) and 2 μl of anti-ubiquitin antibodies (following the manufacturer’s instructions) and rotating overnight at 4 °C. The IP mixture was centrifuged at 1000 r.p.m. for 30 s at 4 °C, and the supernatant was discarded. The beads were washed 3–4 times with 1 ml of RIPA lysis buffer 3 times. Then, 25 μl of 5 × SDS sample buffer was added to the elutions, and the solution was heated at 95 °C for 5 min. The samples were centrifuged at 10 000 × g for 3 min. The supernatants were loaded onto an SDS-PAGE gel, and the supernatant was carefully transferred to a fresh, well-labelled microfuge tube and stored at −80 °C for later use. IPs was separated by SDS-PAGE, and proteins were transferred to PVDF membrane. Samples were probed with appropriate antibodies (anti-Keap-1, anti-LC3, anti-CAT and anti-SOD) for analysis.
siRNA transfection
H9C2 cells (80% confluent) were treated according to the manufacturer’s instructions with Keap-1 siRNAs (mouse; Santa Cruz Biotechnology) for 72 h to inhibit Keap-1 expression. Transfection of H9C2 cells by siRNA was achieved using Lipofectamine 2000 (Invitrogen). In brief, Keap-1 siRNA with the transfection reagent was incubated for 20 min to form complexes, which then were added to plates containing cells and medium. The cells were incubated at 37 °C in a CO2 incubator for further analysis.
Statistical analysis
Data were presented as mean±S.D. Data were first analysed using one-way ANOVA test. Tukey test was used for post hoc comparisons. The threshold of P<0.05 was designated as statistically significant for all tests. All statistical analyses were performed using Prism 5 (GraphPad, La Jolla, CA, USA).
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81370421, 81370330 and 81670344).
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by B Zhivotovsky
The authors declare no conflict of interest.
Supplementary Material
References
- Dokken B. Mechanisms of cardiovascular injury in type 2 diabetes and potential effects of dipeptidyl peptidase-4 inhibition. J Cardiovasc Nurs 2016; 31: 274–283. [DOI] [PubMed] [Google Scholar]
- From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med 2006; 119: 591–599. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006; 75: 333–366. [DOI] [PubMed] [Google Scholar]
- Sato A, Asano T, Isono M, Ito K. Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells. BMC Urol 2014; 14: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med 2011; 51: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Autophagy: a sweet process in diabetes. Cell Metab 2008; 8: 275–276. [DOI] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB. Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy. Antioxid Redox Signal 2015; 22: 1527–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res 2015; 116: 1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann M, Dodson M, Boyer-Guittaut M, Darley-Usmar V, Zhang J. Mitophagy mechanisms and role in human diseases. Int J Biochem Cell Biol 2014; 53: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Bonfili L, Cuccioloni M, Mozzicafreddo M, Rossi G, Buizza L et al. Crosstalk between the ubiquitin-proteasome system and autophagy in a human cellular model of Alzheimer's disease. Biochim Biophys Acta 2012; 1822: 1741–1751. [DOI] [PubMed] [Google Scholar]
- Costes S, Gurlo T, Rivera JF, Butler PC. UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in beta-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy. Autophagy 2014; 10: 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide-mediated cytoprotection. Antioxid Redox Signal 2010; 12: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov D. An outlook on vascular hydrogen sulphide effects, signalling, and therapeutic potential. Arch Physiol Biochem 2013; 119: 189–194. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide and polysulfides as biological mediators. Molecules 2014; 19: 16146–16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin Sci (Lond) 2015; 128: 325–335. [DOI] [PubMed] [Google Scholar]
- Fan W, Tang Z, Chen D, Moughon D, Ding X, Chen S et al. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy 2010; 6: 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Dong S, Li T, Yang F, Yu X, Wu J et al. Exogenous hydrogen sulfide attenuates cardiac fibrosis through reactive oxygen species signal pathways in experimental diabetes mellitus models. Cell Physiol Biochem 2015; 36: 917–929. [DOI] [PubMed] [Google Scholar]
- Zhong X, Wang L, Wang Y, Dong S, Leng X, Jia J et al. Exogenous hydrogen sulfide attenuates diabetic myocardial injury through cardiac mitochondrial protection. Mol Cell Biochem 2012; 371: 187–198. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Huynh K, Qin C, Rosli S, Kiriazis H, Ayer A et al. Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110alpha) signaling. Free Radic Biol Med 2015; 87: 137–147. [DOI] [PubMed] [Google Scholar]
- Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid Redox Signal 2013; 19: 465–481. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 2013; 18: 1906–1919. [DOI] [PubMed] [Google Scholar]
- Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y et al. Hydrogen sulfide induces Keap1 S-sulfhydration and Suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 2016; 65: 3171–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Wang X. Autophagy and the ubiquitin-proteasome system in cardiac dysfunction. Panminerva Med 2010; 52: 9–25. [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Zhang Y, Jin S. Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy 2010; 6: 179–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, Magnaudeix A, Yardin C, Terro F. Autophagy dysfunction and its link to Alzheimer's disease and type II diabetes mellitus. CNS Neurol Disord Drug Targets 2014; 13: 226–246. [DOI] [PubMed] [Google Scholar]
- Yoshida GJ. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J Hematol Oncol 2017; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 2003; 107: 448–454. [DOI] [PubMed] [Google Scholar]
- Kindermann M. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2686 (author reply 2686-2687). [DOI] [PubMed] [Google Scholar]
- Szabo C. Gaseotransmitters: new frontiers for translational science. Sci Transl Med 2010; 2: 59ps54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan A, Liao X, Mo L, Yang C, Yang Z, Wang X et al. Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells. PLoS ONE 2011; 6: e25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang Z, Zhang M, Dong Q, Wang X, Lan A et al. Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-kappaB/COX-2 pathway. PLoS ONE 2011; 6: e21971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zoghbi JF. Editorial focus: oxygen sensors and mediators of the contractile responses of smooth muscle to hypoxia. Focus on: "Hydrogen sulfide mediates hypoxic vasoconstriction through a production of mitochondrial ROS in trout gills". Am J Physiol Regul Integr Comp Physiol 2012; 303: R485–R486. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pei Y, Wang H, Jin Z, Liu Z, Qiao Z et al. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxid Med Cell Longev 2015; 2015: 804603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, Niki I. Significance of hydrogen sulfide production in the pancreatic beta-cell. J Pharmacol Sci 2011; 116: 1–5. [DOI] [PubMed] [Google Scholar]
- Sun WH, Liu F, Chen Y, Zhu YC. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun 2012; 421: 164–169. [DOI] [PubMed] [Google Scholar]
- Tai H, Wang Z, Gong H, Han X, Zhou J, Wang X et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy 2017; 13: 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakka VP, Prakash-babu P, Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol 2016; 53: 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal 2015; 22: 1308–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Gao X, Deeb D, Arbab AS, Gautam SC. Pristimerin induces apoptosis in prostate cancer cells by down-regulating Bcl-2 through ROS-dependent ubiquitin-proteasomal degradation pathway. J Carcinog Mutagen 2013. (suppl 6): 005. [DOI] [PMC free article] [PubMed]
- Dimkovikj A, Fisher B, Hutchison K, Van Hoewyk D. Stuck between a ROS and a hard place: analysis of the ubiquitin proteasome pathway in selenocysteine treated Brassica napus reveals different toxicities during selenium assimilation. J Plant Physiol 2015; 181: 50–54. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Shen W, Shen DM. The changes of ROS and mitochondria membrane potential in HepG2 cells on the pressure of cisplatin. Zhonghua Gan Zang Bing Za Zhi 2005; 13: 531–533. [PubMed] [Google Scholar]
- Xie Z, He C, Zou MH. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 2011; 7: 1254–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ding WY, Wang ZH, Tang MX, Wang F, Li Y et al. Early administration of trimetazidine attenuates diabetic cardiomyopathy in rats by alleviating fibrosis, reducing apoptosis and enhancing autophagy. J Transl Med 2016; 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Wang F, Chen K, Shen M, Dai W, Xu L et al. Hydrogen sulfide ameliorates ischemia/reperfusion-induced hepatitis by inhibiting apoptosis and autophagy pathways. Mediators Inflamm 2014; 2014: 935251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Dai H, Zhuang B, Chai L, Xie Y, Li Y. Exogenous hydrogen sulfide promotes cell proliferation and differentiation by modulating autophagy in human keratinocytes. Biochem Biophys Res Commun 2016; 472: 437–443. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 2014; 10: 2208–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Livneh I, Avni N, Cohen-Rosenzweig C, Ciechanover A. The ubiquitin-proteasome system and autophagy: coordinated and independent activities. Int J Biochem Cell Biol 2016; 79: 403–418. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang X. The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity. Biochim Biophys Acta 2015; 1852: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 2003; 35: 238–245. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 2009; 105: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S et al. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 2013; 304: H1215–H1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S et al. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 2006; 316: 670–678. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008; 322: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang L, Ni R, Cao T, Zheng D, Xiong S et al. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta 2016; 1862: 2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.