Abstract
Lipoprotein (a) [Lp(a)], is an independent risk factor for atherosclerotic cardiovascular disease in patients on chronic hemodialysis. A low concentration of high density lipoprotein cholesterol (HDL-C) and serum albumín are another potential risk factors. The purpose of this study was to explore in patients on chronic hemodialysis, whether Lp(a) elevated levels are influenced by activated acute phase response (APR) and the correlation of Lp(a) with HDL-C and serum albumin. In 69 hemodialysis patients with C-reactive protein (CRP) levels over than 10 mg/L and 101 hemodialysis patients with CRP levels in the normal range, Lp(a), HDL-C and serum albumin were determined in relation to CRP, as a sensitive marker of an APR. Results showed that serum concentration of CRP in 69 hemodialysis patients was significantly higher than in controls (44,62 mg/L versus 8,75 mg/L, p<0,01). Patients with elevated CRP had significantly higher serum levels of Lp(a) and lower serum levels of HDL-C and albumin, than patients with CRP in the normal range (35,39 mg/dl versus 28,6 mg/dl, p<0,01, 0,91 mmol/L versus 1,29 mmol/L, p<0,01 and 33,56 g/L versus 35,86 g/L, p<0,01). Lp(a) levels correlated positively with CRP and negatively with HDL-C and serum albumin, in patients with elevated CRP, but not in healthy controls. According to the results Lp(a) reacts as an acute phase protein, in patients with APR.
Keywords: CRP, acute phase reactant, Lp(a), HDL-C, serum albumin
INTRODUCTION
The morbidity and mortality of cardiovascular disease are substantially higher among hemodialysis patients, than in the general population (1). Atherosclerotic cardiovascular disease accounts for approximately half of deaths in end-stage renal disease and contributes to the extraordinarily high total annual mortality of 23% observed in such patients (2). Lipoprotein(a)[Lp(a)], is an independent risk factor for clinical events attributed to atherosclerotic cardiovascular disease in patients receiving chronic hemodialysis treatment of end-stage renal disease. Lp(a) is a low density lipoprotein (LDL)- like lipoprotein containing a unique apolipoprotein called apo(a). Serum levels of Lp(a) are determined largely by genetic variation in the gene encoding for apo(a). Apo(a) is very homologous to plasminogen (3) and exhibits an extreme size polymorphism with the apo(a) isoproteins, ranging in size from 420 to 840 kDa. Inherited in an autosomal co dominant fashion, the apo(a) isoprotein is closely correlated with serum Lp(a) concentrations, with an inverse correlation between the size of the apo(a) isoprotein and the serum Lp(a) concentrations. Lp(a) has been implicated in the regulation of plasminogen activator inhibitor-1 expression in endothelial cells and shown to inhibit endothelial cell surface fibrinolysis to attenuate plasminogen binding to platelets and to bind to plaque matrix components (4, 5). Autopsy studies in humans have documented the presence of Lp(a) in aortic and coronary atherosclerotic plaques and an apparent co localization with fibrin(ogen). High plasma concentrations of Lp(a) are considered a major risk factor for atherosclerosis and cardiovascular disease (6, 7). Lp(a) levels are frequently elevated in patients receiving chronic hemodialysis treatment of end-stage renal disease (8, 9). Elevated plasma Lp(a) levels in chronic renal failure patients, have been associated with a frequency distribution of apolipoprotein (a) - Lp(a) isoforms, similar to those found in general population. This indicates that elevated Lp(a) levels in these patients are not due to the genetic origin (10). It has been suggested that kidneys have an important role in Lp(a) metabolism. There is decrease in Lp(a) catabolism or increase in Lp(a) production by liver (11). Although it has not been fully explained, high Lp(a) levels in hemodialysis patients may also be due to activated acute phase reactants (12). Approximately 50% of patients on hemodialysis, have evidence of chronic inflammation due to uremia and dialysis. The uremic state is associated with an altered immune response (13, 14, 15) and intermittent stimulation by endotoxins originating from the dialysis water supply and artificial vein grafts or bioincompatibility caused the increased circulating inflammatory proteins, such as plasma CRP (16, 17, 18), a sensitive marker of an APR. CRP is the prototypical acute phase response protein, produced by the liver under the control of various proinflammatory cytokines, namely interleukin-6 (IL-6), interleukin-1 (IL-1) and TNF-α Its uniqueness is due to rapid (within 6 hours) and dramatic increases (up to 1000-fold) in circulating concentrations after a cytokine-mediated response to inflammation. Chronic renal failure results in profound lipid disorders. Decreased HDL-C is one of the main metabolic abnormalities of the lipoprotein profile in chronically uremic patients. Several mechanisms, working in concert, may underlie the reduction in HDL (high density lipoproteins) levels, which is usually indicative of impaired reverse cholesterol transport. Specifically, maturation of HDL is impaired and its composition is altered. Thus, uremic patients usually exhibit decreased levels of apolipoproteins AI and AII (the main protein constituents of HDL), diminished activity of lecithincholesterol acyl-transferase, the enzyme responsible for the esterification of free cholesterol in HDL particles, as well as increased activity of cholesteryl ester transfer protein that facilitates the transfer of cholesterol esters from HDL to triglyceride-rich lipoproteins, thus reducing the serum concentration of high density lipoprotein cholesterol (19). Today the interest on inflammatory biomarkers predicting the risk of clinical event increases (20). Main function of acute phase proteins is expressed by remodeling of HDL creating dysfunction and reduced serum concentration of HDL-C as a result (21, 22). Previous longitudinal studies showed that hypoalbuminemia was associated with cardiovascular disease. Richard and colleagues (23) first reported this association in the general population. They did serum electrophoresis in 7434 middle-aged men free of an atherosclerotic disease at entry, and they monitored the development of cardiovascular disease, during 6,6 years on average. Albumin level was significantly lower among myocardial infarction or sudden death. In hemodialysis patients, hypoalbuminemia is also known to be associated with cardiovascular disease (24, 25) and is a powerful risk factor for cardiovascular mortality. Since albumin is a negative acute-phase reactant, non-nutritional factors like inflammation depress albumin synthesis in hemodialysis patients. Inflammation causes a decrease in albumin synthesis and an increase in albumin fractional rate, providing two mechanisms for hypoalbuminemia (26). Some studies have demonstrated a significant inverse relationship between serum Lp(a) and albumin concentration in hemodialysis patients (27, 28). We therefore hypothesize that an activated acute phase response is responsible for the atherogenic condition in hemodialysis patients and besides Lp(a) we decided to determines serum HDL and albumin, starting from their antiaterogenic effects. The aim of this study was to explore, in patients on chronic hemodialysis treatment, whether the changes in Lp (a) is influenced by activated acute phase reaction and to study the relationship of Lp(a) with CRP, HDL-C and serum albumin.
MATERIALS AND METHODS
In this study were examined 170 blood samples of patients with chronic renal failure (age range from 24 to 62 years) who were treated by hemodialysis in the Clinic of Internal Diseases in Clinical Centre in Prishtina, more than 6 months, which is considered as chronic hemodialysis. In the samples initially were determined CRP and sedimentation rate by two measurements, in a period of 1-3 month, to be sure that CRP levels were not occasionally elevated, but remain elevated over time, as a chronic inflammation. Than the patients were divided in two groups: group of 69 patients with elevated CRP levels over than 10 mg/L and sedimentation rate of over 50mm/h, in both blood samples and group of 101 patients with CRP levels in the normal range. For the first group, based in patients history, angina, possible myocardial infraction, cerebrovascular events were excluded and the concentrations of the second measurement of CRP, were taken in consider. A part of CRP, among all the patients, serum levels of Lp (a), HDL-C and albumin were determined. 50 healthy people, were included as a control group. Measurements of serum CRP, Lp(a) and albumin were performed on fresh samples. The serum concentration of CRP was measured by the turbidimetric method based in combines of CRP with specific antibody to form insoluble antigen antibody complexes. Diazyme’s Lipoprotein (a) assay is based on a latex enhanced immunoturbidimetric method. The normal range for CRP is less than 10 mg/L, and the range for Lp(a) is less than 30mg/dl. HDL-C was measured directly with detergent which solubilizes the HDL lipoprotein particles, releasing HDLcholesterol. Measurement of serum albumin was carried out on serum using the bromocresol green method.
Statistical analysis
The data were analysed using the descriptive statistics for each biochemical parameter that was followed. Statistically significant differences were analysed using the student’s t-test, with the acceptance of statistica! significance at the level p<0,01. Relationships and correlations between biochemical parameters were analysed using Pearson Chi-square(χ2) test.
RESULTS
Mean serum CRP concentration in 69 hemodialysis patients was significantly higher than in controls (44,62 ±18,47 mg/L versus 8,75 ± 4,82, respectively p<0,01; Table 1.). Mean value of Lp(a) was significantly higher in hemodialysis patients than in healthy controls (31,37^mg/ dl versus 19,64 mg/dl, respectively p<0,01; Table 2.). Result showed that mean value of Lp(a) was significantly higher in patients exhibiting elevated CRP than to those patients with CRP levels in the normal range (35,39 mg/L versus 28,6 mg/L; p<0,01; Table 3.). HDL-C levels were significantly lower in hemodialysis patients than in healthy controls, as well as serum albumin (1,14 mmol/L versus 1,35 mmol/L; p< 0,01 and 34,92g/L versus 39,67 g/L; p< 0,01; Table 2.) Patients with elevated CRP had significantly lower serum levels of HDL-C and albumin, in compare with patients with CRP in the normal range (0,91 mmol/L versus 1,29 mmol/L; p<0,01 and 33,56 g/L versus 35,86 g/L; p<0,01; Table 3.).
TABLE 1.
CRP values in hemodialysis patients with activated acute phase response and in healthy controls
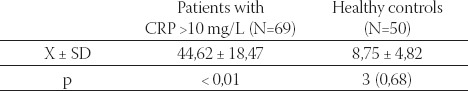
TABLE 2.
Biochemical parameters in all hemodialysis patients and in healthy controls
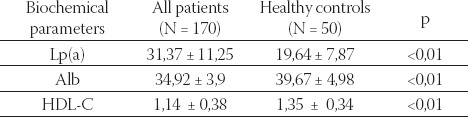
TABLE 3.
Lp(a), serum albumin and HDL-C in hemodialysis patients with normal and elevated levels of CRP

Data are given as mean ± SD. Lp(a) - lipoprotein (a), albalbumin, HDL-C - high density lipoprotein cholesterol Data are given as mean ± SD. Lp(a) -lipoprotein (a), albalbumin, HDL-C - high density lipoprotein cholesterol CRP, in group of patients with levels over than 10 mg/L, correlated positively with Lp(a) (R= + 0,58; p< 0,01; Table 4. and Figure 1.) and negatively with HDL-C(R= -88; p< 0,01;Table 5. and Figure 2.) and serum albumin (R= -87; p< 0,01; Table 7. and Figure 4.). There was no significant correlation of CRP levels with Lp(a), HDL-C and serum albumin in healthy controls. In group of patients with elevated CRP, Lp(a) levels correlated negatively with HDL-C (R= - 0,53; p< 0,01; Table 6. and Figure 3.) and serum albumin (R= -0,57; p< 0,01; Table 8. and Figure 5.) Correlation coefficient was not significant in healthy controls.
TABLE 4.
Correlation of CRP and Lp(a) in hemodialysis patients with elevated CRP levels and in healthy controls
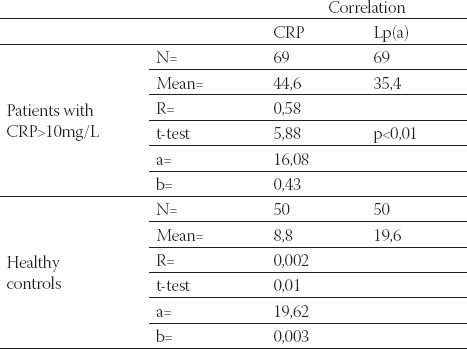
FIGURE 1.
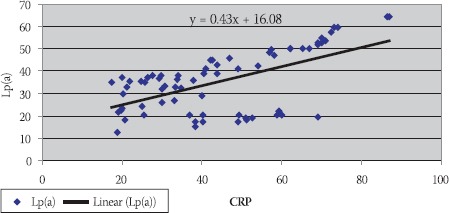
Positive correlation of CRP and Lp(a) in hemodialy-sis patients with elevated CRP levels
TABLE 5.
Correlation of CRP and HDL-C in hemodialysis patients with elevated CRP levels and in healthy controls
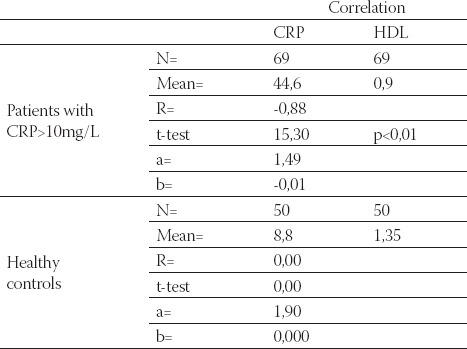
FIGURE 2.
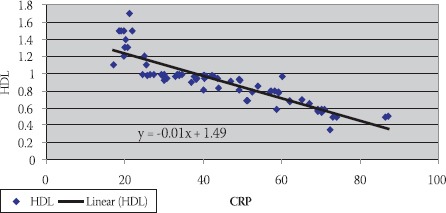
Negative correlation of CRP and HDL-C in hemodialysis patients with elevated CRP levels
TABLE 6.
Correlation of Lp(a) and HDL-C in hemodialysis patients with elevated CRP levels and in healthy controls
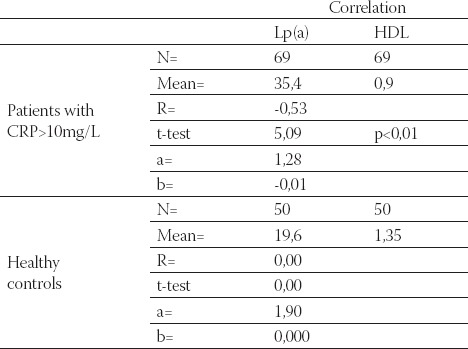
TABLE 7.
Correlation of CRP and serum albumin in hemodialysis patients with elevated CRP levels and in healthy controls
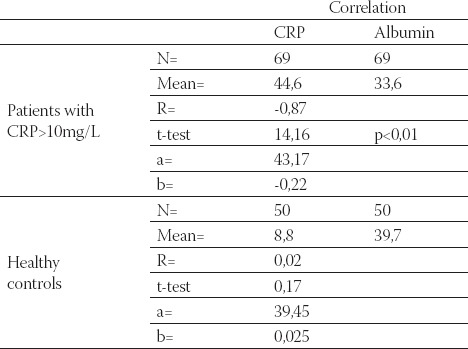
FIGURE 3.
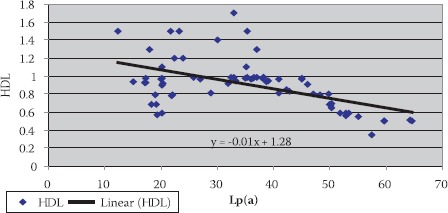
Negative correlation of Lp(a) and HDL-C in hemodialysis patients with elevated CRP levels
FIGURE 4.
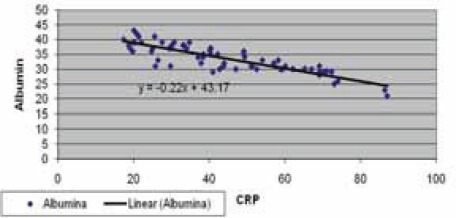
Negative correlation of CRP and serum albumin in hemodialysis patients with elevated CRP levels
TABLE 8.
Correlation of Lp(a) and serum albumin in hemodialysis patients with elevated CRP levels and in healthy controls
FIGURE 5.
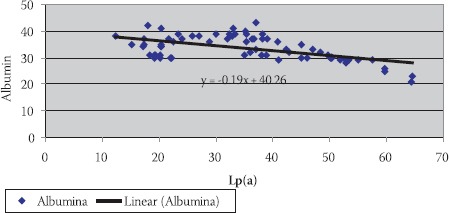
Negative correlation of Lp(a) and serum albumin in hemodialysis patients with elevated CRP levels
DISCUSSION
During recent years, substantial evidence has accumulated suggesting that Lp(a) is another important risk factor for cardiovascular disease in the general population, as well as in dialysis patients. The mechanism of Lp (a) atherogenicity has not been elucidated, but likely involves both its ability to influence plasminogen activation as well as its atherogenic potential as a lipoprotein particle after receptor mediated uptake. Though the concentration of serum Lp(a) is mostly determined by genetic factors, secondary factors such as APR and end stage renal disease also contributes to its increase. Lp (a) levels are frequently elevated in patients receiving chronic hemodialysis treatment (8, 9). In this study mean serum Lp (a) value in hemodialysis patients was significantly higher, in compare with a control group (Table 2.), which confirm that kidney have an important role in Lp(a) metabolism (11). In addition, patients with end-stage renal disease, including dialysis patients, often have inflammation and it has been suggested that impaired renal function may influence the infiammatory response. According to the results considerable number of patients exhibited an activated acute phase response, characterized by an increase of CRP concentration (16, 17, 18). Mean serum CRP value, in 69 patients was significantly higher than in controls (Table 1.) respectively this group of patients, have evidence of chronic inflammation due to uremia and dialysis. Some studies have demonstrated a close relationship between high Lp(a) levels and the APR, as shown by correlations with CRP and IL-6 (29, 30).. Because seven IL-6-respon- sive element sequence motifs can be identified in the 5’ flanking regulatory region of the apo(a) gene on chromosome 6, it is likely that apo(a) responds as an acute phase reactant (31). Uremia can be considered to be a state of activated acute phase response and in the microinflammatory milieu, a number of atherogenic proteins like Lp (a) are elevated and a number of anti-atherogenic factors like HDL-C and serum albumin are diminished. In this study Lp(a) mean value was significantly higher in patients with elevated CRP than to those patients with CRP levels in the normal range (Table 3.). Based on results, APR exhibited higher serum levels of Lp(a), respectively Lp(a) reacts as an acute phase protein in patients with high CRP levels (30). For this reason Lp(a) levels correlated in the positive way with CRP (Figure 1.). It is well known that many hemodialysis patients have low levels of HDL, which is a crucial risk factor for atherosclerosis. Recently, Barter et al. (32) have demonstrated that the serum HDL level is a significant inverse predictor of subsequent major cardiovascular events. In end stage renal disease HDL-C is now emerging as a key entity in both determining risk and providing protection, although none as yet specifies HDL as a target for treatment (19). Inflammation is one of the powerful factors also contribute to its decreased levels (21, 22). Study results showed significantly lower levels of HDL-C in the group of patients with elevated CRP, in compare with the patients with CRP in the normal range (Table 3.), and negative correlation of HDL and CRP (Figure 2.). These results indicate that the inflammatory condition may be responsible for low HDL-C. While serum Lp(a) levels showed a positive correlation with CRP, with HDL-C correlated negatively (Figure 3.). Because of the characteristics of an acute phase reactant, it is meaningful that Lp(a) levels correlated negatively with HDL-C. This correlation is also presented in the published results (33). Hypoalbuminemia is known to be strongly associated with ischemic heart disease in dialysis patients and it is thought to be one of the cardiovascular risk factors (24, 25). Inflammation is an important factor which has an influence on albumin levels (34). Albumin concentration in hemodialysis patients changes with inflammation through its effects on albumin catabolism and synthesis, respectively. According to the results there was the significant difference in albumin levels between a group of the patients with elevated CRP and those patients with CRP values in the normal range. (Table 3.) Albumin levels correlated in the negative way with CRP (Figure 4.), which proved that hypoalbuminemia in hemodialysis patients is partially a consequence of inflammation. Different studies have reported, that by increasing serum albumin levels, in renal failure, serum Lp(a) levels were decreased (35). Based on our results a significant inverse relationship existed between serum albumin and Lp(a) in hemodialysis patients, respectively serum albumin levels correlated in the negative way with Lp(a) in patients with elevated CRP(Figure 5.). According to the literature it is a significant indicator for cardiovascular death of hemodialysis patients (36).
CONCLUSION
According to the study results, end stage renal disease contributes to Lp (a) increases. Changes of the atherogenic risk profile, in hemodialysis patients, namely elevated Lp (a), as well as decreased HDL-C and serum albumin, are partially the consequence of an activated APR. The results further show that Lp (a) reacts as an acute phase reactant in hemodialysis patients with high CRP levels. For this reason Lp (a) correlated positively with CRP and negatively with HDL-C and serum albumin. It is suggested that these correlations might show high cardiovascular risk.
List of Abbreviations
CRP - C-reactive protein
APR - acute phase response
Lp(a) - Lipoprotein (a)
HDL - High density lipoprotein
HDL-C - High density lipoprotein cholesterol
alb. - serum albumin
REFERENCES
- 1.Ronco C, Bowry S, Tetta C. Dialysis Patients and Cardiovascular Problems: Can Technology Help Solve the Complex Equation? Blood Purification. 2006;24(1):39–45. doi: 10.1159/000089435. [DOI] [PubMed] [Google Scholar]
- 2.Longenecker JC, Coresh J, Powe NR, et al. Traditional Cardiovascular Disease Risk Factors in Dialysis Patients Compared with the General Population: The CHOICE Study. J. Am. Soc. Nephrol. 2002;13:1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 3.Hoover-Plow J. Elusive proatherothrombotic role of Lp(a): a new direction? J. Thromb. Haemost. 2006;4(5):971–972. doi: 10.1111/j.1538-7836.2006.01946.x. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989;339(6222):303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- 5.Argraves KM, Kozarsky KF, Fallon JT, Harpel PC, Strickland DK. The atherogenic lipoprotein Lp(a) is internalized and degraded in a process mediated by the VLDL receptor. J. Clin. Invest. 1997;100(9):2170–2181. doi: 10.1172/JCI119753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlén GH, Stenlund H. Lp(a) lipoprotein is a major risk factor for cardiovascular disease: pathogenic mechanisms and clinical significance. Clin. Genet. 1997;52(5):272–280. doi: 10.1111/j.1399-0004.1997.tb04344.x. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DT, Schick BA, Humphries KH, Frohlich J. Lipoprotein(a) Is an Independent Risk Factor for Cardiovascular Disease in Heterozygous Familiar Hypercholesterolemia. Clin. Chem. 2005;51:2067–2073. doi: 10.1373/clinchem.2005.055228. [DOI] [PubMed] [Google Scholar]
- 8.Quashing T, Krane V, Metzger T, Warner C. Abnormalities in Uremia lipoprotein metabolism and its impact on Cardiovascular disease. Am. J. Kid. Dis. 2001;38(4):514–519. doi: 10.1053/ajkd.2001.27384. [DOI] [PubMed] [Google Scholar]
- 9.Longenecker JC, Klag MJ, Marcovina SM, et al. High Lipoprotein(a) Levels and Small Apolipoprotein(a) Size Prospectively Predict Cardiovascular Events in Dialysis Patients. Am. Soc. Nephrol. 2005;16:1794–1802. doi: 10.1681/ASN.2004110922. [DOI] [PubMed] [Google Scholar]
- 10.Greiber S, Wanner C. Lipoprotein (a) in nephrotic syndrome and end stage disease. Miner. Electrol. Metab. 1997;23:161–165. [PubMed] [Google Scholar]
- 11.Dieplinger H, Lacner C, Kronenberg F, et al. Elevated plasma concentrations of lipoprotein (a) in patients with end stage renal disease are not related to the size polymorphism of apolipoprotein (a) J. Clin. Ivest. 1993;91:397–401. doi: 10.1172/JCI116213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riella M. Malnutrition in Dialysis: Malnourishment or uremic inflammatory response. Kidney Int. 2000;57:1211–1233. doi: 10.1038/sj.ki.4491447. [DOI] [PubMed] [Google Scholar]
- 13.Daichou Y, Kurashige S, Hashimoto S. Characteristic Cytokine Products of Th1 and Th2 Cells in Haemodialysis Patients. Nephron. 1999;83:237–245. doi: 10.1159/000045516. [DOI] [PubMed] [Google Scholar]
- 14.Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia Kidney Int. Suppl. 1997;62:S79–S82. [PubMed] [Google Scholar]
- 15.Sester U, Sester M, Heine G. Strong depletion of CD14+CD16+monocytes during haemodialysis treatment. Nephrol. Dial. Transplant. 2001;16:1402–1408. doi: 10.1093/ndt/16.7.1402. [DOI] [PubMed] [Google Scholar]
- 16.Panichi V, Migliori M, De Pietro S, et al. The link of biocompatibility to cytokine production. Kidney Int. 2000;76:S96–S103. doi: 10.1046/j.1523-1755.2000.07612.x. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Garcia R, Rodriguez-Benitez P. Why and how to monitor bacterial contamination of dialysate? Nephrol. Dial. Transplant. 2000;15:760–764. doi: 10.1093/ndt/15.6.760. [DOI] [PubMed] [Google Scholar]
- 18.Zaoui P, Hakim RM. The effects of the dialysis membrane on cytokine release. J. Am. Soc. Nephrol. 1994;4:1711–1718. doi: 10.1681/ASN.V491711. [DOI] [PubMed] [Google Scholar]
- 19.Blaton V. Dyslipidemia at chronic renal failure. New trends in classification, diagnosis and management of kidney diseases. Dubrovnik, Croatia: The 8th EFCC Continuous Postgraduate Course in Clinical Chemistry; 2008. pp. 54–60. [Google Scholar]
- 20.Kleemann R, Zadelaar S, Kloostra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc. Res. 2008;79:340–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker P, Hennekens Ch, Buring J, Rifal N. C-Reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;343(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 22.Van Lenten B, Reddy S, Navah M, Fogelman A. Understanding changes in high density lipoproteins during the acute phase response. Arterioscler. Tromb. Vasc. Biology. 2006;26:1687–1688. doi: 10.1161/01.ATV.0000232522.47018.a6. [DOI] [PubMed] [Google Scholar]
- 23.Richard JL, Warnet JM, Ducimetiere P. Decreased levels of the albumin-alpha-l-globulin fraction of serum proteins, risk factor for ischemic cardiopathy. Rev. Epidemiol. Sante Publique. 1985;33:347–351. [PubMed] [Google Scholar]
- 24.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity and mortality in end-stage renal disease. J. Am. Soc. Nephrol. 1996;7:728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 25.Danielski M, Ikizler A, McMonagle E, Conner Kane J, Pupim L, Morrow J, et al. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am. J. Kidney Dis. 2003;42(2):286–294. doi: 10.1016/s0272-6386(03)00653-x. [DOI] [PubMed] [Google Scholar]
- 26.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin. Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang WS, Kim SB, Min WK, Park S, Lee MS, Park JS. Atherogenic lipid profile and lipoprotein(a) in relation to serum albumin in haemodialysis patients. Nephrol. Dial. Transplant. 1995;10:1668–1671. [PubMed] [Google Scholar]
- 28.Kim SB, Yang WS, Kang ES, Min WK, Park JS. Lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease. Perit. Dial. Int. 1997;17(3):236–242. [PubMed] [Google Scholar]
- 29.Kario K, Matsuo T, Kobayashi H, Matsuo M, Asada R, Koide M. High lipoprotein(a) levels in chronic hemodialysis patients are closely related to the acute phase reaction. Thromb. Haemostasis. 1995;74:1020–1025. [PubMed] [Google Scholar]
- 30.Yun KA, Lee W, Min WK, Chun S, Lee YW, Kim SB, et al. Discrepancy of interleukin-6 levels between end renal disease patients and patients with acute-phase response with increased lipoprotein(a)concentration. Scand. J. Clin. Lab. Invest. 2004;64(3):223–228. doi: 10.1080/00365510410005749. 6. [DOI] [PubMed] [Google Scholar]
- 31.Wade DP, Clarke JG, Lindahl GE, Liu AC, Zysow BR, Meer K, et al. Genetic influences on lipoprotein(a) concentration. Biochem. Soc. Trans. 1993;21:499–502. doi: 10.1042/bst0210499. [DOI] [PubMed] [Google Scholar]
- 32.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. Treating to New Targets Investigators: HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 33.Dursunoĝlu D, Evrengül H, Polat B, Tanriverdi H, Cobankara V, Kaftan A, et al. Lp(a) lipoprotein and lipids in patients with rheumatoid arthritis: serum levels and relationship to inflammation. Rheumatol. Int. 2005;25(4):341–245. doi: 10.1007/s00296-004-0438-0. [DOI] [PubMed] [Google Scholar]
- 34.Panichi V, Migliori M, De Pietro S, Taccola D, Bianchi AM, Giovannini L, et al. C-reactive protein and interleukin-6 levels are related to renal function in predialytic chronic renal failure. Nephron. 2002;91:594–600. doi: 10.1159/000065018. [DOI] [PubMed] [Google Scholar]
- 35.Yang W.S, Kim SB, Min WK, Park JS. Effect of increasing serum albumin on serum lipoprotein(a) concentration in patients on continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1997;30:507–513. doi: 10.1016/s0272-6386(97)90309-7. [DOI] [PubMed] [Google Scholar]
- 36.Koda Y, Nishi Sh I, Suzuki M, Hirasawa Y. Lipoprotein(a) is a predictor for cardiovascular mortality of hemodialysis patients. Kidney International. 1999;56:S251–S253. doi: 10.1046/j.1523-1755.1999.07167.x. [DOI] [PubMed] [Google Scholar]


