Abstract
Postoperative wound infections represent about 16% of hospital-acquired infections. Staphylococcus aureus is the most common cause of nosocomial wound infections. Increased frequency of Methicillin-re- sistant Staphylococcus aureus (MRSA) in hospitalized patients and possibility of vancomycin resistance requires permanent control of MRSA spread in the hospital.
The purpose of this study was to analyse the frequency of Methicillin-resistant Staphylococcus aureus (MRSA) in the swabs taken from the surgical wounds, the presence of MRSA infection in surgical departments and to examine antimicrobial susceptibility of MRSA isolates.
Wound swabs were examined from January 2006 to December 2008. The isolates were identified by conventional methods. Antimicrobial susceptibility testing was performed by Kirby-Bauer disc-diffusion method as per NCCLS guidelines.
A total of 5755 wound swabs were examined: 938 (16,3%) swabs were sterile and 4817 (83,7%) were positive. Staphylococcus aureus was isolated in 1050 (22,0%) swabs and it was the most common cause of wound infections. MRSA was isolated from 12,4% samples in 2006, from 6,7% samples in 2007 and from 3,7% samples during 2008. Wound infections caused by MRSA dominated in the department of plastic surgery (24,4%) and in the department of orthopaedic surgery (24,1%). Antimicrobial susceptibility testing showed that 73% of MRSA isolates were with the same antibiotic sensitivity pattern (antibiotyp)- sensitive only to vancomycin, tetracycline, fucid acid and trimethoprim/sulfamethoxasole.
Our results show decreasing of MRSA infection in the surgical wards. These results appear to be maintained with strategies for preventing nosocomial infection: permanent education, strong application of protocols and urging the implementation of strict infection control policy.
Keywords: MRSA, hospital infections, surgical wound
INTRODUCTION
Despite recent advances in the operative techniques and better understanding of the pathogenesis of the wound infections, postoperative wound infections continúes to be a major source of morbidity and mortality for patients undergoing operative procedures. Surgical site infections (SSIs), as they are called today, accounted for 16% of all hospital-acquired infections, making them the third most frequent type of nosocomial infections in developed countries. The rates were higher in developing countries, and vary from 7% to 40%. The average SSIs prolong the hospital stays by 7,3 days. These infections also contribute greatly to the economic costs of surgical procedures and the estimated range is 1,47-19,1 billion euro. This is a great problem, especially in resource poor countries (1, 2). Infection of surgical wound is considered as nosocomial infection if it occured within 30 days after surgery, or within one year in case of implant. Infection was defined as discharge of pus from the wound, or a clinical suspicion of wound infection, based on inflammatory signs such as raised temperature, redness and tenderness of the wound. Surgical wound infections are prolonging the duration of hospitalization, increase the risk of poor cicatrisation and increase morbidity and mortality. The most critical factors in the prevention of postoperative wound infections are sound judgement and proper technique of the surgeon and surgical team, as well as the general health and disease state of the patient. In order to minimize the postoperative wound infections, it is important to create a safe environment by controlling four main sources of infection i.e. personnel, equipment, the environment and patient’s risk factors (3). Diagnosis and treatment of these infections are very expensive and present an additional load to health insurance funds. It is very useful to explore the causes of these infections in order to timely detect and remove the causative agents (4, 5). Staphylococcus aureus has been recognized for a long time as one of the leading causes of hospital infections all over the world. It is the most common cause of hospital acquired wound infections. Most of its strains are opportunistic pathogens that can colonise individuals, without symptoms, for either short or extended period of time, causing disease when the immune system becomes compromised. Before the antibiotic era diseases caused by Staphy- lococcus aureus had high mortality rates. In 1941 the mortality rate of staphylococcal bacteraemia at the Boston City hospital was reported to be 82% (6). The introduction of benzyl penicillin into chemotherapy in the early 1940s found Staphylococcus aureus fully susceptible and several of the first successes of penicillin therapy were related in the cure of formerly untreatable staphylococcal diseases. But in 1950s, the number of Staphylococcus aureus clinical isolates with resistance to penicillin increased rapidly. The mechanism of penicillin resistance involved the acquisition of a plasmid-borne penicillinase capable of degrading the antibiotic before it reached its cellular targets. Methicillin, originally called celbenine, is a semisynthetic derivative of penicillin chemically modified to withstand the degradative action of penicillinase (7). The drug was introduced into the therapy in Europe in 1959 for combating against hospital strains of penicillinase-pro- ducing Staphylococcus aureus. However, resistance to methicillin was noted shortly thereafter. Methicillin-resistance in Staphylococcus aureus isolates first appeared in the 1960s. Today the major nosocomial pathogen worldwide is Methicillin-resistant Staphylo- coccus aureus (MRSA). Recent surveillance studies in hospitals in various parts of the world indicate a varying incidence of MRSA strains depending on the country and the hospital. In the USA, the National Nosocomial Infections Surveillance System (NNISS) recorded an increase of MRSA in large USA hospitals, from 4% in the 1980s to 50% in the late 1990s. In some hospitals the resistance frequencies as high as 80% have been recorded (8). Methicillin resistance arises following the acquisition of novel DNA, which results in production of a new penicillin-binding protein (PBP), known as PBP2’ or PBP2a, which has low binding affinity for methicillin and other currently available β-lactams. The development of antibiotic resistance by bacteria permanently increases and intermediate vancomycin or glicopeptide resistant Staphylococcus aureus (VISA or GISA) were first detected in Japan in 1997 (9), and subsequently in other countries (10, 11). In June 2002, the first clinical isolate of Staphylococcus aureus fully resistant to vancomycin (VRSA) was isolated in the USA (12). Despite intensive attempts at eradication during the last 20 years, MRSA continues to be the major nosocomial pathogen worldwide (13). The level of hospital MRSA infection is indicative of the overall infection rate of the institution and usually reflects higher concentrations of patients, overcrowding of wards and heavy nursing load. The major route of transmission of MRSA within institutions is from patient-to-patient via the hands of hospital health care workers. Infected and colonised patients are the major reservoirs of the hospital MRSA. Colonization of hospital patients is dependent on the length of hospital stay, nutritional status of the patient, recurrent or recent antibiotic treatment, presence of wound and/ or invasive devices. There is a high patient morbidity and mortality associated with surgical wound infection caused by hospital-acquired MRSA. Currently, most MRSA are hospital acquired and so this organism is a useful indicator of the effects of infection control per se. MRSA do not generally appear to be more virulent than sensitive strains but, because of their resistance patterns, they are more difficult to treat if infection occurs (14, 15). Increased frequency of Methicillin-resistant Staph- ylococcus aureus (MRSA) in hospitalized patients and possibility of vancomycin resistance requires rapid and reliable characterization of isolates and control of MRSA spread in the hospital. The purpose of this study was to analyse frequency of Methicillin-resistant Staphylococcus aureus (MRSA) in swabs taken from the surgical wounds, the presence of MRSA infection in the surgical wards during three years and to examine antimicrobial susceptibility testing of MRSA isolates.
MATERIAL AND METHODS
A total of 5755 swabs taken from surgical wounds were examined from January 2006 to December 2008. All laboratory testing was performed in the Institute for Clinical Microbiology, University of Sarajevo Clinics Centre. Demographic information (age, sex) were collected. The average age of patients was 33,5 years of age-ranging from 1 to 73 years. Gender structure of patients was: 72,2% males and 27,8% females. All swabs were treated following Protocol of work for Laboratory of bacteriology. Blood agar plates (containing Columbia blood agar base by Becton Dickinson and 5% of sheep blood) were used to facilitate the growth of fastidious microorganisms, particularly Gram-positive bacteria. Mac Conkey agar (Becton Dickinson) was used for selective isolation of Enterobacteriacae. These media are specially designed to distinguish lactose fermenting (pink to red) from non lactose-fermenting colonies (colourless or slightly beige). The plates were incubated overnight at 37°C in bacteriological incubators. All isolates were identified by conventional biochemical testing (carbohydrate fermentation patterns and activity of amino acid decarboxylases and other enzymes) according to the Medical Laboratory Manual (Microbiology 1993) and interpretative criteria recommended by the National Committee for Clinical Laboratory Standards (17). Antimicrobial susceptibility testing was performed by Kirby-Bauer disc-diffusion method on Mueller-Hinton agar by NCCLS (National Committee for Clinical Laboratory Standards). Staphylococcus aureus was reported as oxacillin resistant if disk diffusion zone for oxacillin was <10 mm. Antimicrobial susceptibility to oxacillin was confirmed by determining the minimal inhibitory concentration (MIC). MIC was tested with antibiotic gradient strips, E-test (AB Biodisk, Solna, Sweden). For the evaluation of the results, standard statistical methods were used. Statistical analysis was performed by using a Chi-square test. Statistical significance was defined for p<0,05. Test results are presented both graphically and in tabular form.’In our study we observed ethical principles outlined in the World Medical Association Declaration of Helsinki.
RESULTS
A total 5775 samples of wound swabs were examined: 4817 (84%) were positive and 938 (16%) were negative (Graph 1.)
GRAPH 1.

Review of examination samples
Table 1.shows the most common isolates from wound swabs: Staphylococcus aureus in 1050 (22,0%), Pseu- domonas aeruginosa in 428 (8,9%), Escherichia coli in 419 (8,7%), Proteus mirabilis in 313 (6,5%), Kleb- siella pneumoniae in 313 (6,5%), Acinetobacter baumanni in 293 (6,1%), Enterobacter cloacae in 284 (5,9%) and Serratia marscescens in 274 (5,7%).
TABLE 1.
The most common causes of wound infections

MRSA was isolated in 7,9% samples of wound swabs (Graph 2.).
GRAPH 2.

Percentage of MRSA in positive samples
Infections of surgical wound caused by MRSA dominated at the following surgical departments: plastic surgery 24,4%, orthopaedic surgery 24,1% and neurosurgery 12,8% (Graph 3.).
GRAPH 3.
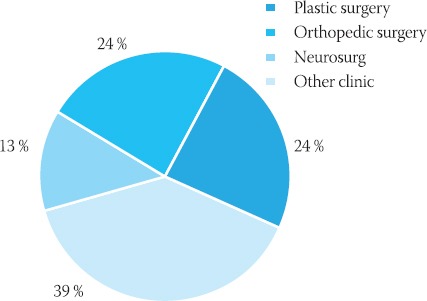
Review of MRSA positive samples in surgical departments
Antimicrobial susceptibility testing showed that Methicillin-resistant Staphylococcus aureus (MRSA) expressed high frequency of resistance to many antimicrobiales (Graph 4.).
GRAPH 4.

The antibiotic sensitivity pattern of MRSA
73% of MRSA isolates were with the same antibiotic sensitivity pattern (antibiotyp)-sen- sitive only to vancomycin, tetracycline, fucid acid and trimethoprim/sulphamethoxasole. We compare frequency of MRSA in wound infections during three years: 205 (12,4%) samples in 2006, 112 (6,7%) samples in 2007 and 64 (3,7%) samples in 2008. This is a statistically significant difference in comparison frequency of MRSA in wound infections in 2006 and in 2008 (p<0,05) (Graph 5, Graph 6).
GRAPH 5.

Percentage of MRSA in positive samples
GRAPH 6.

Decreasing of MRSA in wound infections during three years
χ2=10,605; p=0,001 (p<0,05) statistical significance
DISCUSSION
The Centre for Disease Control and Prevention (CDC) in Atlanta, USA, estimates that 2,7% of surgical procedures are complicated by infections. Surgical site infections (SSIs) represent 16% of all hospital-acquired infections, making them the third most frequent type of nosocomial infections in developed countries. These infections increase morbidity and mortality. It is very useful to explore the causes of these infections due to timely detection and removal of causative agents. Surveillance of surgical site infection is useful to demonstrate magnitude of the problem. Regular feedback of wound infection to the surgeon is very important (1, 3). Staphylococcus aureus is the most common cause of hospital acquired wound infections. Increased frequency of Methicillin-resistant Staphylococ- cus aureus (MRSA) in hospitalized patients and possibility of vancomycin resistance requires rapid and reliable characterization of isolates and control of MRSA spread in hospital. Despite intensive attempts at eradication during the last 20 years, MRSA continues to be the major nosocomial pathogen worldwide. This organism is a useful indicator of the effectiveness of infection control per se (18, 19). In our study we wanted to explore the most common causes of wound infections by examination of wound swabs. Especially, we wanted to analyse frequency of Methicillin-resistant Staphylococcus aureus (MRSA) as a causative agent of wound infections. We examined 5755 swabs taken from surgical wounds: 938 (16,3%) swabs were sterile and 4817 (83,7%) were positive. The most frequently isolated microorganisms were: Staphylococcus aureus 1050 (22,0%), Pseudomonas aeruginosa 428 (8,9%) and Esch- erichia coli 419 (8,7%). Cruse PJE found out the same results in five-year prospective study of 23 649 surgical wounds (20). Nurkić found out the same results in his study of 746 wound swabs conducted in 2005 (21). Methicillin-resistant Staphylococcus aureus (MRSA) was isolated in 7,9% samples. Staphylococ- cus aureus susceptibility data reported from different hospital departments in various parts of the world indicate a variety incidence of MRSA strains depending on the country and the hospital (13, 14). As we compared the percentages of MRSA positive samples in three years, we received important distinction: 205 (12,4%) samples in 2006, 112 (6,7%) samples in 2007 and 64 (3,7%) in 2008. Kalinić described in her report about resistence of bacteria to antibiotics that MRSA infection in Croatia was decreasing from 46,5% in 1997 to 17,8% in 1999 (14). Our results show important decreasing of MRSA wound infections. A few years ago MRSA presented the greatest problem in the surgical departments. There was a high patients morbidity and mortality associated with this kind of nosocomial infections. In order to reduce MRSA infection we started with control measures three years ago. It is important to ensure proper surveillance and monitoring system. We organised patients screening and microbiological surveillance. The screening swabs should be taken from the nose and throat, perineum, operative and wound sites, insertion site of intravascular catethers. Patients with negative culture only entered the departments for programmed operations. Infected patients were treated with antibiotics. Patients with positive culture but without symptoms of disease were decolonized with local mupirocin. With this preventive method the incidence decreased to 6,7% in 2007 and dropped to 3,7 % during 2008. This tendency persists nowadays also. Infections of surgical wounds caused by MRSA dominated at the surgical departments: plastic surgery 24,4%, orthopaedic surgery 24,1% and neurosurgery 12,8%. The strains from wound swabs were susceptible in vitro to vancomycin 100%, tetracycline 73%, fucid acid 80%, gentamycin 60%, trimethoprim/sulfamethoxasole 73%, erithromycin 26% and lincomycin 38%. Antimicrobial susceptibility testing showed that 73% of MRSA isolates were with the same antibiotic sensitivity pattern (antibiotyp)-sensitive only to vancomycin, tetracycline, fucid acid and trimethoprim/sulfamethoxasole. Possibility of vancomycin resistance requires rapid and reliable characterisation of isolates and control of MRSA spread in hospitals. In October 2007 enterococcal resistance to vancomycin was detected in Clinic for Orthopaedic surgery, University of Sarajevo Clinics Centre. Transferable glycopeptide resistance was first reported in 1987 and in 1988 with recognition of VanA enterococci (22). Since that time, six different resistance types have been defined in enterococci. Glycopeptide resistance enterococci (GRE) of all six types produce altered peptidoglycan with decreased binding affinity for glycopeptides. These substantial changes in peptidoglycan composition do not result from acquisition of a single gene; each resistance type is associated with a complex gene cluster (23). Detection and spread of glycopeptide resistance in enterococci suggest concern that this resistance would” escape”into staphylococci. Detection of enterococcal resistance to vancomycin in Clinic for Orthopaedic surgery, University of Sarajevo Clinics Centre is suggesting a critical use of glycopeptides in order to prevent a further rise in enterococcal glycopeptide resistance and spread of the Van genes to Staphylococcus aureus.
CONCLUSION
Our results clearly show reducing of MRSA in surgical site infection. It suggests continuation with strategies for preventing nosocomial infection: education of staff on identifying and reporting new infections; reviewing each wound infection and discussing prevention options; strong application of protocols and urging the implementation of strict infection control rules. The collected data confirm that a constant and active surveillance of this type of nosocomial infection is necessary. The results are useful for comparison of frequency of MRSA infection in other hospitals.
Permanent education, good cooperation and support from the hospital management are extremely important for the successful application of infection control measures.
REFERENCES
- 1.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 1993;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leaper DJ, Van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, Berger A. Surgical site infection-European perspective of incidence and economis burden. Int. Wound J. 2004;1(4):247–273. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damani NN. Prevention of Surgical Site Infections. Manual of Infection Control Procedures. 2003:245–259. [Google Scholar]
- 4.Martone W.J, Nichols RI. Recognition, prevention, surveillance and management of surgical site infection: introduction to the problem and symposium overview. Clin. Infect. Dis. 2001;33(Suppl. 2):567–568. doi: 10.1086/321859. [DOI] [PubMed] [Google Scholar]
- 5.Smyth ETM, Emmerson AM. Surgical site infection surveillance. J. Hosp. Infect. 2000;45:173–184. doi: 10.1053/jhin.2000.0736. [DOI] [PubMed] [Google Scholar]
- 6.Skinner D, Keefer CS. Significance of bacteraemia caused by Staphylococcus aureus. Arch. Inter. Med. 1941;68:851–875. [Google Scholar]
- 7.Dowling HF. The new penicillins. Clin. Pharmacol. Ther. 1961;2:572–580. doi: 10.1002/cpt196125572. [DOI] [PubMed] [Google Scholar]
- 8.Campos ML, Cipriano ZM, Freitas PF. Suitability of the NNIS index for estimeting surgical-site infection risk at small university hospital in Brazil. Infect. Control Hosp. Epidemiol. 2001;22:268–272. doi: 10.1086/501898. [DOI] [PubMed] [Google Scholar]
- 9.Hiramatasu K, Aritaka N, Hanaki H, Ino T, Yabuta K. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 10.Tenover FC, Lancaster MV, Hill BC. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J. Clin. Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MN, Pai CH, Woo Ryu JH, Hiramatsu K. Vancomycin-intermediate Staphylococcus aureus in Korea. J. Clin. Microbiol. 2000;38:3879–3881. doi: 10.1128/jcm.38.10.3879-3881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan A, Dick JD, Perl TM. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 2002;15:430–438. doi: 10.1128/CMR.15.3.430-438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control. 2004;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 14.Kalenić S. The Resistance of Bacteria to Antibiotics. Medicus. 2000;9(2):149–158. [Google Scholar]
- 15.Damani NN. Methicillin-resistant Staphylococcus aureus. Manual of Infection Control Procedures. 2003:121–129. [Google Scholar]
- 16.Gemmell CG, Edwards DI, Fraise AP. Guidellines for the pro-phylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 2006;57:589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 17.Cheesbrough M. Collection, transport and examination of samples: Medical Laboratory Manual, Volume II. Microbiology. Tropical Health Technology Oxford. 1993:146–160. [Google Scholar]
- 18.Dancer SJ. Importance of environment in methicillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect. Dis. 2008;8:101–113. doi: 10.1016/S1473-3099(07)70241-4. [DOI] [PubMed] [Google Scholar]
- 19.Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg. Infect. Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruse PJE, Ford R. A five-year prospective study of 23 649 surgical wounds. Archives of Surgery. 1973;107:206–210. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- 21.Nurkić M. Causes of infection surgical wound. The Fourth Symposium of Hospital Infection Control of Bosnia and Herzegovina. Tuzla, Bosnia and Herzegovina, Juny 14th-16th. 2006:9–19. Proceedings. [Google Scholar]
- 22.Uttley AHC, Collins CH, Naidoo J, George RC. Vancomycin-resistant entrococci. Lancet. 1988;I:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 23.Woodford N. Epidemiology of the genetic elements reponsible for acquired glycopeptide resistance in enterococci. Microb. Drug. Resist. 2001;7:229–236. doi: 10.1089/10766290152652774. [DOI] [PubMed] [Google Scholar]


