Abstract
A combined test performed at the 12th week of gestation enables us to classify the pregnancy as high risk (risk higher than 1:300) or low risk (risk lower than 1:300) for congenital foetal anomalies, with great accuracy of 85 - 90%. According to the available data, the frequency of false positive results is estimated at around 5%. The objective of the study was to examine possible correlation between the serum marker values and amniocentesis results in prenatal diagnostics of congenital foetal anomalies. The study included 745 pregnant women monitored by the Genetic Counselling Service of the Clinic of Gynaecology and Obstetrics of the Clinics Centre Kragujevac. The subjects were included in the study under condition that CRL (embryonic crown-rump length) was from 45 to 84 mm and that the gestational age was at 11-13+6 weeks. Free β HCG and PAPP-A were determined from venous blood using commercial DPS-USA tests. Tests were based on the analytic principle of the immuno-chemiluminescence technique and were performed by application of the automatic Immulite 2000 analyzer by DPC-USA. The foetal nuchal translucency thickness (NT) and CRL were measured by Colour Doppler. The chromosome identification was performed after a certain number of cell divisions by stopping the cell division in metaphase of mitosis when the chromosomes were the most distinguishable. The foetal karyotype was prepared using G bands. In the total sample of pregnant women (n=745), there were six cases of pathological foetal karyotype. A statistical paradox in the frequency of congenital foetal anomalies in favour of younger population was noticed. A high coefficient of Spearman’s rank correlation suggests great importance of the combined test in the detection of congenital foetal anomalies (p<0,05). A high consistency was also proved for components of biochemical screening and ultrasonographic markers. The combined test, as a method of prenatal screening in the first trimester of pregnancy, if used at 11 - 13+6 weeks’ gestation and for CRL of 45-84 mm, has a great importance in the detection of congenital foetal anomalies.
Keywords: prenatal screening, combined test, nuchal translucency, amniocentesis
INTRODUCTION
In our country, no officiai guidelines for screening for Down syndrome exist. However, most perinatologists, after biochemical screening in the first trimester of pregnancy, perform a combined screening in accordance with the recommendations of the Foetal Medicine Foundation - FMF, London. Performing a combined test in the 12th week of gestation which includes a combined assessment of the presence of chromosomopathy by nuchal thickness scan between 11 and 13+6 weeks’ gestation, when the crown to rump length is between 45 - 84 mm, and a biochemical test - detection of serum PAPP-A markers and free β-HCG, the pregnancy can be classified as high risk (risk higher than 1:300) or low risk (risk lower than 1:300) with a great accuracy of 85 - 90%. The frequency of false positive results in the literature is estimated at around 5% (1). Therefore, in recent years, perinatologists worldwide have tried to minimize the number of recommended invasive procedures. Primarily, “additional” ultrasound markers were introduced: nuchal translucency measurement, nasal bone scan, frontal-maxillary personal angle, Ductus venosus Doppler flow during atrial contraction and tricuspid regurgitation. This approach has an accuracy of 95%, with 2% of false positives (2). According to the latest recommendations by Foetal Medicine Foundation, London from 2007 (Theoretical Course for the Certificate of Competence in Ultrasound Examination at 11-13+6 Weeks), high risk pregnancies are those in which the risk for T21 assessed by combined test is higher than 1:50; moderate risk pregnancies are those with the combined test results in the range from 1:51 to 1:1000, while low risk pregnancies are those with the risk less than 1:1001. In this way the primary choice of pregnant women for an invasive procedure based on the test results is further decreased (3). In the last few years, the aim of numerous investigations was to develop non-invasive methods of prenatal diagnosis based on isolation and examination of foetal cells from maternal blood. These studies are based on the presence of foetal free (extracellular) DNA in maternal plasma and quantification of male DNA concentrations in pregnancies with male foetuses using the real time PCR.
Objective
The objective of the paper was to determine the correlation between the serum markers values and amniocentesis results in the prenatal diagnosis of congenital foetal anomalies.
MATERIALS AND METHODS
This intervention observational study was carried out at the Clinic of Gynaecology and Obstetrics of the CC Kragujevac in the period from 2007-2009. In the course of the study, the clinical-experimental method was used. Each patient was given a full explanation of the plan and the objective of the study and all the subjects gave their informed consent to participate. The Ethical Committee of the CC Kragujevac approved the study. It included 745 pregnant women monitored by the Genetic Counselling Service Committee of the Clinic of Gynaecology and Obstetrics of the CC Kragujevac.
The study inclusion criteria involved:
The CRL (crown-rump length) between 45 and 84 mm.
The gestational age of 11-13+6 weeks.
Out of the total number of subjects, 185 underwent a complete laboratory examination with ultrasonographic scan at 11-13+6 weeks’ gestation. The amniocentesis for determination of the fetal karyotype was performed in the whole sample of 745 pregnant women.
The obtained test results and serum and ultrasonographic marker values were compared with the early amniocentesis findings. This gave us an opportunity to establish their sensitivity, specificity and predictive values in the detection of congenital foetal anomalies.
The following parameters were analyzed:
-PAPP-A (multiple of the median - MoM)
-Free β HCG (multiple of the median - MoM)
Free β HCG and PAPP-A were measured from venous blood using commercial DPS-USA tests. The tests were based on the analytic principle of the immuno-chemiluminescence technique and were performed by application of the automatic Immulite 2000 analyzer by DPC-USA The foetal nuchal translucency thickness (NT) and CRL were measured by Colour Doppler. For measurements of the foetal nuchal translucency thickness NT we used a high-resolution ultrasound device with a cine loop which allowed replay and a calliper which provided measurements in decimal form. Only the head and the upper chest were scanned. Since the magnification was maximal, the minimal movement of the calliper changed the measurement only by 0,1 mm. The nuchal translucency was measured on the foetus in the neutral position. The maximum thickness of subcutaneous translucency between skin and soft tissue was measured above the cervical spine. The CRL (crown-rump length) was measured by Colour Doppler. All ultrasound scans were performed using “Aloka prosound 3500” at the Clinic of Gynaecology and Obstetrics of the Clinical Centre Kragujevac.
Cytological methods for chromosome identification
After a certain number of cell divisions, plant alkaloid colchicine that prevents the formation of the mitotic spindle was added to the culture medium. The cells were arrested in the metaphase of mitosis when the chromosomes were the most distinguishable. After additional processing, the chromosomes were stained with Giemsa solution. Each chromosome presents specific and always the same pattern of bands (Gbands - stained with Giemsa). Then, the chromosomes were photographed, cut out and arranged in pairs by size, and the foetal karyotype was established. All the obtained data were stored in a unique database with an obligatory logic control.
The statistic analysis included:
-calculation of the mean value, SD, the value range for each numerical parameter, and analysis of the obtained values in relation to the subgroups,
-Spearman’s coefficient was used for assessment of correlation.
The statistic data processing was performed on a PC using a statistica! program SPSS for Windows and the obtained results were presented in tabular form with commentary.
RESULTS
In the examined population of pregnant women, (n=745), 6 or 0,80% of them had pathological amniocentesis findings. The average age of the examined pregnant women with physiological amniocentesis findings was 35,17±3,45. The median value of fbHCG was 40,21±42,54 mlU/ml, and the median value of PAPPA was 1,89±0,88 mlU/ml. The corrected MoM for fbHCG was 0,85±0,92, and for PAPPA it was 0,92±0,52. The foetal CRL was 64,7±8,57mm, and the nuchal translucency (NT) was 1,76±0,38mm. The average gestation age with normal foetal karyotype was 89,6±11,00 days.
The average age of the examined pregnant women with positive amniocentesis results was 31,4±4,60. In the same group, the median value of fbHCG was 147,4±94,22, and median value of PAPPA was 1,64±1,17 ml/Uml. The corrected MoM for fbHCG was 3,45±1,98, and for PAPA the corrected MoM was 0,63±0,29. The average crown-rump length was 60,3±8,62, and the nuchal translucency thickness was 1,97±0,30mm. The average gestational age was 85,5±3,88 days.
Table 3. shows a high correlation coefficient between the studied variables which is predicative of fetal anomalies in the population of pregnant women with pathological amniocentesis findings.
TABLE 1.
The number of early amniocentesis performed in the period from 2007 to 2009
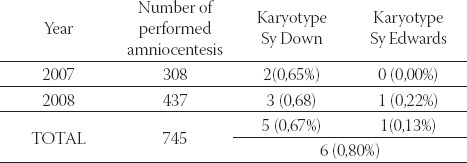
TABLE 2.
The studied parameter values in pregnant women with pathological findings of early amniocentesis
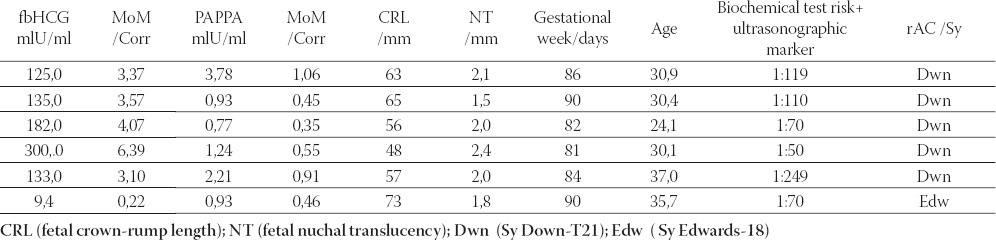
TABLE 3.
Correlation between biochemical markers in the group with foetal pathological karyotype after amniocentesis. Spearman’s rank correlation coefficient (p<0,05), n=6

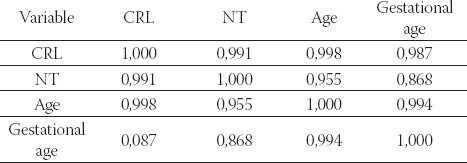
Table 4. shows the importance of ultrasonographic markers in detection of congenital foetal anomalies in the studied pregnancies with high rank correlation.
TABLE 4.
Correlation between ultrasonographic markers and the age in the group with pathological foetal karyotype after amniocentesis. Spearman’s rank correlation coefficient (p<0,05), n=6
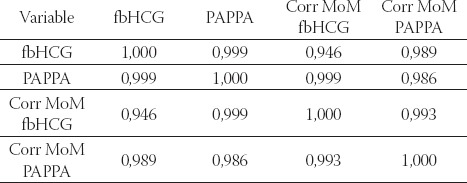
Tables 5. and 6. show that a high correlation between physiological values of biochemical parameters and normal ultrasonographic markers has a great predicative importance in clinical risk assessment of congenital foetal anomalies.
TABLE 5.
Correlation between ultrasonographic markers and age in the group with physiological foetal karyotype after amniocentesis. Spearman’s rank correlation coefficient (p<0,05), n=185

TABLE 6.
Correlation between biochemical parameters in the group with physiological fetal karyotype after amniocentesis. Spearman’s rank correlation coefficient (p<0,05), n=185
DISCUSSION
The foetal nuchal translucency between 11-13+6 weeks of gestation combined with the mother’s age represents an effective screening method for trisomy 21, which detects 75% of trisomic pregnancies with the rate of invasive interventions of 5%. When free β-human chorionic gonadotropin and PAPP-A (pregnancy associated plasma protein A) in maternal blood between 11 and 11-13+6 weeks of gestation are taken into consideration, the detection rate of chromosomopathies rises to 8590% (4). Barclay (5) measured NT in a sample of 11281 pregnant women and discovered 118 congenital foetal anomalies. There were 52 cases of trisomy 21, 71.2% of which had NT above 95th percentile between 11-14 weeks of gestation. In the same study, this percentage decreases to 56 % in the period between 15-16 weeks of gestation (5). In 2005, Manni (6) studied 32000 foetuses of gestational age between 11-14 weeks. He measured NT in pregnant women whose average age was 32. In 854 (5,1%) of the foetuses, NT was above 95th percentile, 744 (87,1%) of which had a normal karyotype. Out of 110 diagnosed chromosomopathies, 72 were trisomy 21 (6). The diameter of foetal nuchal translucency of 3 mm is associated with a threefold increase in the risk of trisomy 21, while the diameters of 4 mm, 5 mm and 6 mm are associated with 18-fold, 28-fold, and 36-fold increases. The nuchal translucency of 6 mm as a separate marker detects Down syndrome in 40 % of the cases. PAPP-A is a protein that is synthesized in intervillous placental cytotrophoblast. It enables binding of the insulin-like growth factor (IGF) to proteins 4 and 5, thus enabling the interaction with cell receptors. The decreased PAPP-A activity causes stagnation in growth, preeclampsia, preterm births and pregnancy loss in his study, Spencer (4) stated that in the sample of 54 722 one foetus pregnancies in the first trimester there was MoM PAPP-A lower than 0,415 or below 5th which resulted in foetal loss before 24 weeks of gestation. Zournatzi (7) stated that the MoM value above 1,83 for fbHCG in the first trimester, together with the MoM for PAPP-A below 0,38 detected trisomy 21 in 50% of the cases. The same author claimed that fbHCG combined with the pregnant woman’s age at the same gestational age had a detection rate of up to 45% for trisomy 21 (7). The combination of both bi’ochemical parameters with the pregnant woman’s age had a detection rate of 65%. According to the published studies the level of free β-HCG in maternal blood normally decreases during gestation (8). In pregnancies affected by trisomy 21, free β-HCG is increased. The maternal blood levels of PAPP-A normally increase with gestation, while with pregnancies affected by trisomy 21 it decreases (9). For a certain gestational age, the levels of β-HCG and PAPP-A present a probability factor that is multiplied with the initial risk in order to calculate the new risk (10). The higher the level of β-HCG and the lower the level of PAPP-A, the higher the risk for trisomy 21 is. In pregnancies with trisomy 21, at 12 week of gestation, the free β-HCG concentration in maternal serum is higher than in pregnancies with foetuses without chromosomal abnormalities (around 2 MoM), while the PAPP-A concentration is lower (around 0,5 MoM). The difference in serum free β-HCG between normal pregnancies and those affected with trisomy 21 increases with gestational duration, and differences in PAPP-A levels decreases with gestational duration. According to the literature, there is no significant relation between the foetal NT and the free β-HCG or PAPP-A in maternal serum neither in normal nor in pregnancies affected by trisomy 21, therefore ultrasound and biochemical markers can be combined in order to make screening more effective than when separate methods are used (11, 12). In order to calculate an individual risk it is necessary to multiply the initial or a priori risk that depends on maternal and gestational age with several probability or risk factors that depend on screening test results done during the pregnancy. The probability or risk factor for an ultrasound finding or biochemical result is calculated when the percentage of foetuses with chromosomopathies is divided with the percentage of normal foetuses with the same findings. Further investigation is needed in order to determine which techniques will improve prenatal screening and enable it to achieve the reliability of invasive methods (13). About 1 in 103-107 nucleated cells in maternal blood is foetal. The number of foetal cells can be increased to about 1 in 10-100 by different techniques such as magnetic cell sorting - MACS or fluorescence activated cell sorting - FACS after binding of magnetically labelled or fluorescent antibodies onto specific cell surface markers (14, 15). However, using chromosome specific DNA probes, as well as fluorescent in situ hybridisation (FISH), it is possible to suspect foetal trisomy by the presence of three-signal nuclei in some of the maternal blood cells enriched for fetal cells. There are some contradictory results regarding the presence of extracellular foetal DNA in pregnancies with trisomy 21 - in some studies the levels were elevated, but in others there was not a significant difference between normal and pregnancies with chromosomopathies. Time will show whether extracellular foetal DNA will become another marker in maternal blood screening for foetal abnormalities.
CONCLUSION
We can conclude that the results of this study are in accordance with those found in the literature. The combined screening test, if used methodologically correctly, has a high predictive value for detection of congenital foetal anomalies, as indicated by the positive correlation coefficient. Statistical paradox seen in higher frequency of foetal anomalies in the population of pregnant women of younger age can be explained by the fact that prenatal diagnostic methods are more rarely used in this population. We must take into serious consideration the age cut-off as a rigid legal framework in implementation of measures in pregnant women’s healthcare. We witness the time when a considerable number of pregnant women does not undergo invasive prenatal diagnosis thanks to screening tests. With the right choice and interpretation of available screening tests, we can prevent loss of recourses and waste of valuable time in making a diagnosis.
List of Abbreviations
CRL - embryonic crown-rump length
NT - fetal nuchal translucency
Fb HCG - free beta-subunit of choriogonadotropin
PAPPA - pregnancy-associated plasma protein a
MoM - Multiple of the median
REFERENCES
- 1.Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am. J. Obstet. Gynecol. 2004;191:45–67. doi: 10.1016/j.ajog.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 2.Bindra R, Heath V, Liao A, Spencer K, Nicolaides Kh. One stop clinic for assessment of risk for trisomy 21 at 11-14 weeks: A pro-spective study of 15,030 pregnancies. Ultrasound Obstet. Gynecol. 2002;20:219–225. doi: 10.1046/j.1469-0705.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- 3.Spencer K, Bindra R, Nix Abj Heath V, Nicolaides Kh. Delta - NT or NT MoM: which is the most appropriate method for calculating accurate patient-specific risks for trisomy 21 in the first trimester? Ultrasound Obstet. Gynecol. 2003;22:142–148. doi: 10.1002/uog.186. [DOI] [PubMed] [Google Scholar]
- 4.Spencer K, Yu Ck, Cowans Nj, Otigbah C, Nicolaides Kh. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free betahCG and with second -trimester uterine artery Doppler. Prenat. Diagn. 2005;25:949–953. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 5.Barclay L. Nuchal translucency predicts Down’s syndrome. Obstet. Gynecol. 2002;100(4):648–654. doi: 10.1016/s0029-7844(02)02145-2. [DOI] [PubMed] [Google Scholar]
- 6.Govanni M, Yoppi MA, Ibba RM, Floris M, Manca F. Nuchal translucency and nasal bone for trisomy 21. Croat. Med. J. 2005;46(5):786–791. [PubMed] [Google Scholar]
- 7.Zournatzi V, Damiilidis A, Karidas C, Tantanasis T, Loufopalus A, Tzafettas J. A prospective two years study of first trimester screening for Down syndrome. Hippokratia. 2008;12(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson SE, Simhan HN. First-trimester pregnancy-associated plasma protein A and subsequent abnormalities of fetal growth. Am. J. Obstet. Gynecol. 2008;198(5):43–45. doi: 10.1016/j.ajog.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Wald NJ, Morris JK, Ibisonj WU T, George LM. Screening in early pregnancy for pre-eclampsia using Down syndrome quadruple test markers. Prenat. Diagn. 2006;26:559–564. doi: 10.1002/pd.1459. [DOI] [PubMed] [Google Scholar]
- 10.Pajkrt E, Van Lith JMM, Mol BWJ, Bleker OP, Bilardo CM. Screening for Down’s syndrome by fetal nuchal translucency measurement in a general obstetric population. Ultrasound Obstet. Gynecol. 1998;12:163–169. doi: 10.1046/j.1469-0705.1998.12030163.x. [DOI] [PubMed] [Google Scholar]
- 11.Adekumle O, Gopee A, El-Sayed M, Thilaganathan B. Increased first trimester nuchal translucency: pregnancy and infant outcomes after routine screening for Down’s syndrome in an un-selected antenatal population. Br. J. Radiol. 1999;857:457. doi: 10.1259/bjr.72.857.10505009. [DOI] [PubMed] [Google Scholar]
- 12.Zoppi MA, Ibba RM, Floris M, Monni G. Fetal nuchal translucency screeninig in 12495 pregnancies in Sardinia. Ultrasound Obstet. Gynecol. 2001;6:649–651. doi: 10.1046/j.0960-7692.2001.00583.x. [DOI] [PubMed] [Google Scholar]
- 13.Pandaya P. Increased nuchal translucency and other chromosomal defects. In: Nicolaides KH, editor. The 11-14 week scan (the diagnosis of fetal abnormalities) New York, London: The Parthenon Publishing Group; 1999. pp. 29–30. [Google Scholar]
- 14.Faraina A, Leshane ES, Lambert-Messerlian GM, Canick JA, Lee T, Louis M, Neveux, et al. Evaluation of Cell-free Fetal DNA as a second-trimester maternal serum marker of Down Syndrome pregnancy. Clin. Chem. 2003;49:239–242. doi: 10.1373/49.2.239. [DOI] [PubMed] [Google Scholar]
- 15.Pan PDI, Peter GM, Lambert-Messerlian JA, Canick DW, Bianchi K. L. Johnson. Cell-free fetal DNA levels in pregnancies conceived by IVF. Hum. Reprod. 2005;20(11):3152–3156. doi: 10.1093/humrep/dei165. [DOI] [PubMed] [Google Scholar]


