Abstract
The melanocortin system has been implicated in the regulation of various physiological functions including melanogenesis, steroidogenesis, energy homeostasis, and feeding behavior. Five melanocortin receptors have been identified to date and belong to the family of G protein-coupled receptors (GPCR). Post-translational modification of the proopiomelanocortin (POMC) prohormone leads to the biosynthesis of the endogenous melanocortin agonists, including α-melanocyte stimulating hormone (α-MSH), β-MSH, γ-MSH, and adrenocorticotropic hormone (ACTH). All the melanocortin agonists derived from the POMC prohormone contain a His-Phe-Arg-Trp tetrapeptide sequence that has been implicated in eliciting the pharmacological responses at the melanocortin receptors. Herein, an alanine (Ala) positional scan is reported for the endogenous α-MSH ligand and the synthetic, more potent, NDP-MSH peptide (Ac-Ser1-Tyr2-Ser3-Nle4-Glu5-His6-DPhe7-Arg8-Trp9-Gly10-Lys11-Pro12-Val13-NH2) at the cloned mouse melanocortin receptors to test the assumption that the structure-activity relationships of one ligand would apply to the other. Several residues outside of the postulated pharmacophore altered potency at the melanocortin receptors, most notably the 1560-, 37-, and 15-fold potency loss when the Glu5 position of α-MSH was substituted with Ala at the mMC1R, mMC3R, and mMC4R, respectively. Importantly, the altered potencies due to Ala substitutions in α-MSH did not necessarily correlate with equivalent Ala substitutions in NDP-MSH, indicating that structural modifications and corresponding biological activities in one of these melanocortin ligands may not be predictive for the other agonist.
Keywords: Melanotropin, alanine positional scan, α-MSH, NDP-MSH, GPCR, obesity
Introduction
The melanocortin system consists of five melanocortin receptors (MC1-5R), discovered to date, that are members of the super family of G protein-coupled receptors (GPCR) and signal through the cyclic adenosine monophosphate (cAMP) stimulatory pathway. The MC1R is primarily expressed in the skin and melanocytes and is involved in pigmentation.1, 2 The MC2R is mainly expressed in the adrenal gland and is involved in steroidogenesis.2 While present in a number of different tissues, expression of the MC3R and MC4R in the central nervous system has been linked to feeding and energy homeostasis.3-11 The MC5R is ubiquitously expressed and has been implicated in exocrine gland function.12-14 The melanocortin receptors are stimulated through endogenous agonists derived from the proopiomelanocortin (POMC) gene transcript.15 Additionally, the MC1R, MC3R, and MC4R are regulated by two endogenous antagonists, agouti and agouti-related protein (AGRP).16-21
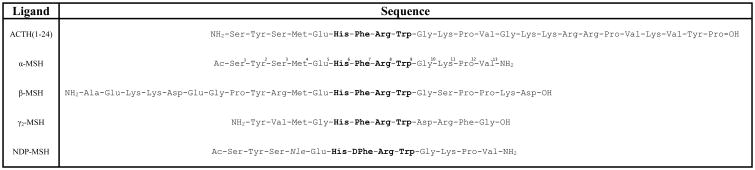
The POMC prohormone is processed by prohormone convertases into several endogenous melanocortin agonists including α-melanocyte stimulating hormone (α-MSH), β-MSH, γ-MSH, and adrenocorticotropic hormone ACTH (Figure 1).22,23 While these endogenous agonists are different lengths and possess varying N- and C-terminal modifications, they all possess a His-Phe-Arg-Trp tetrapeptide sequence postulated to be responsible for stimulating the melanocortin receptors (Figure 1). The minimal sequence H-His-Phe-Arg-Trp-OH was reported to be the smallest peptide to possess activity;24,25 subsequent truncation studies of α-MSH confirmed the Ac-His-Phe-Arg-Trp-NH2 peptide as the minimal fragment to possess activity in the classic frog (Rana pipens) and lizard (Anolis carlinensis) skin bioassays.26,27 This fragment has been demonstrated to possess agonist activity at the cloned mouse receptors, with micromolar potency at the mMC1R, mMC3R, and mMC4R, and sub-micromolar potency reported at the mMC5R.28 While full agonist activity is observed with the Ac-His-Phe-Arg-Trp-NH2 fragment, the potency of this peptide is diminished relative to α-MSH (200- to 200,000-fold depending on the assay and receptor subtype),26-28 indicating additional residues of α-MSH outside the core tetrapeptide sequence contribute to ligand potency.
Figure 1.
Amino acid sequence of the endogenous POMC melanocortin agonists and NDP-MSH synthetic analog. The numbering of α-MSH is indicated. The tetrapeptide sequence His-Phe-Arg-Trp, common to all naturally occurring MCR agonists, is indicated in bold. The more potent NDP-MSH ligand is modified from α-MSH by replacing Met4 with Nle and inverting the stereochemistry at the Phe7 position.
It has previously been shown that modifications at the Met4 and Phe7 positions of α-MSH (numbering defined in Figure 1) may increase the potency of the resulting ligands. The Met4 position is susceptible to oxidation, resulting in loss of activity following attempts to iodinate α-or β-MSH.29,30 A norleucine (Nle) substitution at this position was resistant to oxidative inactivation and demonstrated increased potency compared to α-MSH.30, 31 A 5- to 10-fold increase in potency was also observed when the stereochemistry of the Phe7 position was inverted in the H-His-DPhe-Arg-Trp-Gly-OH pentapeptide derivative of α-MSH relative to the all L amino acid pentapeptide.32 A combination of the Nle4 substitution and stereochemical inversion at the Phe7 position resulted in the synthetic derivative [Nle4, DPhe7]α-MSH, abbreviated to NDP-MSH.33 The more potent and enzymatically stable NDP-MSH was shown to possess prolonged activity compared to α-MSH.33, 34 The enhanced potency and stability of NDP-MSH was translated to a first-in-class therapeutic for adult erythropoietic protoporphria in 2014.35
It has been hypothesized that modification of additional α-MSH amino acids may lead to more potent or selective ligands for the melanocortin receptors. One traditional approach to investigate the importance of every residue in a ligand is to sequentially replace each amino acid with Ala and measure the resulting effect on receptor binding, potency, or efficacy.36-38 Alanine is traditionally selected for this positional scanning approach since it does not usually alter the backbone conformation and does not impose extreme electrostatic or steric effects.36 Alanine positional scanning studies have been reported for many different natural and synthetic ligands, including the gastric inhibitory polypeptide,39 HOE 140 (antagonist at the kinin B2 receptors),40 angiotensin II,38 and bradykinin,37 and γ-MSH.41, 42 There have been two reported Ala positional scans of α-MSH, examining the binding affinities and biological potencies of α-MSH derivatives using B16 murine melanoma cells believed to express the mouse MC1R43 and the binding affinities of Ala-substituted α-MSH ligands with HEK293 cells expressing the rat (r) MC3R.44 An Ala scan has also been reported for the retro-inverso sequence of α-MSH in a human melanoma cell line examining cAMP production.45 To date, there have been no α-MSH Ala scans at the MC4R or MC5R, or at the cloned melanocortin receptors performed in parallel to examine the relative selectivity of the α-MSH derivatives. Additionally, despite first being reported in 1980, there have been no reports of a full Ala positional scan of NDP-MSH. Alanine substitutions of NDP-MSH have been reported at positions 5-9 for the human (h) MC4R,46,47 and Ala replacements at positions 6 and 9 have additionally been reported for the hMC1R and hMC3-5R.48, 49
To systematically examine the contribution of each residue to melanocortin signaling, an Ala positional scan was performed on α-MSH and NDP-MSH at the cloned mouse receptors. As the compounds were examined in parallel, comparisons could be drawn between the different substitutions at the same receptor and each substitution at the different receptors. This approach also permitted a relative comparison between α-MSH, NDP-MSH, and their Ala derivatives to examine if structure-activity relationship studies in one ligand set would translate to the other.
Results & Discussion
Peptide Synthesis and Characterization
All peptides were synthesized on a semi-automated synthesizer (Advanced ChemTech, Louisville, KY) or automated synthesizer (Advanced ChemTech 440MOS, Louisville, KY) using standard fluorenylmethyloxycarbonyl (Fmoc) chemistry.50 The peptides were purified using semi-preparative reverse-phase high-pressure liquid chromatography (RP-HPLC). The purity of these peptides (>95%) was assessed by analytical RP-HPLC in two diverse solvent systems (Table 1). The correct peptide molecular weight was confirmed through mass spectrometry (University of Florida Protein Core Facility). The compounds were assayed for agonist activity using a cAMP-based β-galactosidase reporter assay at the mMC1R and mMC3-5R.51 These studies were performed on the mouse melanocortin receptors since future in vivo studies may first be performed in rodents utilizing wildtype and knockout mice.52 The mMC2R is only stimulated by ACTH and was therefore excluded in this study.53 Due to the inherent error within the assay, peptides that were within a 3-fold potency range were considered to be equipotent. All compounds were full agonists.
Table 1. Analytical Data for the Peptides Synthesized in This Study.
| Peptide | Sequence | HPLC k′ (System 1) | HPLC k′ (System 2) | Calculated (M+1) | Mass Spectral Analysis (M+1) | Purity (%) |
|---|---|---|---|---|---|---|
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.8 | 10.0 | 1664.8 | 1665.0 | >98 |
| [Ala1]α-MSH | Ac-Ala-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.0 | 9.8 | 1648.8 | 1649.0 | >99 |
| [Ala2]α-MSH | Ac-Ser-Ala-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.5 | 9.6 | 1572.8 | 1572.5 | >97 |
| [Ala3]α-MSH | Ac-Ser-Tyr-Ala-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.1 | 10.4 | 1648.8 | 1648.7 | >98 |
| [Ala4]α-MSH | Ac-Ser-Tyr-Ser-Ala-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.4 | 9.2 | 1604.8 | 1604.9 | >98 |
| [Ala5]α-MSH | Ac-Ser-Tyr-Ser-Met-Ala-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.9 | 10.2 | 1606.8 | 1608.0 | >99 |
| [Ala6]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-Ala-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.2 | 10.7 | 1598.8 | 1599.0 | >99 |
| [Ala7]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Ala-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 3.9 | 8.4 | 1588.8 | 1588.9 | >97 |
| [Ala8]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Ala-Trp-Gly-Lys-Pro-Val-NH2 | 5.1 | 10.5 | 1579.7 | 1580.5 | >99 |
| [Ala9]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Ala-Gly-Lys-Pro-Val-NH2 | 3.8 | 8.3 | 1549.8 | 1549.8 | >99 |
| [Ala10]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Ala-Lys-Pro-Val-NH2 | 4.8 | 10.0 | 1678.8 | 1678.4 | >98 |
| [Ala11]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Ala-Pro-Val-NH2 | 5.2 | 10.7 | 1607.7 | 1608.0 | >99 |
| [Ala12]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Ala-Val-NH2 | 4.8 | 9.9 | 1638.8 | 1638.6 | >98 |
| [Ala13]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Ala-NH2 | 4.6 | 9.5 | 1636.8 | 1636.7 | >98 |
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.1 | 11.0 | 1646.8 | 1647.6 | >99 |
| [Ala1]NDP-MSH | Ac-Ala-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.2 | 10.1 | 1630.8 | 1632.5 | >95 |
| [Ala2]NDP-MSH | Ac-Ser-Ala-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.1 | 9.8 | 1554.8 | 1556.2 | >95 |
| [Ala3]NDP-MSH | Ac-Ser-Tyr-Ala-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 6.0 | 10.2 | 1630.8 | 1630.9 | >97 |
| [Ala4]NDP-MSH | Ac-Ser-Tyr-Ser-Ala-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.4 | 8.4 | 1604.8 | 1605.6 | >95 |
| [Ala5]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Ala-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.4 | 9.4 | 1588.8 | 1589.6 | >95 |
| [Ala6]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-Ala-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 5.9 | 12.0 | 1580.8 | 1582.5 | >95 |
| [Ala7]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-Ala-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 4.3 | 8.9 | 1570.8 | 1571.9 | >98 |
| [Ala8]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Ala-Trp-Gly-Lys-Pro-Val-NH2 | 6.0 | 10.9 | 1561.8 | 1562.4 | >95 |
| [Ala9]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Ala-Gly-Lys-Pro-Val-NH2 | 4.2 | 8.7 | 1531.8 | 1532.5 | >95 |
| [Ala10]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Ala-Lys-Pro-Val-NH2 | 5.0 | 9.5 | 1660.9 | 1661.7 | >99 |
| [Ala11]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Ala-Pro-Val-NH2 | 5.5 | 11.8 | 1589.8 | 1590.9 | >96 |
| [Ala12]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Ala-Val-NH2 | 5.5 | 10.9 | 1620.8 | 1621.6 | >95 |
| [Ala13]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Ala-NH2 | 5.0 | 9.6 | 1618.8 | 1619.6 | >95 |
HPLC k′ =[(peptide retention time – solvent retention time)/solvent retention time] in solvent system 1 (10% acetonitrile in 0.1% triflouroacetic acid/water and a gradient to 90% acetonitrile over 35 min) or solvent system 2 (10% methanol in 0.1% triflouroacetic acid/water and a gradient to 90% methanol over 35 min). An analytical Vydac C18 column (Vydac 218TP104) was used for solvent system 1 and a Vydac C4 column (Vydac 214TP104) was used for solvent system 2. For both solvent system used a flow rate of 1.5 mL/min. The peptide purity was determined by HPLC at a wavelength of 214 nm.
α-MSH (Ac-Ser1-Tyr2-Ser3-Met4-Glu5-His6-Phe7-Arg8-Trp9-Gly10-Lys11-Pro12-Val13-NH2)
The endogenous melanocortin agonist α-MSH possessed nonselective nanomolar agonist potencies at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively (Table 2, Figure 2A). The purpose of the present study was to examine the relative change in agonist potency while performing an Ala positional scan over α-MSH. Peptides were analyzed in parallel, so while the absolute potencies of α-MSH and derivatives at the mMCRs may vary from prior reports,28, 54, 55 the relative differences presented herein are expected to remain the same.
Table 2.
Agonist Pharmacology of α-MSH and Analogues at the Mouse Melanocortin Receptors.
| Peptide | Structure | mMC1R | mMC3R | mMC4R | mMC5R | ||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
||
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 31±28 | 1 | 26±13 | 1 | 24±6.2 | 1 | 20±13 | 1 |
| [Ala1]α-MSH | Ac-Ala-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 165±30 | 5 | 17.4±10.4 | 14±5.6 | 46±39 | |||
| [Ala2]α-MSH | Ac-Ser-Ala-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 220±78 | 7 | 6.42±2.56 | -4 | 25±7.9 | 17.2±8.4 | ||
| [Ala3]α-MSH | Ac-Ser-Tyr-Ala-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 800±130 | 26 | 12.1±6.35 | 54±40 | 9.84±4.31 | |||
| [Ala4]α-MSH | Ac-Ser-Tyr-Ser-Ala-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 12400±10800 | 400 | 17.4±6.12 | 120±33 | 5 | 60±55 | ||
| [Ala5]α-MSH | Ac-Ser-Tyr-Ser-Met-Ala-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | >20 μM (48 μM) | 1560 | 220±55 | 37 | 370±25 | 15 | 65±85 | |
| [Ala6]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-Ala-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 1070±115 | 35 | 4270±1960 | 160 | 2750±730 | 110 | 900±350 | 45 |
| [Ala7]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Ala-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 6400±2220 | 200 | 1660±825 | 64 | 4625±390 | 190 | 4200±2100 | 210 |
| [Ala8]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Ala-Trp-Gly-Lys-Pro-Val-NH2 | 6240±3740 | 200 | 670±215 | 26 | 11260±7330 | 470 | 90±20 | 5 |
| [Ala9]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Ala-Gly-Lys-Pro-Val-NH2 | 17700±4860 | 570 | 15700±4870 | 600 | >20 μM (36 μM) | 1500 | >20 μM (27 μM) | 1370 |
| [Ala10]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Ala-Lys-Pro-Val-NH2 | 25±4.80 | 33±11 | 74±20 | 41±17 | ||||
| [Ala11]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Ala-Pro-Val-NH2 | 12±3.4 | 49±33 | 40±15 | 7.10±3.13 | ||||
| [Ala12]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Ala-Val-NH2 | 26±25 | 43±14 | 44±17 | 30±25 | ||||
| [Ala13]α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Ala-NH2 | 47±58 | 42±26 | 32±14 | 30±25 | ||||
The indicated errors represent the standard error of the mean determined from at least three independent experiments. All compounds were full agonists. >20 μM indicates compounds that possessed EC50 values higher than 20 μM, with the calculated EC50 values for these ligands recorded in parenthesis.
Figure 2.
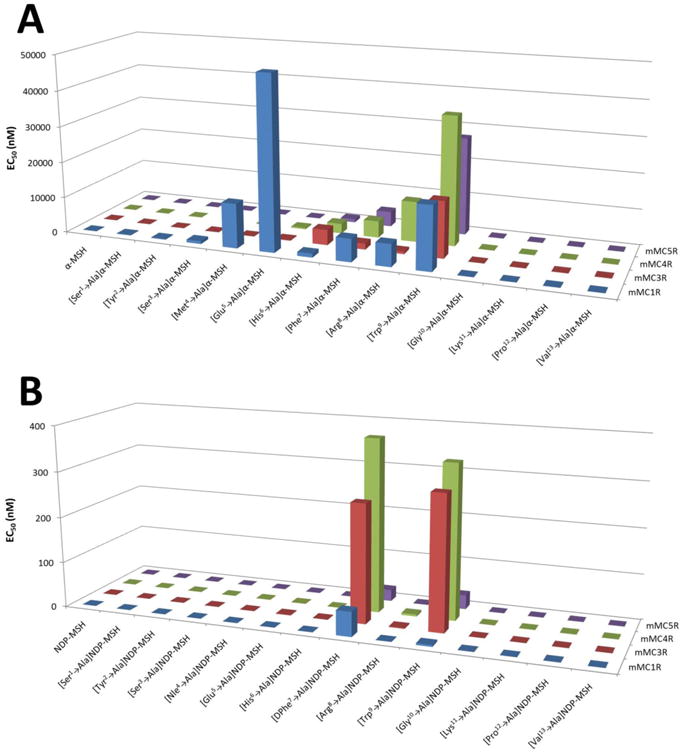
(A) Graphical illustration summarizing the ligand potency of an Ala positional scan of α-MSH at the mouse melanocortin receptors. (B) Graphical illustration summarizing the ligand potency of an Ala positional scan of NDP-MSH at the mouse melanocortin receptors.
Substitution at the Ser1 position ([Ala1]α-MSH) resulted in a 5-fold potency loss at the mMC1R, while no differences were observed at the mMC3-5R as compared to the reference compound α-MSH (Table 2, Figure 2A). A similar 7-fold potency loss at the mMC1R was observed for [Ala2]α-MSH, which was more potent at the mMC3R (4-fold) compared to α-MSH. Alanine substitution at position 3 ([Ala3]α-MSH) resulted in decreased potency at the mMC1R (26-fold), but was within experimental error of α-MSH at the mMC3-5R. The replacement at the Met4 with Ala to generate [Ala4]α-MSH decreased potency 400- and 5-fold at the MC1R and mMC4R, respectively, but did not alter potency at the mMC3R and mMC5R. Interestingly, [Ala5]α-MSH decreased potency 1560-fold relative to α-MSH at the mMC1R. Decreased potencies at the mMC3R (37-fold) and mMC4R (15-fold) were also observed for this peptide, though potency at the mMC5R was unaltered. Alanine substitutions at the next four positions, His6-Phe7-Arg8-Trp9, resulted in decreased agonist potency at all four receptors. Replacing His6 with Ala resulted in [Ala6]α-MSH, which possessed 35-, 160-, 110-, and 45-fold decreased agonist potency at the mMC1R and mMC3-5Rs, respectively. Substitution at Phe7, generating [Ala7]α-MSH, resulted in 200-, 64-, 190-, and 210-fold decreased potency at the mMC1R and mMC3-5Rs compared to α-MSH. The replacement at the basic Arg8 residue with Ala ([Ala8]α-MSH) also resulted in decreased agonist potency at the mMC1R and mMC3-4Rs, with a 200-, 26-, 470-fold decrease, respectively. This peptide was also less potent (5-fold) at the mMC5R. Substitution at the Trp9 residue to generate [Ala9]α-MSH resulted in decreased agonist potency at the mMC1R (570-fold) and the mMC3-5Rs (600-, 1500-, and 1370-fold, respectively) as compared to α-MSH. Substitution at the four residues (Gly10-Lys11-Pro12-Val13) on the C-terminal portion of the peptide did not alter agonist potency at the four melanocortin receptors tested.
Alanine positional scans of α-MSH have been previously reported using B16 murine melanoma cells that are thought to express the mMC1R and HEK293 cells expressing the rat MC3R.43, 44 In those reports, substitution at the first position of α-MSH did not affect affinity or potency as compared to a 5-fold potency loss in the present report at the mMC1R. The [Ala1]α -MSH peptide is a naturally occurring variant in the Xenopus laevis amphibian,56-58 and this substitution may be postulated to not modify biological activity. The second position was also reported to maintain equipotent binding and potency at the mMC1R while a 7-fold binding loss was reported at the rMC3R.43, 44 In the present report, a 7-fold potency loss was observed at the mMC1R and 4-fold increased potency was observed at the mMC3R. Alanine substitution at the Ser3 position resulted in a 26-fold potency loss at the mMC1R in the present study, deviating from the equipotent binding at the mMC1R and rMC3R and potency at the mMC1R previously reported.43, 44 Overall, Ala substitution at the first three residues of α-MSH appeared to minimally affect agonist potency, in agreement with the prior Ala scans of α-MSH.43, 44
Replacement of the fourth position Met4 with Ala has previously been associated with 140-fold decreased binding affinity and 59-fold decreased tyrosinase potency at the mMC1R and 14-fold decreased affinity at the rMC3R.43, 44 In a retro-inverso Ala scan of α-MSH, replacement of the Met position resulted in a significant reduction of cAMP accumulation of approximately 35% relative to α-MSH.45 In the present study, [Ala4]α-MSH reduced potency at the mMC1R 400-fold and 5-fold at the mMC4R, though this substitution did not alter potency at the mMC3R or mMC5R. Unexpectedly, the Glu5 to Ala substitution reduced potency at the mMC1R (1560-fold), mMC3R (37-fold) and mMC4R (15-fold) relative to α-MSH. The previous reports of [Ala5]α-MSH indicated equipotent binding and potency relative to α-MSH.43, 44 Furthermore, truncation studies of α-MSH did not indicate any difference in the relative potency of the Ac-Glu-His-Phe-Arg-Trp-Gly-NH2 versus the Ac-His-Phe-Arg-Trp-Gly-NH2 fragments in the Anolis carolinensis or Rana pipiens skin assays, an observation that suggested this position may not be important for potency.26, 27 Based on these cumulative data, it could be speculated that this substitution may only affect the full length α-MSH peptide when examined at the cloned mouse receptors. Further substitutions at this position may help clarify the importance of this side chain in the full-length α-MSH sequence.
The next four positions constitute the purported His-Phe-Arg-Trp pharmacophore of α-MSH, and Ala substitutions in this tetrapeptide sequence have previously been reported to decrease potency. Alanine replacement at positions 6-9 decreased binding affinity 83-, 500-, 2080-, and 2000-fold and tyrosinase potency 6-, 260-, 100-, and 120-fold at the mMC1R.43 A similar decrease in binding affinity (6-, 91-, 200-, and 83-fold for positions 6-9, respectively) was also reported for the rMC3R.44 These four positions were also found to significantly decrease potency in a retro-inverso scan of α-MSH, resulting in 30% - 80% less cAMP accumulation.45 In the present study, Ala substitutions at these four positions resulted in decreased potencies at all four receptor subtypes, highlighting the importance of this tetrapeptide sequence of α-MSH as previously postulated.26,27 The present study also did not indicate any alteration in potency when positions 10-13 were substituted. The previous Ala positional scans indicated minimal alterations in potency at these positions, with [Ala12]α-MSH decreasing binding affinity 10-fold.43
a-MSH Receptor Selectivity
Previous reports of α-MSH at the cloned mouse receptors have indicated that α-MSH possessed less than 15-fold selectivity for the melanocortin receptor subtypes.28, 54, 55 No selectivity of α-MSH was observed in the present study, though it was observed that specific Ala substitutions significantly (>100-fold) altered the selectivity profile at the mouse melanocortin receptors examined. The loss of potency at the mMC1R when Met4 was substituted with Ala resulted in 710-, 100-, and 210-fold selectivity for the mMC3R, mMC4R, and mMC5R over the mMC1R, respectively, for [Ala4]α-MSH. Similarly, [Ala5]α-MSH was 220-, 130-, and 740-fold selective for the mMC3R, mMC4R, and mMC5R over the mMC1R. The [Ala8]α-MSH peptide was also found to be 120-fold selective for the mMC5R over the mMC4R. The altered selectivity profiles observed for this set of peptides may aid in the design of future α-MSH based ligands for the melanocortin receptors.
NDP-MSH (Ac-Ser1-Tyr2-Ser3-Nle4-Glu5-His6-DPhe7-Arg8-Trp9-Gly10-Lys11-Pro12-Val13-NH2)
An Ala positional scan was also performed on NDP-MSH, a more potent synthetic analogue of α-MSH in which Nle is substituted for Met4 and the stereochemistry of the Phe7 is inverted to DPhe. These modifications have been reported to result in sub-nanomolar potency at the cloned mMCRs.28, 54 Additionally, NDP-MSH was found to possess <10-fold selectivity to the mMC1R over the mMC3-5R (Table 3, Figure 2B). While different Ala substitutions modified the selectivity profile in the present study, no significant changes in receptor selectivity (>100-fold) were observed.
Table 3.
Agonist Pharmacology of NDP-MSH and Analogues at the Mouse Melanocortin Receptors.
| Peptide | Structure | mMC1R | mMC3R | mMC4R | mMC5R | ||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
EC50 (nM) |
fold difference |
||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.023±0.010 | 1 | 0.24±0.05 | 1 | 0.16±0.02 | 1 | 0.14±0.03 | 1 |
| [Ala1]NDP-MSH | Ac-Ala-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.016±0.007 | 0.16±0.017 | 0.17±0.03 | 0.095±0.010 | ||||
| [Ala2]NDP-MSH | Ac-Ser-Ala-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.028±0.006 | 0.22±0.06 | 0.32±0.02 | 0.085±0.003 | ||||
| [Ala3]NDP-MSH | Ac-Ser-Tyr-Ala-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.024±0.0040 | 0.34±0.080 | 0.27±0.01 | 0.14±0.009 | ||||
| [Ala4]NDP-MSH | Ac-Ser-Tyr-Ser-Ala-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.11±0.020 | 5 | 0.36±0.040 | 0.39±0.10 | 0.053±0.004 | |||
| [Ala5]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Ala-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.053±0.011 | 1.18±0.40 | 5 | 0.58±0.03 | 4 | 0.25±0.012 | ||
| [Ala6]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-Ala-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.025±0.001 | 0.55±0.12 | 0.32±0.04 | 0.29±0.16 | ||||
| [Ala7]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His- Ala-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 54±21 | 2350 | 260±64 | 1080 | 380±64 | 2370 | 25±11 | 180 |
| [Ala8]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Ala-Trp-Gly-Lys-Pro-Val-NH2 | 0.14±0.08 | 6 | 2.07±0.42 | 9 | 4.56±0.57 | 29 | 0.32±0.09 | |
| [Ala9]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Ala-Gly-Lys-Pro-Val-NH2 | 4.05±0.72 | 180 | 295±92 | 1230 | 340±140 | 2120 | 31.0±3.34 | 220 |
| [Ala10]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Ala-Lys-Pro-Val-NH2 | 0.037±0.012 | 0.13±0.06 | 0.20±0.03 | 0.11±0.02 | ||||
| [Ala11]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Ala-Pro-Val-NH2 | 0.010±0.003 | 0.17±0.02 | 0.23±0.03 | 0.094±0.005 | ||||
| [Ala12]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Ala-Val-NH2 | 0.096±0.034 | 4 | 0.36±0.06 | 0.83±0.18 | 5 | 0.18±0.03 | ||
| [Ala13]NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Ala-NH2 | 0.058±0.021 | 0.30±0.06 | 0.35±0.09 | 0.24±0.04 | ||||
The indicated errors represent the standard error of the mean determined from at least three independent experiments. All compounds were full agonists.
No potency differences were observed when Ala replaced one of the first three amino acids in NDP-MSH at any mMCRs (Table 3, Figure 2B). A 5-fold decrease was observed for [Ala4]NDP-MSH at the mMC1R, while no differences were observed at the mMC3-5Rs. Decreased potency was also observed for [Ala5]NDP-MSH at the mMC3R (5-fold) and mMC4R (4-fold), though no change was observed at the mMC1R and mMC5R. Replacement of His6, resulting in [Ala6]NDP-MSH, did not alter agonist potency relative to NDP-MSH, despite being in the postulated His-Phe-Arg-Trp melanocortin agonist tetrapeptide domain. Potency decreases were observed at all four receptors when DPhe7 was substituted with Ala, generating [Ala7]NDP-MSH, resulting in a 2350-, 1080-, 2370-, and 180-fold decrease in agonist EC50 values relative to NDP-MSH at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively. Substitution at the Arg8 position ([Ala8]NDP-MSH) decreased agonist potency 6-, 9-, and 29-fold at the mMC1R, mMC3R, and mMC4R, respectively. This substitution did not affect the agonist potency at the mMC5R. The Trp9 to Ala substitution ([Ala9]NDP-MSH) decreased potencies 180-, 1230-, 2120-, and 220-fold at the mMC1R and mMC3-5Rs relative to NDP-MSH. The potencies were unaltered for [Ala10]NDP-MSH, [Ala11]NDP-MSH, or [Ala13]NDP-MSH at any of the cloned receptors, while [Ala12]NDP-MSH resulted in decreased potency at the mMC1R (4-fold) and mMC4R (5-fold).
To the authors' knowledge, there are no prior reports of a complete Ala positional scan of NDP-MSH. A previous truncation study demonstrated that removal of the first three amino acids decreased agonist potency at the mMC5R (5-fold relative to NDP-MSH) and did not affect the mMC1R and the mMC3-4R, suggesting these amino acids are not critical for potency.28 The present report is consistent with this finding, since Ala substitutions at the first three positions did not alter ligand potency. A 5-fold decrease at the mMC1R was observed in the present study when Nle4 was substituted with Ala. Similar 5- and 4-fold decreases at the mMC3R and mMC4R were observed when Glu5 was replaced with Ala. Truncation of the first five positions from NDP-MSH has been reported to decrease potency at the mMC1R (16-fold) and the mMC3R (68-fold) relative to NDP-MSH.28 This same truncation did not alter potency in the frog skin bioassay relative to NDP-MSH,59 implying these residues may play a small, but not critical role, in ligand potency. The [Ala5]NDP-MSH peptide has previously been assayed at the hMC4R and possessed 4-fold decreased agonist potency relative to NDP-MSH, in agreement with the results observed herein.47
The next four residues, His-DPhe-Arg-Trp, are postulated to be the key agonist sequence of NDP-MSH; surprisingly, substitution at the His6 residue did not alter the agonist potency at any of the receptor subtypes. Prior publications of the [Ala6]NDP-MSH peptide reported a 4-fold potency loss47 or an equipotent ligand that was 40% as efficacious as NDP-MSH at the hMC4R.46 When binding was examined in the COS cell-line expressing human melanocortin receptors, [Ala6]NDP-MSH was reported to decrease binding affinity by 8-fold at the hMC1R and 6-fold at the hMC3R.49 Relatively modest decreases in potency and efficacy have been reported for the [Ala6]NDP-MSH ligand compared to no significant differences observed in the present study, which may be due to differences in cellular assay conditions or differences between the mouse and human receptors.
Substitution at the DPhe7 position with Ala resulted in potency losses (180- to 2370-fold) at the melanocortin receptors. Previous reports of a DAla substitution at this position observed potency losses of 1190-fold47 and 770-fold46 at the hMC4R compared to NDP-MSH. Both LAla and DAla have similar potency losses when substituted at position seven, highlighting the importance of the DPhe residue and that the stereochemistry of the substituting Ala at this position appears to have little effect. An interesting comparison can be made between [Ala7]NDP-MSH and [Ala7]α-MSH, as the only difference in these peptides is the Nle versus Met at the fourth position. These ligands (within experimental error) are equipotent at the mMC1R and mMC5R, and unexpectedly the [Ala7]α-MSH is 10- and 16-fold more potent at the mMC3R and mMC4R, respectively, than [Ala7]NDP-MSH. These data suggest that Nle substitution at the fourth position may not always be a potency enhancing modification.
The Arg8 to Ala substitution also decreased agonist potency at the mMC1R, mMC3R, and mMC4R, though not on the same order of magnitude as either the aromatic Phe7 or Trp9 positions. Previously, a 32-fold agonist potency loss was reported for [Ala8]NDP-MSH at the hMC4R,46 similar to the present 29-fold decreased potency observed at the mMC4R. The smaller potency changes associated with the His6 and Arg8 substitutions imply these residues may be less critical for melanocortin agonist potency relative to the DPhe7 and Trp9 positions. This has also been observed in the melanocortin agonist MTII, where substitution at the equivalent His and Arg positions with Ala, Glu, or Lys were better tolerated than the DPhe or Trp positions of the key tetrapeptide sequence.60, 61
Substitution at the Trp9 position generated [Ala9]NDP-MSH that possessed 180- to 2120-fold decreased agonist potency relative to NDP-MSH. This peptide was reported not to activate the hMC4R at concentrations up to 100 μM.46 An additional investigation reported this peptide to possess some agonist activity at the hMC3R (34% of NDP-MSH maximum signal at 1 μM) and the hMC5R (26% at 1.5 μM), was a partial agonist at the hMC4R (22% maximum signal with EC50 = 2.5 μM), and was a full agonist at the hMC1R (3-fold less potent than NDP-MSH).48 These reported alteration in agonist activity correlate with the current data, underscoring the importance of this position in the agonist signaling of NDP-MSH.
Only substitution at the Pro12 position with Ala in the final four positions altered agonist potency compared to NDP-MSH, with the [Ala12]NDP-MSH peptide possessing a 4- and 5- fold potency loss at the mMC1R and mMC4R, respectively. Truncation of these NDP-MSH residues have been reported to affect the mMC5R (6-fold potency loss compared to NDP-MSH)28 and resulted in a 13-fold potency loss in the frog skin bioassay,59 suggesting these residues are not critical for agonist potency.
Template Comparison of α-MSH and NDP-MSH
A comparison of the Ala positional scans of α-MSH and NDP-MSH illustrates some differences in the resulting structure-activity relationships (Figure 3), in contradiction to previous assumptions by some scientists in the field. At the mMC1R, Ala replacement at the Met4, Glu5, Arg8, and Trp9 positions of α-MSH resulted in 400-, 1560-, 200-, and 570-fold potencies losses compared to α-MSH, while Ala substitutions at the Nle4, Glu5, Arg8, and Trp9 positions in NDP-MSH were 5-, <3-, 6-, and 220-fold less potent, indicating that substitution at more residues in α-MSH may alter agonist potency. At the same receptor, Ala substitution at the DPhe7 position in NDP-MSH possessed 2350-fold decreased potency, while Ala replacement at the Phe7 position of α-MSH decreased potency 210-fold, indicating that the seventh position of NDP-MSH may be more sensitive to substitution than α-MSH.
Figure 3.
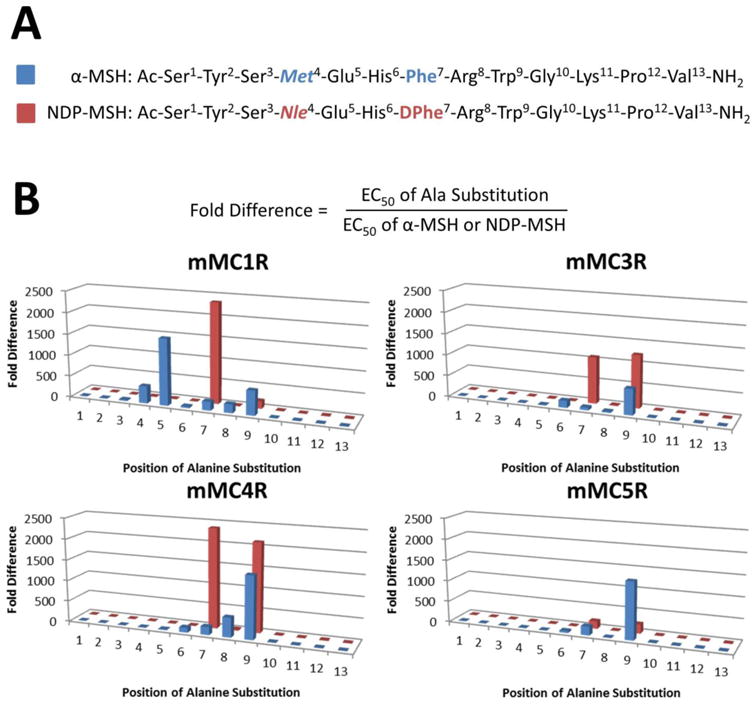
(A) Amino acid sequence and numbering of α-MSH and NDP-MSH. (B) Graphical illustration comparing the fold difference of Ala substitutions in α-MSH and NDP-MSH at the mMC1R, mMC3R, mMC4R, and mMC5R. Fold difference is defined as the EC50 value of a particular substitution divided by the EC50 value of the native sequence of the parent ligand.
A similar trend at the seventh position was observed at the mMC3R, where DPhe7 replacement with Ala in NDP-MSH resulted in 1080-fold decreased agonist potency and substitution at Phe7 with Ala in α-MSH decreased potency 64-fold. Substitution of Ala at the Trp9 and His6 positions also resulted in different potency losses between the two templates at the mMC3R. Peptide [Ala9]NDP-MSH possessed 1230-fold potency loss compared to NDP-MSH while [Ala9]α-MSH was 600-fold less potent α-MSH. Alanine substitution at His6 in α-MSH resulting in 160-fold decreased potency relative to α-MSH compared to the equipotency of [Ala6]NDP-MSH and NDP-MSH.
At the mMC4R, substitution of Ala at the four residues of the postulated His6-(D)Phe7-Arg8-Trp9 pharmacophores altered the SAR between the two ligand templates. Replacement at His6 with Ala in α-MSH decreased potency 110-fold while the same substitution in NDP-MSH resulted in an equipotent agonist compared to NDP-MSH. Alanine substitution at Phe7 decreased potency 190-fold in α-MSH, while Ala replacement of DPhe7 in NDP-MSH decreased potency 2370-fold. At the Arg8 position, Ala substitution in α-MSH decreased agonist potency 470-fold, while the same substitution in NDP-MSH decreased potency 29-fold. Exchanging Ala at the Trp9 position decreased potency 1500-fold for α-MSH and 2120-fold for NDP-MSH. This same Ala substitution at Trp9 was the only position that possessed different SAR at the mMC5R, with [Ala9]α-MSH possessing 1370-fold and [Ala9]NDP-MSH 220-fold decreased potency.
The results of these Ala positional scans performed in parallel indicate several substituted residues that result in different magnitudes of agonist potency change between α-MSH and NDP-MSH, experimental demonstrating differential SAR between α-MSH and NDP-MSH. These positions are found within and outside the postulated His-(D)Phe-Arg-Trp pharmacophores of the melanocortin agonists. At the mMC1R, Ala substitution at the Met4 and Glu5 positions (residues not part of the tetrapeptide melanocortin core sequence) resulted in 400- and 1560- fold potency loss, respectively, while the equivalent positions in NDP-MSH (Nle4 and Glu5) were 5-fold less potent and equipotent (Figure 3B). Replacing one of the four residues in the purported melanocortin agonist pharmacophore [His-(D)Phe-Arg-Trp] resulted in different potency fold changes between α-MSH and NDP-MSH at the mMC4R (Figure 3B).
These observations are in contrast to the dogma in the field that the α-MSH and NDP-MSH templates would possess similar structure-activity relationships. In the previous reported Ala positional scans of α-MSH, the Ala substitution at the Arg8 position had the largest effect on binding.43, 44 The [Ala8]α-MSH peptide possessed 2080-fold decreased affinity in mouse melanoma cells and 200-fold decreased affinity in HEK293 cells expressing the rMC3R;43, 44 the [Ala8]α-MSH peptide also possessed 100-fold decreased tyrosinase potency in the mouse melanoma cells.43 These results have been interpreted to indicate “that Arg8 and Trp9 are the most important residues in the MSH core for receptor binding”62 and “the importance of Arg8 in the activation of MC1R by Arg8-containing melanotropin derivatives in various species.”63 While the 200- and 470-fold potency losses possessed by the [Ala8]α-MSH peptide at the mMC1R and mMC3R observed in the present study support the importance of the Arg8 position, the 6- and 29-fold potency losses reported for the [Ala8]NDP-MSH peptide at the mMC1R and mMC3R does not support claims of the importance of the Arg8 position. These observation imply that specific residues should not be assumed to possess similar activities across different melanocortin ligands and broad conclusions should not be drawn from studies performed on one ligand. In order to investigate how well structure-activity relationships translate between ligands, the ligands have to be investigated in parallel in studies like the present one. While Ala substitution at many residues result in similar potency changes in both α-MSH and NDP-MSH, the experimental data presented herein demonstrate that modifications at every residue does not result in similar functional effects between these two ligands and should be interpreted separately. Similarly, these results should be used cautiously in the design and data interpretation of other melanocortin agonists based upon β-MSH, γ-MSH, and other peptide templates.
Conclusion
The present study was undertaken to perform, in parallel, an Ala positional scan of α-MSH at the cloned mouse receptors, as well as report for the first time, a complete Ala positional scan of NDP-MSH at the same receptors. While two residues of the purported pharmacophore of NDP-MSH were shown to have a large influence on agonist potency, additional residues outside the key agonist tetrapeptide sequence of α-MSH were also shown to affect potency. In particular, the Met4 and Glu5 positions of α-MSH were shown to decrease agonist potency 400- and 1560-fold at the mMC1R, and this knowledge may be useful in the development of future selective melanocortin ligands based upon α-MSH. Perhaps most importantly, by performing an Ala scan of both ligands in parallel it was shown that different positions influenced agonist potency in α-MSH and NDP-MSH. This suggests that the structure-activity relationships from one of these ligands may not be valid for the other and should not be assumed across different melanocortin ligands.
Methods
Peptide synthesis
Peptides were synthesized on a semi-automated synthesizer (Advanced ChemTech, Louisville, KY) using standard Fmoc methodology.50 The amino acids Fmoc-Trp(Boc), Fmoc-Arg(Pbf), Fmoc-Phe, Fmoc-His(Trt), Fmoc-Tyr(tBu), Fmoc-Val, Fmoc-Lys(Boc), Fmoc-Pro, Fmoc-Gly, Fmoc-Met, Fmoc-Glu(OtBu), Fmoc-Nle, Fmoc-DPhe, and Fmoc-Ser(tBu), coupling reagents 2-(1-H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and 1-hydroxybenzotriazole (HOBt), and the rink-amide-MBHA resin were purchased from Peptides International. Dichloromethane (DCM), methanol (MeOH), acetonitrile (ACN) and anhydrous ethyl ether were purchased from Fisher (Fair Lawn, NJ, USA). N,N-Dimethylformamide (DMF) was purchased from Burdick and Jackson (McGaw Park, IL, USA). Trifluoroacetic acid (TFA), acetic anhydride, pyridine, 1,3-diisopropylcarbodiimide (DIC) and piperidine were purchased from Sigma (St. Louis, MO, USA). N,N-Diisopropylethylamine (DIEA), triethylsilane (Et3SiH), triisopropylsilane (TIS), p-cresol and p-thiocresol were purchased from Aldrich (Milwaukee, WI, USA). All reagents and chemicals were ACS grade or better and were used without further purification.
The peptides were assembled on a rink-amide-MBHA resin (0.40 meq/g substitution), using a 0.1 mmol scale. The syntheses consisted of the following steps: (i) removal of the Fmoc group by 25% piperidine in DMF (1 × 5 min, 1 × 20 min) and (ii) coupling the Fmoc-amino acid (3 eq) first with DIC (3 eq) and HOBt (3 eq) for 1 h, followed by a second coupling step with the Fmoc-amino acid (3 eq), HBTU (3 eq), and DIEA (5.1 eq) for 1 h. The presence or absence of the free N-α-amino group was monitored using the Kaiser test (the chloranil test was used for the secondary amine of proline residues).64, 65 Following removal of the terminal Fmoc group, the N-α-amine was acetylated by mixing with a 2:1:1 solution of acetic anhydride, pyridine and DMF for 30 min. After synthesis completion, peptides were cleaved from the resin and side chain deprotected using either a 89.9% TFA, 5% water, and 5% Et3SiH, 0.05% p-cresol and 0.05% p-thiocresol cleavage cocktail (α-MSH analogs) or a 95% TFA, 2.5% water, and 2.5% TIS solution (NDP-MSH analogs) for 3 h. Peptides were precipitated and washed using cold (4 °C) anhydrous ethyl ether. The crude peptide yields ranged from 60 to 90% of the theoretical yields. All peptide were purified by RP-HPLC using a Shimadzu chromatography system with a photodiode array detector and a semi-preparative RP-HPLC C18 bonded silica column (Vydac 218TP1010, 1.0 × 25 cm) and lyophilized. The purified peptides were at least >95% pure as determined by analytical RP-HPLC in two diverse solvent systems and had the correct molecular mass (University of Florida Protein Core Facility).
β-Galactosidase cAMP Functional Bioassay
HEK293 cells stably expressing the melanocortin receptors were transfected with 4 μg CRE/β-galactosidase reporter gene as previously described.51 Briefly, 5,000 to 15,000 post-transfection cells were planted into 96 well Primera plates (Falcon) and incubated overnight. Forty-eight hours post-transfection the cells were stimulated with 100 μL of peptide (10-4 to 10-12 M) or forskolin (10-4 M) control in assay medium (DMEM containing 0.1 mg/mL BSA and 0.1 nM isobutylmethylxanthine) for 6 hrs. Peptides were initially dissolved in water at 10-2 M and serially diluted with assay medium before being added to the cells. The assay media was aspirated and 50 μL of lysis buffer (250 mM Tris-HCl pH=8.0 and 0.1% Triton × 100) was added. The plates were stored and -80 °C overnight. The plates containing the cell lysates were thawed the following day. Aliquots of 10 μL were taken from each well and transferred to another 96-well plate for relative protein determination. To the cell lysate plates, 40 μL of phosphate buffered saline with 0.5% BSA was added to each well. Subsequently, 150 μL of substrate buffer (60 mM sodium phosphate, 1 mM MgCl2, 10 mM KCl, 5 mM β-mercaptoethanol, 2 mg/mL ONPG) was added to each well and the plates were incubate at 37 °C. The sample absorbance, OD405, was measured using a 96-well plate reader (Molecular Devices). The relative protein was determined by adding 200 μL of 1:5 dilution Bio Rad G250 protein dye:water to the 10 μL cell lysate sample taken previously, and the OD595 was measured on a 96-well plate reader (Molecular Devices). Data points were normalized both to the relative protein content and non-receptor dependent forskolin stimulation.
Data Analysis
The EC50 values represent the mean of duplicate replicates performed in at least three independent experiments. The EC50 estimates and their associated standard errors of the mean (SEM) were determined by fitting the data to a nonlinear least-squares analysis using the PRISM program (v4.0, GraphPad Inc.). The results are not corrected for peptide content.
Acknowledgments
The authors would like to thank Skye R. Doering for his design of the TOC graphic.
Funding Sources: This work has been supported by NIH Grants R01DK57080 and R01DK91906 (C.H.-L.). Aleksandar Todorovic is a recipient of the American Heart Association Predoctoral Fellowship. Mark Ericson is a recipient of an NIH F32 Postdoctoral Fellowship (F32DK108402).
Abbreviations
- ACTH
Adrenocorticotropin Hormone
- Fmoc
9-fluorenylmethoxycarbonyl
- AGRP
Agouti-Related Protein
- GPCR
G Protein-Coupled Receptor
- cAMP
cyclic 5′-adenosine monophosphate
- MC1R
Melanocortin-1 Receptor
- MC2R
Melanocortin-2 Receptor
- MC3R
Melanocortin-3 Receptor
- MC4R
Melanocortin-4 Receptor
- MC5R
Melanocortin-5 Receptor
- MCR
Melanocortin Receptor
- MSH
Melanocyte Stimulating Hormone
- POMC
Proopiomelanocortin
- α-MSH
Alpha-Melanocyte Stimulating Hormone
- β-MSH
Beta-Melanocyte Stimulating Hormone
- γ-MSH
Gamma-Melanocyte Stimulating Hormone
- μM
Micromolar
- NDP-MSH (4-Norleucine-7-D-Phenylalanine)
Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2
- Nle
norleucine
- RP-HPLC
reverse-phase high-pressure liquid chromatography
- SAR
structure-activity relationships
Footnotes
Author Contributions: AT. and C.H.-L. designed the research. A.T., R.P., N.B.S., M.S.W. and Z.X. performed the experiments. A.T., M.D.E. and C.H.-L. analyzed the data. M.D.E. wrote the manuscript with the help of A.T. and C.H.-L.
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 2.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 3.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 4.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 5.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 6.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinol. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 7.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 8.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 9.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irani BG, Xiang ZM, Yarandi HN, Holder JR, Moore MC, Bauzo RM, Proneth B, Shaw AM, Millard WJ, Chambers JB, Benoit SC, Clegg DJ, Haskell-Luevano C. Implication of the melanocortin-3 receptor in the regulation of food intake. Eur J Pharmacol. 2011;660:80–87. doi: 10.1016/j.ejphar.2010.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 14.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 16.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 17.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 18.Yang YK, Thompson DA, Dickinson CJ, Wilken J, Barsh GS, Kent SB, Gantz I. Characterization of Agouti-related protein binding to melanocortin receptors. Mol Endocrinol. 1999;13:148–155. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]
- 19.Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: Identification of Agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochem. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 20.Xiang ZM, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the Agouti-related protein (AGRP) antagonist. Biochem. 2006;45:7277–7288. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- 21.Xiang ZM, Proneth B, Dirain ML, Litherland SA, Haskell-Luevano C. Pharmacological characterization of 30 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists, synthetic agonists, and the endogenous Agouti-related protein antagonist. Biochem. 2010;49:4583–4600. doi: 10.1021/bi100068u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eipper BA, Mains RE. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem. 1978;253:5732–5744. [PubMed] [Google Scholar]
- 23.Eipper BA, Mains RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980;1:1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka H, Inouye K. Syntheses of peptides related to the N-terminal structure of corticotropin III. The synthesis of L-histidyl-L-phenylalanyl-L-arginyl-L-tryptophan, the smallest peptide exhibiting the melanocyte-stimulating and the lipolytic activities. Bull Chem Soc Jpn. 1964;37:1465–1471. [Google Scholar]
- 25.Otsuka H, Inouye K. The synthesis of an MSH-active tetrapeptide, L-histidyl-L-phenylalanyl-L-arginyl-L-tryptophan. Bull Chem Soc Jpn. 1964;37:289–290. [Google Scholar]
- 26.Castrucci AM, Hadley ME, Sawyer TK, Wilkes BC, al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao KR, Hruby VJ. α-melanotropin: the minimal active sequence in the lizard skin bioassay. Gen Comp Endocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 27.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, Castrucci AM, Hintz MF, Riehm JP, Rao KR. α-Melanotropin: the minimal active sequence in the frog skin bioassay. J Med Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 28.Haskell-Luevano C, Holder JR, Monck EK, Bauzo RM. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. J Med Chem. 2001;44:2247–2252. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 29.Heward CB, Yang YCS, Sawyer TK, Bregman MD, Fuller BB, Hruby VJ, Hadley ME. Iodination associated inactivation of β-melanocyte stimulating hormone. Biochem Bioph Res Co. 1979;88:266–273. doi: 10.1016/0006-291x(79)91725-x. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer TK, Yang YCS, Bregman MD, Hruby VJ, Heward CB, Fuller BB, Hadley ME. Structure-function studies of melanophore stimulating hormones (α-MSH and β-MSH) and their analogs on melanoma plasma membrane adenylate cyclase: comparison with frog skin melanophores. In: Gross E, Meienhofer J, editors. Sixth American Peptide Symposium. Pierce Chemical Company: Georgetown University; Washington D.C: 1979. pp. 1017–1020. [Google Scholar]
- 31.Medzihradszky K. Synthesis and biological activity of adrenocorticotropic and melanotropic hormones. In: Bognar R, Bruckner V, Szantay C, editors. Recent developments in the chemistry of nautral carbon compounds. Akademiai Kiado; Budapest, Hungary: 1976. pp. 119–250. [Google Scholar]
- 32.Schnabel E, Li CH. The synthesis of L-histidyl-D-phenylalanyl-L-arginyl-L-tryptophylglycine and its melanocyte-stimulating activity. J Am Chem Soc. 1960;82:4576–4579. [PubMed] [Google Scholar]
- 33.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-phenylalanine-α-melanocyte-stimulating hormone -a highly potent α-melanotropin with ultralong biological-activity. Proc Natl Acad Sci USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haskell-Luevano C, Miwa H, Dickinson C, Hadley ME, Hruby VJ, Yamada T, Gantz I. Characterizations of the unusual dissociation properties of melanotropin peptides from the melanocortin receptor, hMC1R. J Med Chem. 1996;39:432–435. doi: 10.1021/jm950407s. [DOI] [PubMed] [Google Scholar]
- 35.Luger TA, Bohm M. An α-MSH analog in erythropoietic protoporphyria. J Invest Dermatol. 2015;135:929–931. doi: 10.1038/jid.2015.16. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 37.Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- 38.Regoli D, Park WK, Rioux F. Pharmacology of angiotensin. Pharmacol Rev. 1974;26:69–123. [PubMed] [Google Scholar]
- 39.Hinke SA, Manhart S, Speck M, Pederson RA, Demuth HU, McIntosh CH. In depth analysis of the N-terminal bioactive domain of gastric inhibitory polypeptide. Life Sci. 2004;75:1857–1870. doi: 10.1016/j.lfs.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Quartara L, Ricci R, Meini S, Patacchini R, Giolitti A, Amadesi S, Rizzi C, Rizzi A, Varani K, Borea PA, Maggi CA, Regoli D. Ala scan analogues of HOE 140. Synthesis and biological activities. Eur J Med Chem. 2000;35:1001–1010. doi: 10.1016/s0223-5234(00)01182-x. [DOI] [PubMed] [Google Scholar]
- 41.Grieco P, Balse-Srinivasan P, Han G, Weinberg D, MacNeil T, Van der Ploeg LH, Hruby VJ. Synthesis and biological evaluation on hMC3, hMC4 and hMC5 receptors of γ-MSH analogs substituted with L-alanine. J Pept Res. 2002;59:203–210. doi: 10.1034/j.1399-3011.2002.01966.x. [DOI] [PubMed] [Google Scholar]
- 42.Langouche L, Pals K, Denef C. Structure-activity relationship and signal transduction of γ-MSH peptides in GH3 cells: further evidence for a new melanocortin receptor. Peptides. 2002;23:1077–1086. doi: 10.1016/s0196-9781(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 43.Sahm UG, Olivier GW, Branch SK, Moss SH, Pouton CW. Synthesis and biological evaluation of α-MSH analogues substituted with alanine. Peptides. 1994;15:1297–1302. doi: 10.1016/0196-9781(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 44.Sahm UG, Qarawi MA, Olivier GWJ, Ahmed ARH, Branch SK, Moss SH, Pouton CW. The melanocortin (MC3) receptor from rat hypothalamus -Photoaffinity labeling and binding of alanine-substituted α-MSH analogs. FEBS Lett. 1994;350:29–32. doi: 10.1016/0014-5793(94)00725-x. [DOI] [PubMed] [Google Scholar]
- 45.Weeden T, Stefano J, Duan S, Edling A, Hou LH, Chuang WL, Perricone MA, Pan C, Dzuris JL. A retro-inverso α-melanocyte stimulating hormone analog with MC1R-binding selectivity. J Pept Sci. 2011;17:47–55. doi: 10.1002/psc.1306. [DOI] [PubMed] [Google Scholar]
- 46.Fleck BA, Ling N, Chen C. Substituted NDP-MSH peptides paired with mutant melanocortin-4 receptors demonstrate the role of transmembrane 6 in receptor activation. Biochem. 2007;46:10473–10483. doi: 10.1021/bi700406k. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van der Ploeg LHT, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochem. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 48.Bednarek MA, MacNeil T, Tang R, Fong TM, Cabello MA, Maroto M, Teran A. Potent and selective peptide agonists for human melanocortin receptors 1b and 5. J Pept Sci. 2008;14:401–408. [Google Scholar]
- 49.Schioth HB, Tesfaye A, Mutulis F, Rudzish R, Mutule I, Muceniece R, Watanobe H, Wikberg JES. Subtype selective binding properties of substituted linear melanocyte stimulating hormone analogues. Neuropeptides. 2002;36:427–434. doi: 10.1016/s0143-4179(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 50.Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J Am Chem Soc. 1970;92:5748–5749. [Google Scholar]
- 51.Chen WB, Shields TS, Stork PJS, Cone RD. A colorimetric assay for measuring activation of G(s)-coupled and G(q)-coupled signaling pathways. Anal Biochem. 1995;226:349–354. doi: 10.1006/abio.1995.1235. [DOI] [PubMed] [Google Scholar]
- 52.Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19:206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JES. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 54.Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. 1. Modifications at the His position. J Med Chem. 2002;45:2801–2810. doi: 10.1021/jm0104872. [DOI] [PubMed] [Google Scholar]
- 55.Joseph CG, Yao H, Scott JW, Sorensen NB, Marnane RN, Mountjoy KG, Haskell-Luevano C. γ2-Melanocyte stimulation hormone (γ2-MSH) truncation studies results in the cautionary note that γ2-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31:2304–2313. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martens GJM. Expression of 2 proopiomelanocortin genes in the pituitary-gland of Xenopus laevis - Complete structures of the 2 preprohormones. Nucleic Acids Res. 1986;14:3791–3798. doi: 10.1093/nar/14.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martens GJM. Structural organization of the proopiomelanocortin gene in Xenopus laevis 5′-end homologies within the toad and mammalian genes. Eur J Biochem. 1987;165:467–472. doi: 10.1111/j.1432-1033.1987.tb11462.x. [DOI] [PubMed] [Google Scholar]
- 58.Martens GJM, Civelli O, Herbert E. Nucleotide-sequence of cloned cDNA for proopiomelanocortin in the amphibian Xenopus laevis. J Biol Chem. 1985;260:3685–3689. [PubMed] [Google Scholar]
- 59.Haskell-Luevano C, Sawyer TK, Hendrata S, North C, Panahinia L, Stum M, Staples DJ, Castrucci AM, Hadley MF, Hruby VJ. Truncation studies of α-melanotropin peptides identify tripeptide analogues exhibiting prolonged agonist bioactivity. Peptides. 1996;17:995–1002. doi: 10.1016/0196-9781(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 60.Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van der Ploeg LH, Weinberg DH. Analogs of lactam derivatives of α-melanotropin with basic and acidic residues. Biochem Biophys Res Commun. 2000;272:23–28. doi: 10.1006/bbrc.2000.2589. [DOI] [PubMed] [Google Scholar]
- 61.Bednarek MA, Silva MV, Arison B, MacNeil T, Kalyani RN, Huang RR, Weinberg DH. Structure-function studies on the cyclic peptide MT-II, lactam derivative of α-melanotropin. Peptides. 1999;20:401–409. doi: 10.1016/s0196-9781(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 62.Schioth HB, Mutulis F, Muceniece R, Prusis P, Wikberg JES. Selective properties of C- and N-terminals and core residues of the melanocyte-stimulating hormone on binding to the human melanocortin receptor subtypes. Eur J Pharmacol. 1998;349:359–366. doi: 10.1016/s0014-2999(98)00212-x. [DOI] [PubMed] [Google Scholar]
- 63.Cheung AWH, Danho W, Swistok J, Qi L, Kurylko G, Franco L, Yagaloff K, Chen L. Structure-activity relationship of linear peptide Bu-His-DPhe-Arg-Trp-Gly-NH2 at the human melanocortin-1 and-4 receptors: Arginine substitution. Bioorg Med Chem Lett. 2002;12:2407–2410. doi: 10.1016/s0960-894x(02)00459-6. [DOI] [PubMed] [Google Scholar]
- 64.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 65.Christensen T. Qualitative test for monitoring coupling completeness in solid-phase peptide-synthesis using chloranil. Acta Chem Scand, Ser B. 1979;33:763–766. [Google Scholar]