Sir,
Dutasteride is used off-label worldwide in the treatment of androgenetic alopecia (AGA) and has proved its efficacy in different studies.[1] Mesotherapy using dutasteride (MD) has also been used in patients with AGA.[2,3,4] Therapy schedules reported involve frequent treatment sessions,[3,4] what is a limitation for some patients and may difficult the treatment adherence. The objective of our study was to evaluate the effectiveness and safety of a new treatment protocol with MD performing sessions each 3 months.
A prospective clinical study including patients diagnosed with AGA was designed. MD was performed with 1 mL of intradermal dutasteride 0.01% injections (dutasteride 0.01%, Mesotherapy Worldwide, Australia) through 6 months with a one-session treatment every three months; a total of three sessions.
Assessment of the response was done 9 months after the first injection by three dermatologists who evaluated the degree of improvement as follows: −1 = worsening, 0 = no change, 1 = improvement. To evaluate the safety of the therapy, a strict surveillance for adverse effects was done. In addition, laboratory investigations (total and free testosterone, 5-alpha-dihydritestosterone, and 3-alpha-androstanediol glucuronide) were performed before and after treatment to analyse if the treatment produced any systemic hormonal modification. Statistical analysis with the Wilcoxon signed-rank test was performed to detect significance between laboratory results.
Six patients (five men and one woman) with a mean age of 36.7 years (range 31–63) were included in the study. An improvement of AGA was observed with MD in all cases with an increase of hair density and hair diameter in trichoscopy [Figure 1]. No adverse effects were recorded during the treatment sessions and follow-up period. Laboratory tests showed no differences between serum hormone levels before and after treatment. The Wilcoxon signed-rank test confirmed that there were no differences statistically significative (P > 0.05).
Figure 1.
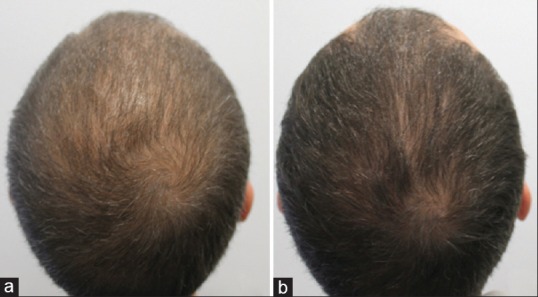
Androgenetic alopecia in a 33-year-old man before (a) and after (b) treatment with dutasteride injections through 6 months with a one-session treatment every 3 months
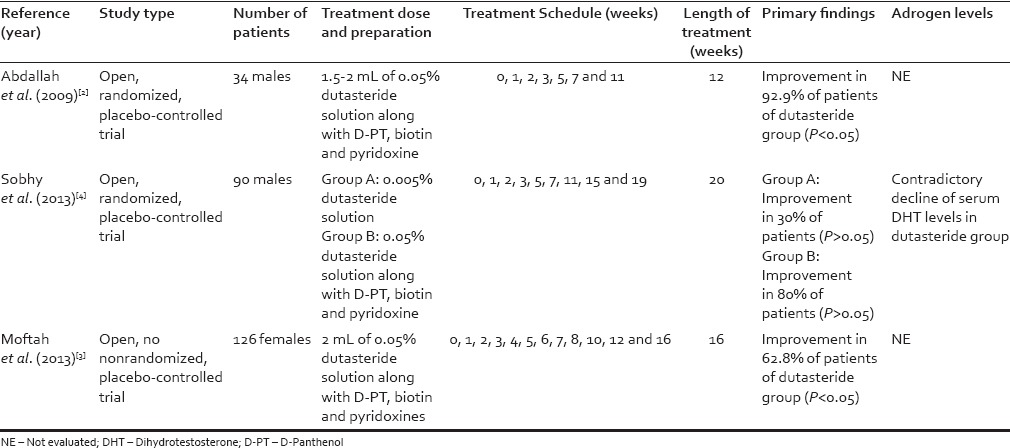
Some trials compared mesotherapy of dutasteride-containing solution with placebo [Table 1][2,3,4] and they detected a significant improvement of hair. Treatment schedule in all these studies involves intensive treatment sessions, with weekly injections for the 1st month in all patients. Dutasteride has a long half-life (approximately four weeks) and it is more powerful than finasteride in inhibiting Type I and II 5-alpha-reductase.[5] From our point of view, an easier treatment schedule may be carried out without entailing a loss of efficacy.
Table 1.
Main studies of treatment of androgenetic alopecia with mesotherapy with dutasteride reported in the literature
The role of 5-alpha-reductase inhibitors disrupting hormonal levels is a controversial issue. Oral dutasteride (0.5 mg/day) decreases serum dihydrotestosterone levels by more than 90% and increases serum testosterone levels.[5] It is believed that systemic absorption after mesotherapy is equal to after oral dutasteride because the scalp is highly vascular.[4] We did not detect significant differences between serum hormone levels before and after treatment, suggesting that the absorption of dutasteride after a nonintensive treatment protocol is worthless. The limitations of our study are the small sample size and the short follow-up. Nevertheless, we consider of interest our preliminary results that may be the basis for future research.
In conclusion, mesotherapy with dutasteride may be an effective therapy for patients with AGA even with less intensive treatment schedules, as sessions once every 3 months.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252–8. doi: 10.1016/j.jaad.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah MA, El-Zawahry KA, Besar H. Mesotherapy using dutasteride-containing solution in male pattern hair loss: A controlled pilot study. J Pan Arab Leag Dermatol. 2009;20:137–45. [Google Scholar]
- 3.Moftah N, Moftah N, Abd-Elaziz G, Ahmed N, Hamed Y, Ghannam B, et al. Mesotherapy using dutasteride-containing preparation in treatment of female pattern hair loss: Photographic, morphometric and ultrustructural evaluation. J Eur Acad Dermatol Venereol. 2013;27:686–93. doi: 10.1111/j.1468-3083.2012.04535.x. [DOI] [PubMed] [Google Scholar]
- 4.Sobhy N, Aly H, El Shafee A, El Deeb M. Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males. Our Dermatol Online. 2013;4:40–5. [Google Scholar]
- 5.Azzouni F, Godoy A, Li Y, Mohler J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv Urol. 2012;2012:530121. doi: 10.1155/2012/530121. [DOI] [PMC free article] [PubMed] [Google Scholar]


