Abstract
Leuprolide acetate (LEU), also known as Lupron, is commonly used to treat prostate cancer in men. As a gonadotropin-releasing hormone (GnRH) receptor agonist, it initially stimulates the release of gonadal hormones, testosterone (T) and estradiol. This surge eventually suppresses these hormones, preventing the further growth and spread of cancer cells. Individuals receiving this treatment often report anxiety and cognitive changes, but LEU’s effects on the neural mechanisms that are involved in anxiety during the trajectory of treatment are not well known. In this study, we examined the acute effects of LEU on fear extinction, hypothesizing that increased T levels following a single administration of LEU will facilitate extinction recall by altering neuronal activity within the fear extinction circuitry. Two groups of naïve adult male rats underwent a 3-day fear conditioning, extinction, and recall experiment. The delayed group (n=15) received a single injection of vehicle or LEU (1.2mg/kg) 3 weeks before behavioral testing. The acute group (n=25) received an injection one day after fear conditioning, 30 minutes prior to extinction training. Following recall, the brains for all animals were collected for c-fos immunohistochemistry. Blood samples were also collected and assayed for T levels. Acute administration of LEU increased serum T levels during extinction training and enhanced extinction recall 24h later. This enhanced extinction memory was correlated with increased c-fos activity within the infralimbic cortex and amygdala, which was not observed in the delayed group. These results suggest that the elevation in T induced by acute administration of LEU can influence extinction memory consolidation, perhaps through modification of neuronal activity within the infralimbic cortex and amygdala. This may be an important consideration in clinical applications of LEU and its effects on anxiety and cognition.
Keywords: testosterone, Lupron, leuprolide acetate, anxiety, fear extinction, c-fos
Graphical Abstract
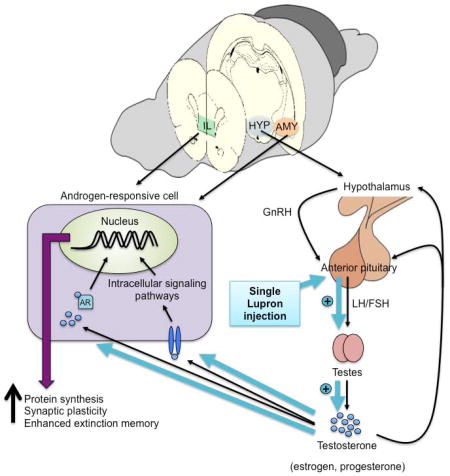
1. Introduction
There is growing evidence that gonadal hormones influence fear and anxiety symptoms. The anxiolytic effects of testosterone (T) have been demonstrated across numerous studies (Berglund et al., 2011; Carrier et al., 2015; Fernández-Guasti and Martínez-Mota, 2005; McHenry et al., 2014; Wainwright et al., 2016; Wang et al., 1996); however, little is known about its influence on fear extinction circuitry. There is substantial evidence suggesting that low T may be a vulnerability factor for pathological anxiety in men. Hypogonadal men (those suffering from decreased gonadal function) are more likely than men with normal T levels to develop anxiety-and stress-related disorder (Shores et al., 2004; DiBlasio et al., 2008; Zarrouf et al., 2009). The risk of developing pathological anxiety can also be reversed with T administration. In hypogonadal men, or men taking T-reducing agents, including most prostate cancer drugs (such as Lupron), T replacement therapy improves affect and reduces anxiety and depression (Wang et al., 1996; Pope et al., 2003; Kanayama et al., 2007; Zarrouf et al., 2009). Moreover, in castrated male rodents, T replacement ameliorates anxiety behaviors (Hodosy et al., 2012; Khakpai, 2014). Within the clinical population, there is evidence that T is reduced in men with post-traumatic stress disorder (PTSD). Cerebrospinal fluid levels of T are significantly reduced in veterans with PTSD compared to healthy volunteers (Mulchahey et al., 2001), and soldiers undergoing exercises that induce psychological stress showed reduction in T levels (Morgan et al., 2000). A single T administration was also found to reduce the startle reflex, further implicating a role for T in PTSD (Hermans et al., 2006).
We have previously shown that endogenous elevation, or exogenous administration, of estrogen (E2) in female rodents and women significantly improved fear extinction retention and increased functional activation of the fear extinction network (Milad et al., 2009; Zeidan et al., 2011; Graham and Milad, 2013). This may be indirectly related to levels of T, as it is converted to E2 via the enzyme aromatase. We have shown that when fadrozole, an aromatase inhibitor, is administered to males, extinction memory consolidation is impaired, an effect that can be reversed with E2 administration (Graham and Milad, 2014). We have also found that in men, extinction learning is better acquired in the morning and that the higher the T level (relative to cortisol), the greater the extinction learning (Pace-Schott et al., 2013). These findings strongly suggest that testosterone can modulate extinction learning; however, we have not previously examined T in our fear extinction protocol.
Leuprolide acetate (LEU), also known as Lupron, is commonly prescribed to treat prostate cancer in men, while also used to treat gonadal hormone-related diseases such as ovarian cancer, endometriosis, and uterine fibroids in women. Its mechanism of action involves binding to the (gonadotropin-releasing hormone) GnRH receptors in the anterior pituitary gland, initiating the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The surge in LH and FSH induces the production and increased secretion of gonadal hormones (testosterone, progesterone, and estradiol) from the testes or ovaries. The increased release of gonadal hormones is involved in a negative feedback loop that saturates and desensitizes the receptors in the pituitary that ultimately suppress the release of gonadal hormones to induce a chemical castration. LEU administration has also been associated with increased levels of anxiety and cognitive impairments in both men and women (Warnock and Bundren, 1997; Almeida et al., 2004; Cherrier et al., 2009). Although the peripheral effects of LEU have been well studied, its influences in the brain are not well understood. The first aim of this study was to delineate the trajectory of serum testosterone levels following acute LEU treatment. Using this information, we then aimed to localize the central effects of LEU during fear extinction to not only identify the neural underpinnings of its effects on emotion and fear-related symptoms, but to also utilize LEU’s mechanism of action, which allowed us to examine the effects of manipulating endogenous levels of gonadal hormones on fear extinction circuitry without using invasive strategies.
Here we investigated how a single administration of LEU: 1) alters serum testosterone levels over several time points, 2) acutely modulates fear extinction learning and retention, 3) mediates fear extinction long after treatment, and 4) influences neuronal activity as measured by immediate early gene c-fos within critical brain regions of the fear extinction network. We hypothesized that elevated levels of testosterone induced by LEU treatment would enhance extinction memory consolidation. Based on previous literature, we also hypothesized that this enhancement would be associated with increased neuronal activity within the infralimbic cortex, which would strengthen modulation of its downstream targets.
2. Material and methods
2.1 Subjects
Adult male Sprague Dawley rats (250–300g) were obtained from Harlan Laboratories. Following one week of acclimation to the animal holding facility at Massachusetts General Hospital, the animals were handled daily for one week. Animals in Experiment 1 were held and handled daily for 5 minutes each. Handling for Experiment 2 rats consisted of an additional massaging of the tails and brief restraint as described in the blood collection procedure. This was done to minimize potential stress during blood collections. For the acute serum testosterone data, we used the same animals from Experiment 2 to: 1) reduce the number of animals used in the study and 2) avoid any potentially disruptive and confounding effects of collecting blood multiple times during the behavioral tasks across the 3 days of the experiment. All procedures conducted in this study were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital and abide by the guidelines set forth by the Institutional Animal Care and Use Committee.
2.2 Serum collection and analysis
For experiment 1, blood samples were collected prior to transcardial perfusions via cardiac puncture. These blood samples were collected 24 h after injections. For experiment 2, tail vein blood sampling was used. For the tail vein blood collections, tap water was warmed to approximately 45°F, and the rat tails were submerged in the water for at least 1 minute for dilation of blood vessels. After locating the lateral tail vein, a 25-gauge butterfly needle connected to a 1mL luer-lok syringe was inserted bevel-up, and blood was collected via the tubing and syringe. All blood samples were allowed to clot for at least 90 minutes prior to centrifugation at 4,000 g for 5 minutes. For Experiment 2, baseline blood samples were taken 2 days before the injections of vehicle or LEU. Tail vein blood was then taken at the following time points after injections: 30 minutes, 65 minutes, 100 minutes, 4 hours, 1 day, and then before extinction and recall at 21 days and 22 days, respectively. The 30, 65, and 100 minute time points were chosen to align with the beginning, middle, and end of the extinction training session. The 4 hour time point was included because we have previously found effects of estradiol on extinction memory occur within this time window after extinction (Zeidan et al., 2011). All serum samples for Experiment 2 were processed using the mouse/rat testosterone enzyme-linked immunosorbent assay (ELISA; Calbiotech, Spring Valley, CA).
2.3 Behavior
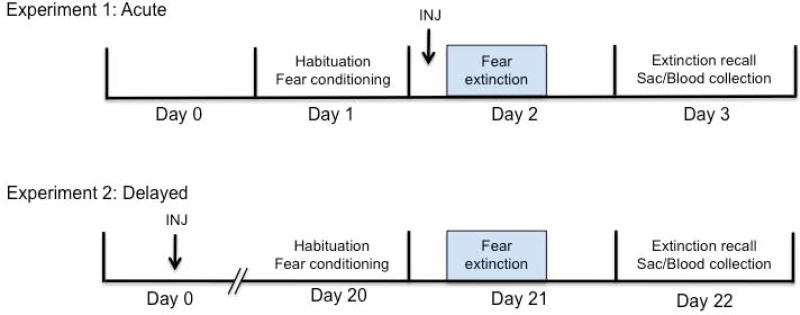
Following daily handling for at least 1 week, all rats were pre-exposed to the experimental chambers for one 30-minute session per day for 3 days. Each animal was assigned to a specific chamber for the pre-exposure sessions and was tested in the same chamber for the duration of the experiment. The behavioral chamber was made of Plexiglas and contained a house light, an audio speaker for the conditioned stimulus (CS) presentations, and a video camera to record behavior. The experiment consisted of 3 phases: habituation/fear conditioning, fear extinction learning, and fear extinction recall. The context and stimulus parameters did not change across these phases. All trials were run with a variable intertrial interval averaging 3 minutes. These behavioral protocols were carried out using GraphicState 3.03 (Coulbourn Instruments, Whitehall, PA). For both experiments 1 and 2, all rats underwent 6 trials of the CS (30-s, 4-kHz, 80-dB tone) alone habituation trials and were then fear conditioned with 6 trials of CS paired with the unconditioned stimulus (US; 0.5-s, 0.5-mA footshock) on day 1. For the paired CS-US presentations during fear conditioning, the CS coterminated with the US. On day 2, all animals underwent extinction training, which consisted of 20 trials of CS alone presentations. For experiment 1, the animals were injected subcutaneously with either 0.9% saline (vehicle; Sigma-Aldrich, St. Louis, MO) or LEU (1.2mg/kg; Sigma-Aldrich, St. Louis, MO) thirty minutes before the extinction session on day 2. The 1.2mg/kg dose was selected based on results from our dose response test to confirm this was the lowest dose to induce the T surge, as well as previous reports examining the castration effects of LEU with chronic administration at doses from 1–3mg/kg for days to months (Ichikawa et al., 1988; Kostanski et al., 2000; Yamamoto et al., 2004). On day 3 for both experiments, the animals were returned to the chamber and presented with 3 CS-alone trials for the extinction recall test. For experiment 2, the 3-day paradigm took place 20 days after the drug injection. An experimental timeline for both experiments has been provided in Figure 1.
Figure 1.
Experimental timeline for Experiment 1 (acute) and Experiment 2 (delayed). In both experiments, the behavioral testing took place over 3 days, animals were sacrificed after recall, and brains were processed for c-fos immunohistochemistry. In the acute experiment, animals received drug injections 24h after fear conditioning and 30min prior to the extinction session. In the delayed experiment, drug injections took place 21 days before the extinction session.
2.4 c-fos immunohistochemistry
One hour following the completion of the recall session, all animals were injected with a lethal dose of Fatal-Plus solution (Vortech Pharmaceutical Ltd., Dearborn, MI). The animals were transcardially perfused with 0.9% saline for 15 minutes and then fixed with 4% paraformaldehyde for 20 minutes, both at a speed of 20ml/min. Brains were removed and stored in 4% paraformaldehyde overnight at 4° C. The brains were then transferred to 20% sucrose potassium phosphate buffered saline (KPBS) solution for 24h and then stored in 30% sucrose KPBS solution at 4°C for cryoprotection. For sectioning on the cryostat, the brains were embedded in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and rapidly frozen. Brain sections were 40-µm thick coronal slices collected at −21 °C.
To process tissue containing the medial prefrontal cortex (mPFC), amygdala, and hippocampus for c-fos immunohistochemistry, we used a modified version of the protocol described in Do-Monte et al. (2015). The brain sections were washed in KPBS (7min × 5), which was followed by an 18h incubation period in a 2% normal goat serum (NGS), 3% Triton-X, KPBS solution with 1:20,000 polyclonal rabbit anti-c-fos antibody (Calbiochem, Billerica, MA) at room temperature (RT). The slices were then incubated in biotinylated goat anti-rabbit IgG secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA), for 2h at RT. After mixing in avidin-biotin horseradish peroxidase complex solution (1:200; Vectastain Elite ABC kit, Vector Laboratories) for 90min at RT, the tissue was placed in 0.02% 3,3'-diaminobenzidine (DAB), 0.3% nickel-ammonium sulfate, 1.2% glucose oxidase sodium PBS solution reacted with D-glucose (10%) for 15–20min. When darkly stained c-fos immunopositive nuclei were observed, the reaction was stopped with KPBS (5min × 5). The sections were then mounted onto gelled slides, which underwent dehydration steps, were cleared with Citrosolv (Fisher Scientific, Pittsburgh, PA), and were cover slipped with DPX medium (Electro Microscopy Sciences, Hatfield, PA).
2.5 c-fos quantification
For positive c-fos expression analyses, we targeted the known critical nodes of the fear extinction circuit and their subregions. Within the mPFC, we examined the prelimbic (PL) and infralimbic (IL) areas. We also looked at c-fos expression within the amygdalar nuclei: basolateral (BLA), centrolateral (CeL), centromedial (CeM), and lateral (LA) amygdala. Finally, we included the dorsal dentate gyrus (DDG), dorsal CA1 (DCA1), dorsal CA3 (DCA3), ventral dentate gyrus (VDG), ventral CA1 (VCA1), and ventral CA3 (VCA3) in these analyses as well. All regions were imaged with a CCD color camera (Moticam Pro 252A, British Columbia, Canada) that was attached to a compound microscope (Motic BA410, British Columbia, Canada) at 20X magnification. Photomicrographs were taken according to areas identified in Paxinos and Watson (2007) for sections of the PL and IL (+3.72mm to +3.00mm from Bregma), amygdala (−2.04mm to −3.24mm from Bregma), dorsal hippocampus (−2.92mm to −3.60mm from Bregma), and ventral hippocampus (−5.40mm to −5.64mm from Bregma). We determined the average c-fos-positive cell counts from 2–4 sections per brain region and used this value to assess neuronal activation within these brain structures. For all images, a rectangular region of interest (ROI) box with dimensions of 268 × 329µm was created for each target brain structure, and c-fos-positive cells within this box were counted using ImageJ software (NIH, Bethesda, MD) by a rater blind to the experimental condition.
2.6 Data analysis
Freezing behavior was represented as a percentage of time spent freezing over the duration of the trial, (seconds animal spent immobile/30s)*100. A mixed-factor analysis of variance (ANOVA) was performed to analyze behavior as blocks of 2-trial averages, using drug as the between-subjects variable and blocks as the within-subjects variable. Freezing behavior during extinction recall was analyzed using an independent samples t-test. In the event that equal variances could not be assumed, we reported the corrected values. For the c-fos results for each brain region, an independent samples t-test was also performed to analyze differences between the drug groups.
3. Results
3.1 Serum testosterone
Blood was collected from Experiment 2 animals 2 days before vehicle or LEU injections for baseline measurements of serum T. Within the same day of the injections, blood was sampled 30min, 65min, 100min, and 4h post-injection to assess the acute effects of a single LEU (1.2mg/kg) injection. A repeated measures ANOVA revealed a main effect of time point [F(3.37, 26.94)=24.72, p=0.000] and drug treatment [F(1,8)=29.89, p=0.001], as well as an interaction between time point and drug treatment [F(3.37, 26.94)=13.34, p=0.000]. An independent samples t-test indicated that serum T levels did not differ between drug groups at baseline [t(11)=-1.125, p=0.285] and 30min [t(13)=-0.300, p=0.769]. However, LEU-treated rats exhibited significantly elevated serum T levels at 65min [t(13)=-2.546, p=0.024], 100min [t(11)=-4.472, p=0.006], and 4h [t(12)=-8.907, p=0.000], time points that coincided with the middle to 4 hours after the end of the extinction training session. These effects were no longer present one day after the injection [t(13)=0.441, p=0.667]. Blood was also sampled at 21 and 22 days after injections to determine the effect of LEU on serum T levels during the delayed extinction training (21d) and extinction recall (22d) sessions of Experiment 2 (Figure 2). An independent samples t-test revealed that serum T levels were significantly reduced with LEU administration at 21 days [t(13)=2.945, p=0.018], but were not affected 22 days post-injection [t(13)=0.380, p=0.710]. The sensitivity, inter-assay, and intra-assay coefficients of variation (CV) were 0–18ng/mL, 9.85% and 6.95%, respectively.
Figure 2.
Effect of Lupron (LEU) on serum testosterone levels across different time points. Blood samples were taken from animals at baseline (2 days before injections), then multiple time points after injections (30min, 65min, 100min, 4h, 1d, 21d, and 22d). Compared to LEU injections (1.2mg/kg) increased serum testosterone (T) levels at the 65min, 100min, and 4h time points. In contrast, LEU administration decreased T levels at 21d. *p<0.05.
3.2 Behavior
3.2.1 Experiment 1
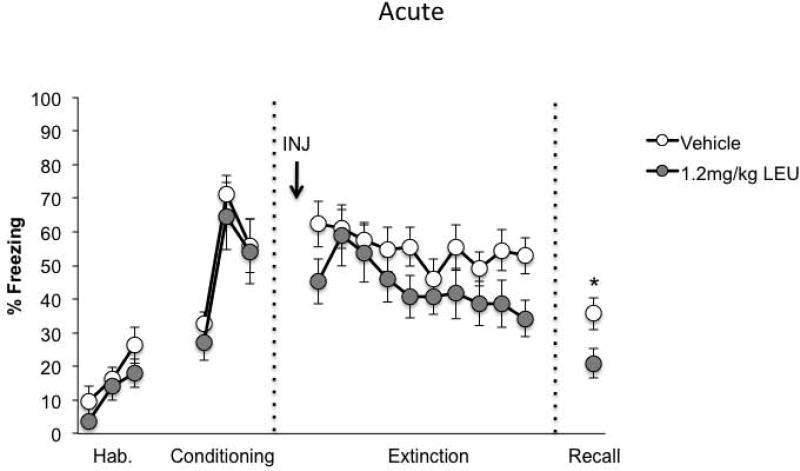
Freezing behavior did not differ between drug groups during the habituation, fear conditioning, and fear extinction phases. However, male rats injected with LEU at the 1.2mg/kg dose exhibited reduced freezing during extinction recall compared to vehicle-treated rats (Figure 3; Vehicle, n=15; LEU, n=10). Behavioral data were analyzed as blocks of 2-trial averages. During the habituation phase, a repeated measures ANOVA revealed a main effect of block [F(2,46)=10.151, p=0.000], but no effect of drug [F(1,23)=1.311, p=0.264] or drug × block interaction [F(2,46)=0.396, p=0.676]. There was also a main effect of fear conditioning block [F(2,46)=25.57, p=0.000], but no effect of drug [F(1,23)=0.320, p=0.577] or drug × block interaction [F(2,46)=0.109, p=0.897]. This suggests that fear learning occurred but was matched across the groups. Similarly, for the extinction session, there was no effect of drug [F(1,23)=2.315, p=0.142] or drug × block interaction [F(9,207)=0.915, p=0.513], but there was a main effect of block [F(9,207)=3.340, p=0.001], indicating that extinction learning occurred. During extinction recall, LEU-treated animals froze significantly less compared to those treated with saline [t(23)=2.118, p=0.045].
Figure 3.
Freezing behavior for animals in Experiment 1 (acute). The percentage of time the animal spent freezing during the trial did not differ between groups during habituation and fear conditioning. LEU-treated animals exhibited reduced freezing during the last block of fear extinction. Freezing was also reduced with Lupron treatment during extinction recall. *p<0.05.
3.2.2 Experiment 2
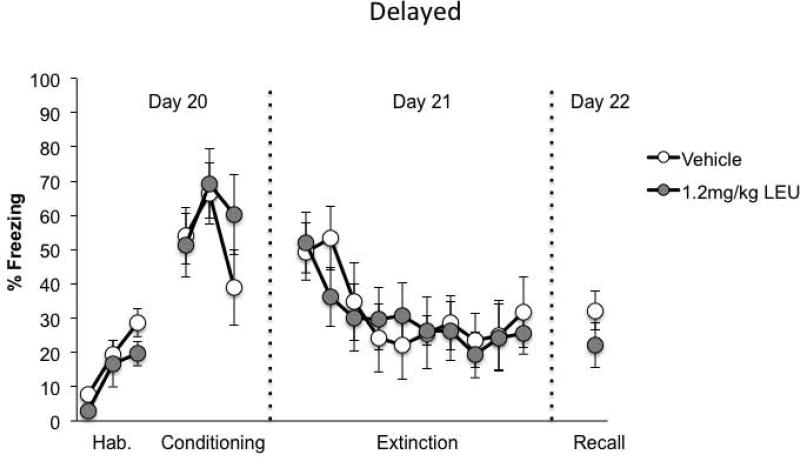
Similar to the behavioral results in Experiment 1, freezing behavior did not differ between the drug groups across the habituation [F(1,13)=2.138, p=0.167], fear conditioning [F(1,13)=0.338, p=0.571], and fear extinction sessions [F(1,13)=0.028, p=0.871] (Figure 4; Vehicle, n=8; LEU, n=7). Likewise, there were no drug × block interactions across the 3 phases: habituation [F(2,26)=0.396, p=0.677], fear conditioning [F(2,26)=2.366, p=0.114], and fear extinction [F(9,117)=0.841, p=0.580]. A repeated measures ANOVA revealed only a main effect of block for these phases: habituation [F(2,26)=13.735, p=0.000], fear conditioning [F(2,26)=5.684, p=0.009], and extinction [F(9,117)=5.850, p=0.000]. In contrast to Experiment 1, the LEU-treated group did not differ from the vehicle-treated animals during extinction recall [t(13)=1.168, p=0.264].
Figure 4.
Freezing behavior for animals in Experiment 2 (delayed). Animals that received LEU injections 20d before the start of the behavioral testing displayed freezing behavior that was not different from those that were injection with saline vehicle across all phases of the experiment.
3.3 c-fos
3.3.1 Experiment 1
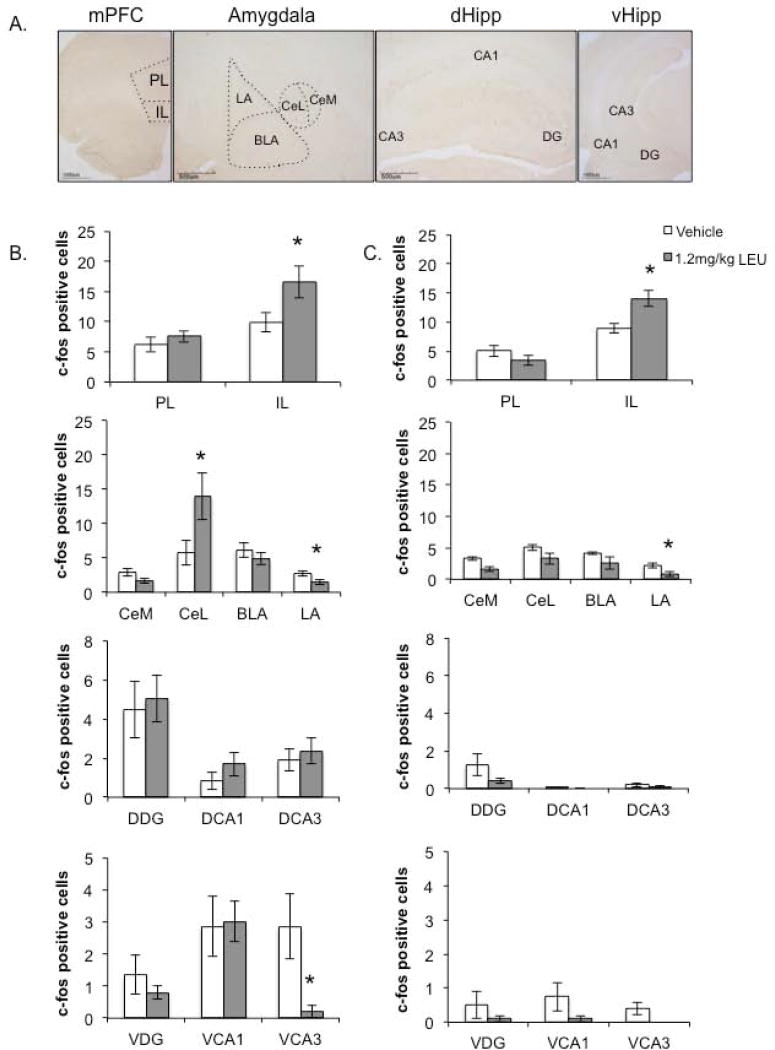
C-fos expression was assessed in the mPFC, amygdala, dorsal hippocampus, and ventral hippocampus (subregions outlined in Figure 5A). An independent samples t-test revealed that male rats that were administered Lupron acutely (Figure 5B) showed no differences in PL c-fos activity [t(22)=-0.735, p=0.410], but significantly increased c-fos expression within the IL compared to those that received vehicle injections [t(22)=-2.314, p=0.030]. Within the amygdala, LEU treatment increased CeL activity [t(21)=-2.325, p=0.030], while reducing c-fos expression in the LA [t(20)=2.095, p=0.032] with no effects in the CeM [t(23)=1.913, p=0.068] and BLA [t(21)=0.840, p=0.410]. Moreover, LEU administration did not induce differences across any of the dorsal hippocampal regions: DDG [t(16)=-0.278, p=0.785], DCA1 [t(16)=-1.188, p=0.252], or DCA3 [t(16)=-0.528, p=0.604]. Similarly, there was no effect of LEU within the ventral hippocampal regions VDG [t(10)=0.749, p=0.471] and VCA1 [t(10)=-0.115, p=0.911], but LEU significantly reduced VCA3 c-fos activation [t(10)=2.172, p=0.039].
Figure 5.
The influence of LEU on c-fos expression within the fear extinction network. A, Photomicrographs of c-fos positive cells within the mPFC, amygdala, and dorsal and ventral hippocampi illustrate the regions of interest (ROIs). B, C-fos expression across ROIs for the acute (Experiment 1) behavioral testing after LEU administration. LEU administration significantly increased c-fos expression within the IL and CeL, while reducing activity within the LA compared to vehicle treatment. C, C-fos expression across ROIs for the delayed (Experiment 2) behavioral testing after Lupron administration. LEU-treated animals exhibited significantly increased IL c-fos activity, but reduced LA activity, compared to their vehicle-treated counterparts. *p<0.05.
3.3.2 Experiment 2
Compared to their vehicle-treated counterparts, rats that were injected with LEU 22 days prior to sacrifice (Figure 5C) exhibited no differences in PL activity [t(11)=1.275, p=0.229], but displayed significantly increased IL c-fos activation [t(11)=-3.210, p=0.008]. For the amygdala, LEU treatment had an effect only on LA activity, inducing a reduction in c-fos expression [t(10)=2.624 p=0.025]. Moreover, administration of LEU had no effect across all of the dorsal and ventral hippocampal subregions: DDG [t(12)=1.498, p=0.160], DCA1 [t(12)=1.000, p=0.356], DCA3 [t(12)=0.846, p=0.414], VDG [t(8)=1.000, p=0.347], VCA1 [t(8)=1.511, p=0.198], and VCA3 [t(8)=2.138, p=0.099].
3.4 c-fos-behavior correlation
Correlational analyses to examine the relationship between c-fos expression and behavior (illustrated in Figure 6) revealed that there was a significant negative correlation between IL c-fos expression and percent freezing during extinction recall for Experiment 1 (r=-0.420, p=0.041). This was not the case for the delayed animals in Experiment 2 in which there was no correlation between c-fos activity and behavior (r=-0.486, p=0.092). There was also a significant negative correlation between CeL c-fos expression and fear expression during extinction recall for animals in Experiment 1 (r=-0.428, p=0.042). However, in Experiment 2, CeL c-fos activity was not correlated with freezing behavior during extinction recall (r=0.112, p=0.730). Therefore, with increased neuronal activity within the IL and CeL, freezing behavior decreased, suggesting that elevated T levels may strengthen the modulatory influence of these extinction-related structures to enhance extinction memory. These were the only sites that exhibited a correlation between neuronal activity and freezing behavior and/or differences between Experiment 1 and Experiment 2.
Figure 6.
Correlational analyses between c-fos activity and freezing behavior during extinction recall. IL (top panel) and CeL (bottom panel) c-fos expression is negatively correlated with freezing behavior during extinction recall in the acute but not delayed experiment. *p<0.05.
4. Discussion
We have demonstrated that a single administration of the GnRH agonist, LEU, improves fear extinction memory retention when extinction takes place close in time with the LEU injection (acute), but has no effect when fear is extinguished weeks after drug treatment (delayed). Interestingly, only in the acute experiment (Experiment 1) was the effect on behavior correlated with increased IL neuronal activity as measured by c-fos expression. Treatment with LEU appeared to alter neuronal activity during extinction recall within certain regions of the fear extinction network compared to controls. Males injected with LEU had significantly more c-fos positive cells within the IL and fewer c-fos positive cells within the LA compared to their vehicle-injected counterparts in both the acute and delayed experiments. In addition, CeL c-fos activity was only significantly increased in the acute experiment. Using a subcutaneous injection of 1.2 mg/kg LEU, we found that in LEU-treated animals serum levels of testosterone were increased. They reached their peak at time points coinciding with the middle and end of extinction training of Experiment 1, when most of the extinction learning has occurred (as observed in this study). At these time points, rapid molecular mechanisms may be activated to begin the memory consolidation process (synaptic consolidation) (Bramham and Messaoudi, 2005). Previous studies have demonstrated that manipulations conducted immediately at the end of training have significant influences on memory consolidation (Aubry et al., 2016; Clopath, 2012; Zeidan et al., 2011). It was only in this acute experiment (Experiment 1) that we observed the significantly enhanced fear extinction memory; there was no difference in extinction recall in the delayed experiment (Experiment 2) in which serum T levels were significantly reduced when extinction training took place. Together, these data suggest that elevated T during extinction training strengthens extinction memory 24h later, but the reduction in T during extinction, as in the delayed group, does not necessarily impair extinction recall. To our knowledge, this is the first study that has characterized the hormonal profile of the acute effects of a single administration of LEU on serum testosterone levels and fear extinction and its neural correlates.
4.1 Influence of testosterone on fear extinction circuitry
Studies have reported fear-reducing properties of testosterone administration (Frye and Seliga, 2001; Aikey et al., 2002). However, very few have examined T’s role in fear extinction and related brain network. The current study begins to delineate the effect of testosterone on the neurocircuitry underlying enhanced fear extinction memory consolidation, finding changes in neuronal activity within the IL and LA. Lupron-induced elevation in T appeared to increase c-fos activity in the IL and decrease LA c-fos activity during extinction recall, which was evident in the acute (24h after injections) experiment and remained in the delayed (22 days after injections) experiment. Thus, it seems that the IL and LA are not critical sites for testosterone action enhancing fear extinction memory. The CeL may be the activation site necessary for the beneficial effects of testosterone on extinction, as CeL neuronal activity was increased during extinction recall only in the acute experiment, which is where we observed the enhanced extinction memory. These data seem to be consistent with other findings that report that androgen receptors in the medial prefrontal cortex or hippocampus (where we also found no effects of Lupron) are not necessary for the anxiolytic effects of testosterone ((Chen et al., 2016)).
We have previously found that exogenous administration of estrogen during extinction training reduces freezing during extinction recall, thereby strengthening the extinction memory in female rats (Zeidan et al., 2011). Furthermore, injecting male rats with an aromatase inhibitor Fadrozole, which prevents the conversion of testosterone to estrogen, was found to impair extinction recall, an effect that was reversed with estrogen administration prior to the fear extinction session (Graham and Milad, 2014). Although it was not examined in this study, these data suggest that testosterone may play a role in modulating fear extinction memory, possibly through its conversion to estrogen, which has also been suggested by Carrier et al. (2015). Recently, we found that estrogen treatment modifies CeL c-fos expression during extinction training to enhance extinction recall, increasing CeL neuronal activity as we found in this study (Maeng et al., 2017). Furthermore, Lynch et al. (2016) demonstrated that contextual fear generalization, another symptomatic feature of PTSD, is more pronounced in males that had been administered Fadrozole and thus had lower levels of estrogen. This finding, together with those of the present study, strongly suggests that testosterone’s conversion to estrogen plays a critical role in enhancing the consolidation of the fear extinction memory in males. Testosterone, like estrogen, can act through either classical (slow) or non-classical (fast) molecular signaling pathways (Papakonstanti et al., 2003; Phan et al., 2011; Walker, 2011; Woolley, 2007). Based on this study’s results, it appears that there may be both an initial non-genomic transcriptional activation by T during extinction training and a longer-lasting genomic process during consolidation to produce the enhanced extinction memory the day after training. The molecular mechanisms underlying T modulation of extinction memory consolidation warrant further investigation to better understand how T improves extinction memory retrieval in our present study.
4.2 Testosterone and anxiety disorders
Epidemiological evidence indicates that men are less likely than women to develop anxiety or stress-related psychiatric disorders. This may be partially due to the fact that males lack the natural fluctuations in gonadal hormones that females experience. Numerous studies have suggested that T may have anxiolytic and neuroprotective properties similar to those that have been reported for estrogen (Carrier et al., 2015; Filova et al., 2015; Almehmadi et al., 2016). For instance, men with higher levels of T reported better quality of life measures than those with lower levels (Cohen et al., 2016). These effects are further supported by reports of positive effects of LEU on mood and memory during the earlier weeks following treatment; however, after some time from the start of treatment, presumably when T levels are significantly suppressed, patients describe negative effects such as anxiety. Since, as previously mentioned, testosterone can be converted to estrogen via aromatization, it is possible that T’s beneficial effects in men may be due to the indirect effects of estrogen via this conversion. In fact, women taking LEU report similar side effects of enhanced estrogen stimulation and the subsequent deprivation, highlighting a role for estrogen. Here we have begun to delineate how a systemic LEU injection affects the brain in males, and these modified circuits appear to underlie fear extinction processes involved in anxiety behaviors. Consistent with our results, previous investigations of LEU acutely administered intracerebroventricularly have also reported anxiolytic effects in male mice (Umathe et al., 2008a, 2008b). The clinical implications of our findings suggest that an acute LEU-induced increase in T may improve the efficacy of exposure therapy sessions for anxiety and stress-related psychiatric disorders. Due to its castration-like effects with chronic administration, administering LEU prior to multiple or daily sessions of therapy may not be beneficial for symptom improvement. However, understanding how acute LEU can strengthen extinction processes that are the basis for exposure therapy may be the key to better treatment outcomes. Given the similar reported effects of LEU in women, it may be important to know whether LEU affects the same brain systems in the same way.
4.3 Conclusions
In summary, LEU administration allowed us to manipulate the natural pulsatile release of gonadal hormones of the hypothalamic-pituitary-gonadal (HPG) system to investigate how testosterone might play a role in fear extinction and its neurocircuitry. Here we first focused on the initial stimulatory effect of LEU, but further study of its castrating effects should be investigated, which is clinically relevant to its current use as treatment and reports of resulting increased feelings of anxiety, mood disturbances, and cognitive impairments. For the purpose of this study and comparison with our previous estrogen findings, we chose to investigate the acute response to LEU treatment and the influence of elevated testosterone levels on fear extinction memory consolidation. As we have previously shown the rapid effects of estrogen in enhancing fear extinction memory, and it is known that LEU stimulates gonadal hormones beyond testosterone, determining whether LEU also concomitantly elevates estrogen levels appears to be a logical next step. Increased testosterone levels could also lead to increased aromatization of testosterone to estrogen, elevating estrogen levels as well. It is quite possible that the positive effects of LEU we observed in fear extinction recall may be driven by increased levels of estrogen or the change in testosterone to estrogen ratio. We were unable to conduct a within-subject analysis of the acute serum testosterone (or estrogen) levels during extinction training due to technical limitations. Future studies could address this issue perhaps using a chronic venous catheterization to facilitate blood collections and reduce stress induced by the procedure during behavioral recordings.
Moreover, LEU is typically prescribed to treat sex hormone disorders as a depot injection with treatments continuing for months. In this chronic administration, testosterone levels are no longer elevated, but suppressed to castration levels. During this period of hormonal suppression, some patients have experienced heightened feelings of anxiety and impaired cognitive effects. Women who are prescribed LEU express having similar experiences. Future experiments examining the influence of this chronic LEU-induced chemical castration on fear extinction and neurocircuitry in both males and females could provide valuable insight into LEU’s central and psychological side effects, which have not been fully investigated prior to this study. Doing so may also reveal critical sex differences that may need to be considered in future LEU treatment regimens.
GnRH agonist Lupron treatment acutely elevates serum testosterone (T) levels.
T elevation during extinction training improves extinction memory recall.
Freezing during recall is negatively correlated with IL and CeL c-fos activity.
Endogenous T modulation by Lupron influences fear extinction neurocircuitry.
Acknowledgments
We thank the members of the Milad lab for their review and comments on this manuscript.
Funding
This work was supported by the National Institute of Mental Health (NIMH) grant 1R01MH097880-001 and departmental funds from the Department of Psychiatry at Massachusetts General Hospital to Mohammed R. Milad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm. Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Almehmadi Y, Yassin AA, Nettleship JE, Saad F. Testosterone replacement therapy improves the health-related quality of life of men diagnosed with late-onset hypogonadism. Arab J. Urol. 2016;14:31–36. doi: 10.1016/j.aju.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Aubry AV, Serrano PA, Burghardt NS. Molecular Mechanisms of Stress-Induced Increases in Fear Memory Consolidation within the Amygdala. Front. Behav. Neurosci. 2016:10. doi: 10.3389/fnbeh.2016.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund LH, Prytz HS, Perski A, Svartberg J. Testosterone levels and psychological health status in men from a general population: the Tromsø study. Aging Male Off. J. Int. Soc. Study Aging Male. 2011;14:37–41. doi: 10.3109/13685538.2010.522276. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The Anxiolytic and Antidepressant-like Effects of Testosterone and Estrogen in Gonadectomized Male Rats. Biol. Psychiatry. 2015;78:259–269. doi: 10.1016/j.biopsych.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CV, Brummet JL, Jordan CL, Breedlove SM. Down, But Not Out: Partial Elimination of Androgen Receptors in the Male Mouse Brain Does Not Affect Androgenic Regulation of Anxiety or HPA Activity. Endocrinology. 2016;157:764–773. doi: 10.1210/en.2015-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C. Synaptic consolidation: an approach to long-term learning. Cogn. Neurodyn. 2012;6:251–257. doi: 10.1007/s11571-011-9177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Lapin B, Wang CH, Helfand B, Victorson D, Novakovic K. Variation in Testosterone Levels and Health-related Quality of Life in Men Diagnosed With Prostate Cancer on Active Surveillance. Urology. 2016 doi: 10.1016/j.urology.2016.03.056. [DOI] [PubMed] [Google Scholar]
- DiBlasio CJ, Hammett J, Malcolm JB, Judge BA, Womack JH, Kincade MC, Ogles ML, Mancini JG, Patterson AL, Wake RW, Derweesh IH. Prevalence and predictive factors for the development of de novo psychiatric illness in patients receiving androgen deprivation therapy for prostate cancer. Can. J. Urol. 2008;15:4249–4256. discussion 4256. [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Filova B, Malinova M, Babickova J, Tothova L, Ostatnikova D, Celec P, Hodosy J. Effects of testosterone and estradiol on anxiety and depressive-like behavior via a non-genomic pathway. Neurosci. Bull. 2015;31:288–296. doi: 10.1007/s12264-014-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn. Mem. Cold Spring Harb. N. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol. Psychiatry. 2013;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J. A single administration of testosterone reduces fear-potentiated startle in humans. Biol. Psychiatry. 2006;59:872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hodosy J, Zelmanová D, Majzúnová M, Filová B, Malinová M, Ostatníková D, Celec P. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol. Biochem. Behav. 2012;102:191–195. doi: 10.1016/j.pbb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Akimoto S, Shimazaki J. Effect of leuprolide on growth of rat prostatic tumor (R 3327) and weight of male accessory sex organs. Endocrinol Jpn. 1988;35:181–187. doi: 10.1507/endocrj1954.35.181. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Amiaz R, Seidman S, Pope HG. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp. Clin. Psychopharmacol. 2007;15:529–538. doi: 10.1037/1064-1297.15.6.529. [DOI] [PubMed] [Google Scholar]
- Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav. Brain Res. 2014;263:9–15. doi: 10.1016/j.bbr.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Kostanski JW, Dani BA, Schrier B, DeLuca PP. Effect of the Concurrent LHRH Antagonist Administration with a LHRH Superagonist in Rats. Pharm. Res. 2000;17:445–450. doi: 10.1023/a:1007581004844. [DOI] [PubMed] [Google Scholar]
- Lynch JF, Vanderhoof T, Winiecki P, Latsko MS, Riccio DC, Jasnow AM. Aromatized testosterone attenuates contextual generalization of fear in male rats. Horm. Behav. 2016;84:127–135. doi: 10.1016/j.yhbeh.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebrón-Milad K. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J. Neurosci. Res. 2017;95:163–175. doi: 10.1002/jnr.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 2014;35:42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Wang S, Mason J, Southwick SM, Fox P, Hazlett G, Charney DS, Greenfield G. Hormone profiles in humans experiencing military survival training. Biol. Psychiatry. 2000;47:891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- Mulchahey JJ, Ekhator NN, Zhang H, Kasckow JW, Baker DG, Geracioti TD. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001;26:273–285. doi: 10.1016/s0306-4530(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC, Vijayakumar S, Ahmed NAK, Verga PW, Orr SP, Pitman RK, Milad MR. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J. Psychiatr. Res. 2013;47:1776–1784. doi: 10.1016/j.jpsychires.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol. Endocrinol. Baltim. Md. 2003;17:870–881. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Academic Press; 2007. [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am. J. Psychiatry. 2003;160:105–111. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch. Gen. Psychiatry. 2004;61:162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- Umathe SN, Bhutada PS, Jain NS, Dixit PV, Wanjari MM. Effects of central administration of gonadotropin-releasing hormone agonists and antagonist on elevated plus-maze and social interaction behavior in rats. Behav. Pharmacol. 2008a;19:308–316. doi: 10.1097/FBP.0b013e328308f1fb. [DOI] [PubMed] [Google Scholar]
- Umathe SN, Bhutada PS, Jain NS, Shukla NR, Mundhada YR, Dixit PV. Gonadotropin-releasing hormone agonist blocks anxiogenic-like and depressant-like effect of corticotrophin-releasing hormone in mice. Neuropeptides. 2008b;42:399–410. doi: 10.1016/j.npep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Wainwright SR, Workman JL, Tehrani A, Hamson DK, Chow C, Lieblich SE, Galea LAM. Testosterone has antidepressant-like efficacy and facilitates imipramine-induced neuroplasticity in male rats exposed to chronic unpredictable stress. Horm. Behav. 2016;79:58–69. doi: 10.1016/j.yhbeh.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–120. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J. Clin. Endocrinol. Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- Warnock JK, Bundren JC. Anxiety and mood disorders associated with gonadotropin-releasing hormone agonist therapy. Psychopharmacol. Bull. 1997;33:311–316. [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sasaki S, Tatsura H, Umemoto Y, Kubota H, Kamiya H, Kawai T, Kang K, Kohri K. Penile apoptosis in association with p53 under lack of testosterone. Urol. Res. 2004;32:9–13. doi: 10.1007/s00240-003-0358-6. [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J. Psychiatr. Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]