Abstract
Background:
The role fine-needle aspiration (FNA) in the diagnosis of salivary gland lesions has evolved over the years. Although clinical and radiological parameters help to narrow the differential diagnosis the tissue diagnosis still remains the gold standard.
Materials and Methods:
This study is from January 2013 to December 2015 in our Department of Pathology where 170 salivary gland lesions were aspirated. The aim of the present study was to analyze adequacy rate in relation to the size of lesion and to evaluate varied cytological spectrum of salivary gland lesions with emphasis on differential diagnosis and to correlate cytological diagnosis with age, gender and anatomical site.
Results:
The 170 cytological smears were categorized into two groups: Group 1 adequate aspirations (88.2%), Group 2 inadequate aspirations (11.7%). The adequate aspirations were subdivided as neoplastic (53.33%) and nonneoplastic (46.66%). The distribution of the various neoplastic lesions (80; 53.33%) were 66 (82.5%) benign, 12 (15%) were malignant and 2 (2.5%) were suspicious of malignancy. Among benign neoplasms, the pleomorphic adenoma (62; 93.3%) was the most frequent followed by Warthins tumor (4; 6%). The most common malignant neoplasms were adenoid cystic carcinoma (6; 50%), followed by mucoepidermoid carcinoma (4; 33.3%), malignant lymphoma (1; 8.3%) and metastatic carcinomatous deposits (1; 8.3%). In two cases, cytological picture indicated suspicion for malignancy however specific tumor typing could not be done. The neoplasms occurred more frequently in the parotid gland (65%), followed by submandibular gland (21.3%) and minor salivary glands (13.8%). The nonneoplastic lesions (70) included 68.6% cases of chronic sialadenitis, 17.1% cases were reported as mucocele, 11.4% cases of acute sialadenitis 2.9% cases as tubercular granulomas.
Conclusion:
FNA cytology provides useful information on the management of salivary gland lesions and prevents unnecessary surgery in cases of nonneoplastic lesions and identification of malignancy helps the surgeon in deciding type and extent of surgery.
Keywords: Fine-needle aspiration cytology, salivary gland lesions, salivary gland neoplasms
INTRODUCTION
Fine-needle aspiration cytology (FNAC) is the first tissue-based procedure applied before any surgical intervention. It has now been widely accepted by head and neck surgeons as an excellent though challenging, the primary method of evaluating the space occupying lesions of salivary gland.
A nodule or diffuse enlargement of the salivary glands may be caused by inflammation, cystic lesion, degenerative process, or benign/malignant neoplasm. Salivary gland neoplasms account for 2%–6.5% of all the head and neck neoplasms.[1] The apparent increase in the incidence of salivary gland lesions in some places has been the reason attributed to the current widespread practice of fine-needle aspiration (FNA) in the diagnosis of salivary gland lesions. FNA renders specific diagnosis in the majority of cases, thus helps the surgeon to appropriately plan treatment which ranges from conservative management for nonneoplastic lesions, wide local excision for benign neoplasms, radical surgery for malignant tumors and chemotherapy/radiotherapy for metastasis and lymphoproliferative disorders. The cytological evaluation of salivary gland tumors is sometimes limited by wide range and heterogeneous nature of benign and malignant tumors arising in this area, many of which share overlapping cytological features, thus making the diagnosis problematic at times. The accuracy of cytological diagnosis depends on multiple factors that include aspirator experience, size of the lesion, clinical and radiological data about the lesion and utilization of Romanowsky type of stain for FNA of salivary gland lesions.
The aim of the present study was to analyze adequacy rate in relation to size of the lesion and to evaluate varied cytological spectrum of salivary gland lesions with emphasis on differential diagnosis and their correlation with age, gender and anatomical site.
MATERIALS AND METHODS
This was a prospective study over a period of 3 years (from January 2013 to December 2015) comprising 170 patients with salivary gland space occupying lesions who underwent FNAC in our Department of Pathology. The clinical data pertaining to patients’ age, sex and anatomical site were recorded. The patients’ consent was taken before the procedure.
All the aspirations were performed by cytopathologists using a 22-gauge needle, and the smears were prepared on clean glass slides. The air-dried smears and ethanol fixed smears were stained with May Grunwald's Giemsa, Papanicolaou and Hematoxylin and Eosin, respectively. The special stains such as Ziehl–Neilson (ZN) were used as and when required. The stained FNA smears were examined by two cytopathologists independently for cytomorphological findings, diagnosis and differential diagnosis where needed.
The SPSS 17 software version (IBM, Inc.,) was used for statistical analysis, and Chi-square test was used to access the association of cytological diagnosis with gender, age and anatomical site in both nonneoplastic as well as neoplastic lesions. P ≤ 0.05 was considered statistically significant.
RESULTS
During the present study, 170 patients underwent FNA of salivary gland lesions. The cytological smears were categorized into two groups: Group 1 - adequate aspirations (150, 88.2%) when smears had sufficient cellularity and were suggestive of a definitive diagnosis. Group 2 - inadequate aspirations (20, 11.7%) when smears did not show any epithelial cells and comprised blood/necrosis only.
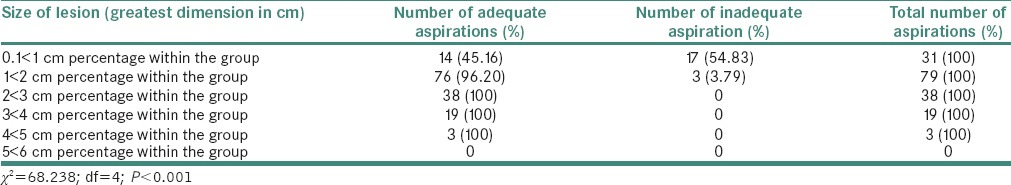
The adequate aspirations (150, 88.2%) were categorized as neoplastic (80, 53.33%) and nonneoplastic (70; 46.66%). An association between the size of lesion and adequacy of FNAC aspirations is found with highest number of inadequate and inconclusive aspirations (54.83%) in the size of lesion measuring 0.1 < 1.0 cm followed by 3.79% inadequate and inconclusive aspirations in the group where size was 1 < 2 cm. A Chi-square statistics with degree of freedom (4) with Chi-square value (68.238) shows that size of the lesion has a significant level of association (P < 0.001) with the positive outcome of FNAC as shown in Table 1.
Table 1.
Distribution of adequate and inadequate salivary gland aspirations according to the size of lesion
Neoplastic lesions
The distribution of the various neoplastic lesions (80; 53.33%) was 66 (82.5%) benign, 12 (15%) were malignant and 2 (2.5%) were suspicious of malignancy as shown in Tables 2 and 3. Among benign neoplasms, the pleomorphic adenoma (62; 93.3%) was the most frequent followed by Warthin's tumor (4; 6%). The most common malignant neoplasms were adenoid cystic carcinoma (6; 50%), followed by mucoepidermoid carcinoma (MEC) (4; 33.3%), malignant lymphoma (1; 8.3%) and metastatic carcinomatous deposits (1; 8.3%). In two cases, cytological picture indicated suspicion for malignancy however specific tumor typing could not be done.
Table 2.
Distribution of salivary gland tumors according to sex and age in eighty cases
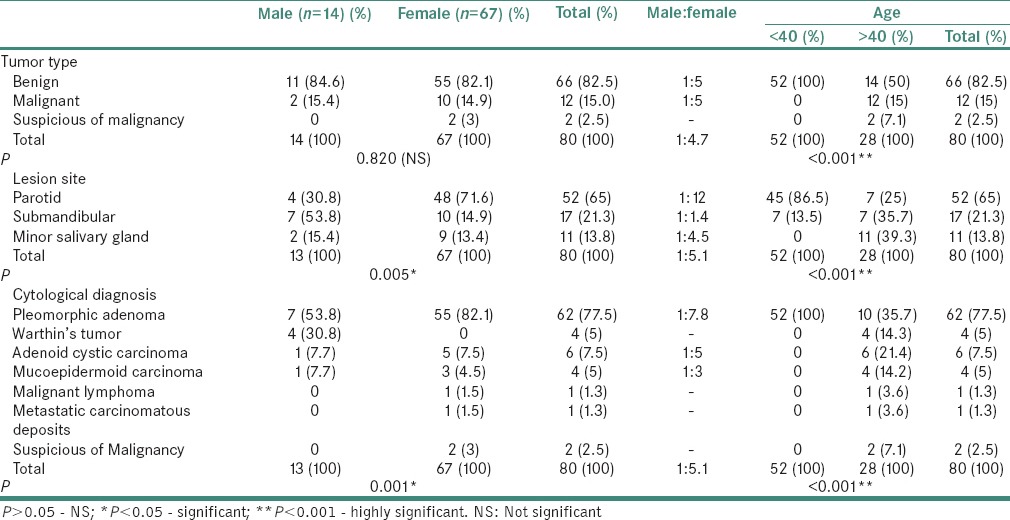
Table 3.
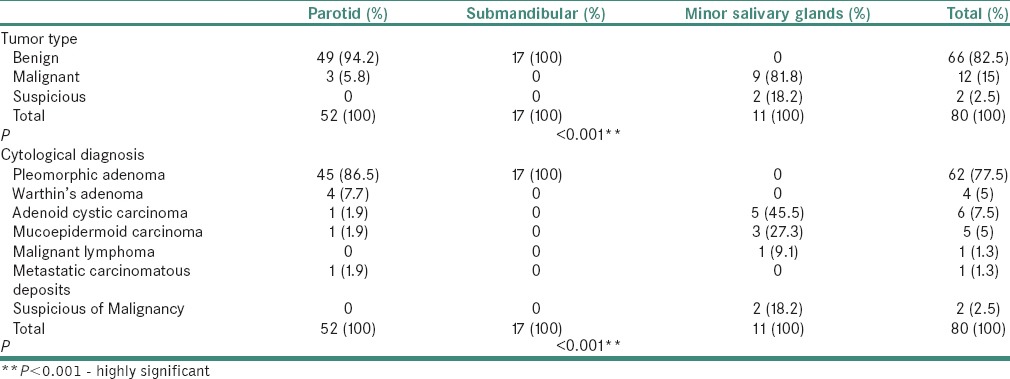
Distribution of salivary gland tumor (eighty cases) according to site
The female predominance was seen in all neoplasms except in Warthin's tumor, which was more frequent in males in our study; however, no statistically significant association was seen between the type of neoplasms (benign/malignant) and gender (P = 0.820) as shown in Table 2.
The male to female ratio in benign and malignant neoplasms was 1:5 and 1:6, respectively. We observed benign neoplasms occurred more frequently in patients with age <40 as compared to malignant tumors were commonly seen in patients older than 40. A strong positive association was seen between the type of neoplasms (benign/malignant) and age (P < 0.001).
The parotid gland (52%) was the most commonly involved by neoplasms, followed with submandibular gland (17%) and minor salivary glands (11%). The benign tumors were significantly more frequent in the parotid gland (49%), whereas the malignant was significantly more frequent in the minor salivary glands (11%). It was observed that type of neoplasms (i.e., benign/malignant) had a positive correlation with the anatomical site (P < 0.001) as shown in Table 3.
Pleomorphic adenoma (was more frequent in females (88.7%) in contrast Warthin's tumor which occurred exclusively in males. The pleomorphic adenoma was more common in age <40 in contrast all the four cases of Warthins occurred in age group >40. The pleomorphic adenoma most commonly occurred in parotid gland (72.5%) and small fraction involved submandibular gland (27.41%). However, Warthin's tumor involved parotid gland exclusively. The age distribution and site of involvement of benign neoplasms is shown in Tables 2 and 3.
The adenoid cystic carcinoma and MEC showed female predominance with majority of patients were older than 40 and frequently involved minor salivary gland. The malignant lymphoma and metastatic carcinomatous were observed in one patient each with age more than 40 and involved minor salivary gland and parotid gland, respectively, as shown in Tables 2 and 3.
The degree of association of specific tumor subtype with gender, age and anatomical site was P = 0.001, P < 0.001 and P < 0.001, respectively. The anatomical site showed positive correlation with gender as well with age among neoplastic lesions (P = 0.005 and P < 0.001).
Nonneoplastic lesions
The distribution of the nonneoplastic lesions is summarized in Tables 4 and 5. The nonneoplastic lesions (70) included 48 (68.6%) cases of chronic sialadenitis, 12 (17.1%) cases were reported as mucocele, 8 (11.4%) cases of acute sialadenitis and 2 (2.9%) cases as tubercular granulomatous. The females (57.1%) were more frequently involved by nonneoplastic lesions and 42 cases occurred in patients with age <40. The parotid gland (51.4%) was more frequently involved by nonneoplastic lesions followed by submandibular gland in (44.2%) and minor salivary glands in (4.2%). No statistically significant association was found between cytological subtype of nonneoplastic lesion and gender (P = 0.938) as well as among anatomical site with cytological subtype (P = 0.557) and age (0.110) as shown in Table 4.
Table 4.
Distribution of nonneoplastic salivary gland aspirates (70) in accordance with gender, age and site
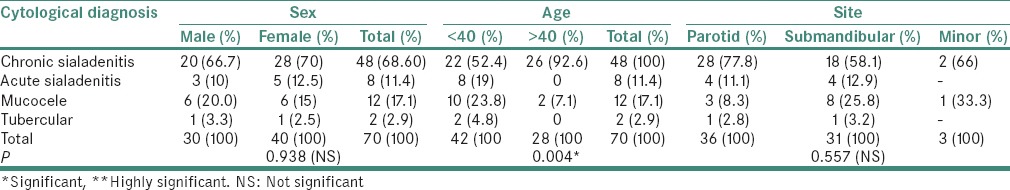
Table 5.
Correlation of site with gender and age among nonneoplastic lesions of salivary glands (70)
A positive correlation was seen between site and gender (P = 0.001) in cases of nonneoplastic lesions and cytological subtype of nonneoplastic lesion with age (P = 0.004) as depicted in Table 5.
DISCUSSION
The role FNA in the diagnosis of salivary gland lesions has evolved over the years. Although clinical and radiological parameters help to narrow the differential diagnosis, the tissue diagnosis still remains the gold standard. The aim of FNA was to distinguish nonneoplastic from neoplastic and further subtype where ever possible. FNAC provides useful information on the management of salivary gland lesions and prevents unnecessary surgery in cases of nonneoplastic lesions and identification of malignancy helps the surgeon in deciding type and extent of surgery. A stepwise approach in FNA of salivary glands starts from clinical examination of the lesion, type of aspirate aspirated and microscopic examination of smears.
The cytological analysis in the present study revealed 88.2% as adequate for evaluation and 11.7% as inadequate aspirations. A study conducted by Das et al.[2] on 712 patients of salivary gland aspirates also showed 96% aspirates as adequate for evaluation. Similar study results were obtained by Nguansangiam et al.,[3] who evaluated 290 salivary gland aspirates and found 5.2% aspirates as inadequate for evaluation. In the current study, it was observed that number of inadequate aspirations (54.83%) were seen in the lesions of size (0.1 < 1.0 cm), followed by 3.79% in the group (1 < 2 cm). This showed relative difficulty in obtaining the aspirate from smaller lesions. In the current study, 150 aspirates were regarded as adequate for evaluation, out of which 80 (53.3%) were reported as neoplastic, 70 (46.6%) as nonneoplastic. Studies done by Singh Nanda et al.[4] on 127 salivary gland aspirates revealed 44.1% aspirates as neoplastic and 55.9% as nonneoplastic.
Neoplastic lesions
The eighty aspirates which were reported as neoplastic were further categorized as benign (66; 82.5%) malignant (12; 15%) and suspicious for malignancy (2; 2.5%). This is in concordance with studies conducted by Nguansangiam et al.,[3] who reported 89.47% as benign, 8.27% as malignant and 2.25% as suspicious of malignancy. Salivary gland tumors - showed female predominance with an overall male:female ratio of 1:5.1 and male:female ratio among benign and malignant neoplasms was 1:5 and 1:6, respectively. Al-khateeb and Ababneh[5] and Vargas et al.[6] described male:female ratio varying from 1:1.2 to 2:3. The present study showed no statistical correlation between tumor type (benign/malignant) and gender (P = 0.832) which is in concordance with study conducted by de Oliveira et al.[7] The benign neoplasms (52 cases) involved patients who were younger than 40 years in contrast to malignant tumors (14 cases) which were more frequently seen in patients older than 40. We observed statistically positive correlation between tumor type and age (P < 0.001) which is similar to the results obtained by de Oliveira et al.[7] William et al.[8] reported peak incidence for benign tumors in fourth decade of life while for malignant neoplasms as seventh decade. In present analysis, benign neoplasms frequently involved major salivary glands while malignant neoplasms occurred in minor salivary glands (P < 0.001). Previously reported study also observed that the likelihood of a salivary gland being malignant is inversely propotional to size of the gland.[9]
Among benign neoplasms, pleomorphic adenoma was most common, comprising 77.5% of all neoplasms and 93.93% of benign neoplasms. This is in concordance with a study conducted by Vargas et al.[6] and Ito et al.,[10] who reported the incidence of pleomorphic adenoma as 67.7% and 67.8%, respectively. The majority of pleomorphic adenoma occurred in parotid gland (45 cases) and the remaining in submandibular gland (17 cases). They were frequent in females with age <40. The pleomorphic adenoma is the most notorious neoplasm. Although most of these neoplasms were readily identified because of their biphasic pattern, comprising epithelial/myoepithelial cells and magenta chondromyxoid stroma [Figure 1]. These tumors exhibit wide morphological spectrum varying from predominantly epithelial types to predominantly stromal types which often poses diagnostic challenge to cytopathologists. If epithelial component predominates in smears, then the tumor needs to be differentiated from monomorphic adenoma, myoepithelioma and low-grade carcinoma.[11,12] However, in cases of increased stroma with or without hyaline globules, it has to be differentiate from adenoid cystic carcinoma.[11,12] The smears from pleomorphic adenoma with metaplastic squamous cell and scant mucoid materials may be misinterpreted as MEC.[13] If myxoid material is abundant and epithelial cells are sparse, then it can be mistaken for retention cyst[14] cases. The myoepithelial cells have tremendous potential for differentiating into various cytomorphologic forms, a finding which is more evident on cytologic smears and are seen as plasmacytoid, spindle and stellate cells. The smears with predominance of plasmacytoid myoepithelial cells need to be differentiated from malignant lymphoma and plasma cell proliferations.[15]
Figure 1.
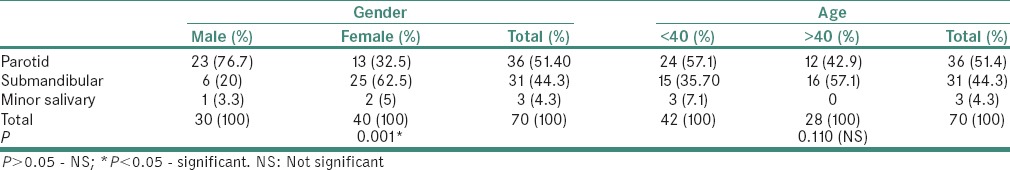
Cytological smear shows epithelial cells embedded in chondromyxoid stroma in case of pleomorphic adenoma, MGG, ×40
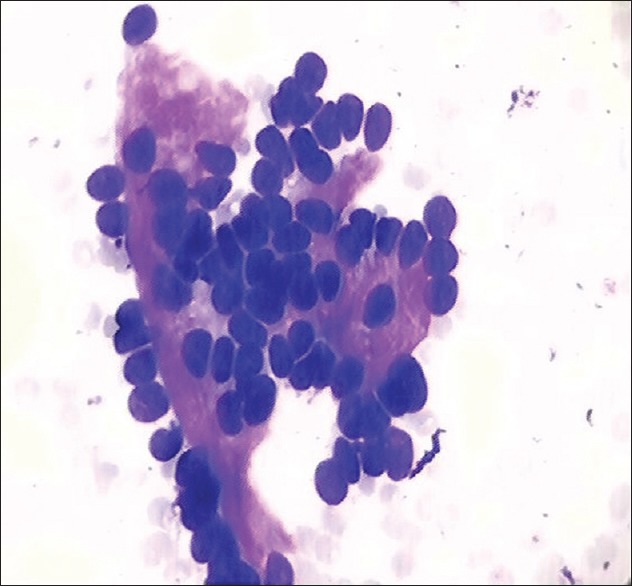
We reported ten cases of pleomorphic adenoma exhibiting squamous cell metaplasia and 22 cases with plasmacytoid differentiation [Figure 2] and one case which had hyaline globule in addition to chondromyxoid stroma.
Figure 2.
Smear shows myoepithelial cells exhibiting plasma cell differentiation in case of pleomorphic adenoma, H&E, ×40
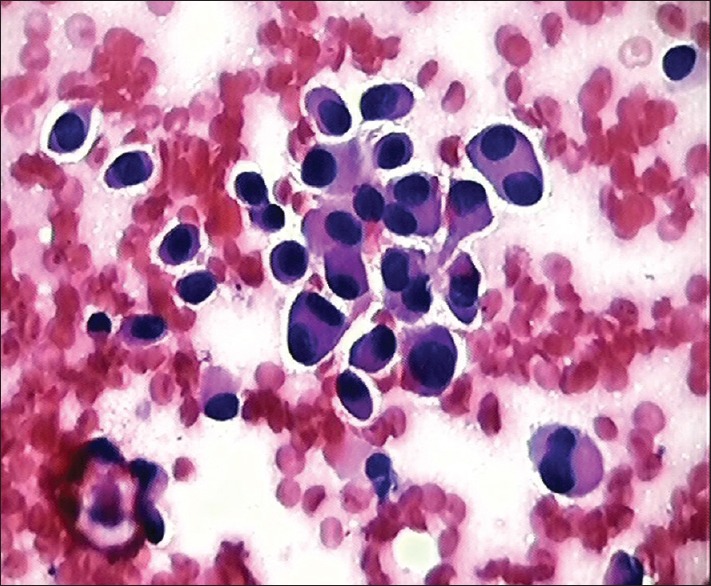
Warthin's tumor was third most common neoplasm (5%) in our study, affecting only males and involving parotid gland. The similar results were obtained by Li et al.[16] and de Oliveira et al.[7] The three main components that characterize the cytological smears of Warthin's tumor are oncocytes, lymphocytes and dirty fluid background [Figure 3]. The differential diagnosis of Warthin's tumor includes lymphoepithelial cysts of salivary gland, chronic inflammatory and obstructive duct lesions exhibiting oncocytic metaplasia and lymphocytes.[17,18] Its needs to be differentiated from oncocytic neoplasms which has oncocytes and variable number of lymphocytes however cells clusters in oncocytic neoplasms are bigger and three dimensional.[15] It is important to always reaspirate any residual mass after initial drainage of fluid from Warthin's tumor.
Figure 3.
Smear shows oncocytes admixed lymphomononuclear cells against dirty background in case of Warthins tumor, H&E, ×40
Adenoid cystic carcinoma was the most common malignant tumor constituting 7.5% of all neoplasms. This is in concordance with the findings of Souza Lima S et al.[19] The aspirates from adenoid cystic carcinoma usually had two components: epithelial cells and acellular basement membrane material seen as homogenous spherical structures [Figure 4]. The hyaline globules are not unique for adenoid cystic carcinoma, it may be found in cases of polymorphous low-grade adenocarcinoma, basal cell adenomas, pleomorphic adenoma and even in epithelial-myoepithelial carcinoma and basal cell adenocarcinoma.[20]
Figure 4.
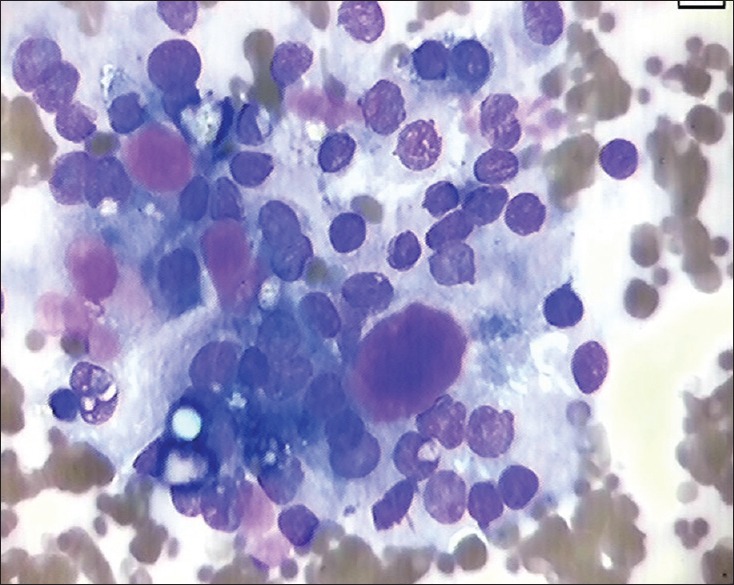
Cytological smear shows tumor cells along with hyaline globules in case of adenoid cystic carcinoma, MGG, ×40
MEC constituted 2.66% of all neoplasms in our study. Satko et al.[21] and Lima et al.[22] in their series reported the incidence of mucoepidermoid as 5.2% and 4.4%, respectively. The cytological smears from a MEC show the presence of mucus producing cells and intermediate cells as shown in Figure 5. The cells exhibit varying degree of atypia according to which the tumor is categorized as low, intermediate and high grade. Low-grade tumor accounts for 80% of all MECs and is characterized by cystic growth pattern.[15] The aspiration of low-grade MEC usually yields mucoid fluid and smears are typically hypocellular having bland cytologic features.[15] A low-grade tumor has to be differentiated from Warthins tumor, benign salivary gland cyst, branchial cleft cyst, sialolithiasis and pleomorphic adenoma with excess of mucoid stroma.[15] An extensive search for mucus producing goblet cells is required in these cases. Klijanienko and Vielh[23] reviewed fifty cases of MEC and suggested FNAC is an accurate technique for diagnosing high- or intermediate-grade tumors; however, it remains unsatisfactory in detecting low-grade tumors.
Figure 5.
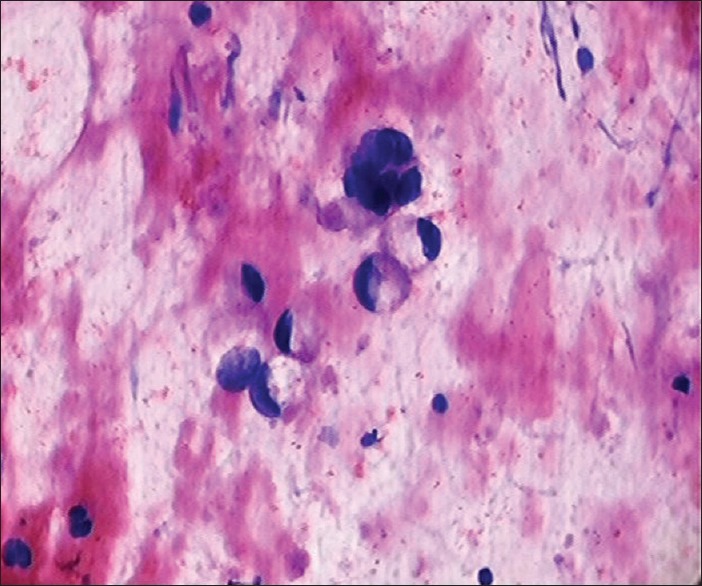
Cytological smears show mucous secreting tumors cells in case of mucoepidermoid carcinoma, MGG, ×40
We reported one case each of metastatic carcinomatous deposits from larynx in our analysis. Nguansangiam et al.[3] reported 2.2% salivary gland aspirates as metastasis. It is important to differentiate a primary neoplasm from a secondary malignant neoplasm to avoid unnecessary surgery and to guide subsequent radiotherapy and chemotherapy. One case of lymphoma was diagnosed in our work Das et al.[2] reported almost similar incidence of 3.5% salivary gland aspirates as lymphoma.
In our current study, we concluded that specific tumor type exhibited statistically positive correlation with age (P < 0.001), gender (P = 0.001) and site of lesion (P < 0.001). de Oliveira et al.[7] in their study showed positive correlation of specific tumor type with age (P < 0.05), gender (P < 0.05) and site of lesion (P < 0.05). This is in concordance with our study.
Nonneoplastic lesions
In the current work, nonneoplastic lesions constituted 46.66% of adequate salivary gland aspirates as shown in Table 5. A studies conducted by Singh Nanda et al.[4] and Rajwanshi et al.[24] revealed the presence of nonneoplastic lesions as 55.9% and 66.8%, respectively. Chronic sialadenitis was seen in 68.6% followed by mucocele in 17.1%, acute sialadenitis in 11.4% and tuberculosis in 4.8% in the present analysis. Similar results were obtained by Singh Nanda et al.,[4] who studied patients and found chronic sialadenitis in 83% of cases, mucocele in 9.8% cases and abscess in 5.6% cases. Gupta et al.[25] quoted that out of 74 patients of salivary gland aspirates 57.4% had chronic sialadenitis, 19.1% had mucocele, 12.7% had acute sialadenitis and 2% had granulomatous sialadenitis and 1.8% presented with sialadenosis. A higher incidence was seen in females with male:female ratio 3:4; however, male predominance was seen in study conducted by Gupta et al.[25] The age range for nonneoplastic lesions was from 6 to 57 years. The parotid gland was most commonly involved followed by submandibular and minor salivary glands as shown in Table 5.
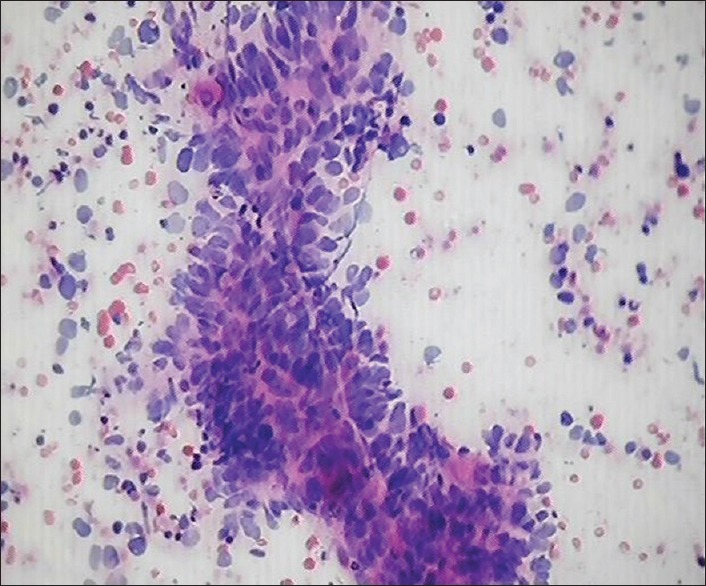
The cytological smears of chronic sialadenitis comprised clusters of ductal epithelial cells along with the presence of lymphomononuclear cells in the background. The cases diagnosed as acute sialadenitis showed numerous polymorphonuclear neutrophils and cell debris. The aspirate in cases of mucocele was watery in consistency and smears exhibited numerous foamy macrophages admixed with lymphomononuclear cells. The two cases were reported as tuberculosis of major salivary gland, which on microscopic examination showed the presence of epitheloid cell granuloma [Figure 6] and exhibited acid-fast bacilli on ZN staining.
Figure 6.

Smear shows epithelioid cell granuloma in case of tuberculosis of submandibular gland, H&E, ×40
CONCLUSION
Our study highlighted FNAC as a simple rapid, safe, minimally invasive technique for diagnosis of salivary gland lesions. Adequate sampling, high-quality smear preparation and experienced cytopathologist can diagnose majority of nonneoplastic and common benign and malignant neoplasms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51–8. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 2.Das DK, Petkar MA, Al-Mane NM, Sheikh ZA, Mallik MK, Anim JT. Role of fine needle aspiration cytology in the diagnosis of swellings in the salivary gland regions: A study of 712 cases. Med Princ Pract. 2004;13:95–106. doi: 10.1159/000075637. [DOI] [PubMed] [Google Scholar]
- 3.Nguansangiam S, Jesdapatarakul S, Dhanarak N, Sosrisakorn K. Accuracy of fine needle aspiration cytology of salivary gland lesions: Routine diagnostic experience in Bangkok, Thailand. Asian Pac J Cancer Prev. 2012;13:1583–8. doi: 10.7314/apjcp.2012.13.4.1583. [DOI] [PubMed] [Google Scholar]
- 4.Singh Nanda KD, Mehta A, Nanda J. Fine-needle aspiration cytology: A reliable tool in the diagnosis of salivary gland lesions. J Oral Pathol Med. 2012;41:106–12. doi: 10.1111/j.1600-0714.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khateeb TH, Ababneh KT. Salivary tumors in North Jordanians: A descriptive study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e53–9. doi: 10.1016/j.tripleo.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Vargas PA, Gerhard R, Araújo Filho VJ, de Castro IV. Salivary gland tumors in a Brazilian population: A retrospective study of 124 cases. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:271–6. doi: 10.1590/s0041-87812002000600005. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira FA, Duarte EC, Taveira CT, Máximo AA, de Aquino EC, Alencar Rde C, et al. Salivary gland tumor: A review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271–5. doi: 10.1007/s12105-009-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams NP, Boyd DL, Choy L, Hanchard B. Salivary gland lesions: A Jamaican perspective. West Indian Med J. 2001;50:62–5. [PubMed] [Google Scholar]
- 9.Shemen LJ. Salivary glands: Benign and malignant disease. In: Lee KJ, editor. Essential Otolaryngology, Head and Neck Surgery. 7th ed. Stamford: Appleton and Lange; 1999. pp. 506–7. [Google Scholar]
- 10.Ito FA, Ito K, Vargas PA, de Almeida OP, Lopes MA. Salivary gland tumors in a Brazilian population: A retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34:533–6. doi: 10.1016/j.ijom.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Klijanienko J, Vielh P. Fine-needle sampling of salivary gland lesions. I. Cytology and histology correlation of 412 cases of pleomorphic adenoma. Diagn Cytopathol. 1996;14:195–200. doi: 10.1002/(SICI)1097-0339(199604)14:3<195::AID-DC1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Stewart CJ, Hamilton S, Brown IL, MacKenzie K. Salivary epithelial-myoepithelial carcinoma: Report of a case misinterpreted as pleomorphic adenoma on fine needle aspiration (FNA) Cytopathology. 1997;8:203–9. [PubMed] [Google Scholar]
- 13.Stanley MW, Lowhagen T. Mucin production by pleomorphic adenomas of the parotid gland: A cytologic spectrum. Diagn Cytopathol. 1990;6:49–52. doi: 10.1002/dc.2840060111. [DOI] [PubMed] [Google Scholar]
- 14.Young JA. Fine Needle Aspiration Cytopathology. 1st ed. London: Blackwell Scientific Publications; 1993. pp. 48–65. [Google Scholar]
- 15.Mukunyadzi P. Review of fine-needle aspiration cytology of salivary gland neoplasms, with emphasis on differential diagnosis. Am J Clin Pathol. 2002;118:S100–15. doi: 10.1309/WVVR-30E4-13TW-494D. [DOI] [PubMed] [Google Scholar]
- 16.Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008;44:187–92. doi: 10.1016/j.oraloncology.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Ballo MS, Shin HJ, Sneige N. Sources of diagnostic error in the fine-needle aspiration diagnosis of Warthin's tumor and clues to a correct diagnosis. Diagn Cytopathol. 1997;17:230–4. doi: 10.1002/(sici)1097-0339(199709)17:3<230::aid-dc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Elliott JN, Oertel YC. Lymphoepithelial cysts of the salivary glands. Histologic and cytologic features. Am J Clin Pathol. 1990;93:39–43. doi: 10.1093/ajcp/93.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Lima SS, Soares AF, de Amorim RF, Freitas Rde A. Epidemiologic profile of salivary gland neoplasms: Analysis of 245 cases. Braz J Otorhinolaryngol. 2005;71:335–40. doi: 10.1016/S1808-8694(15)31332-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley MW, Horwitz CA, Rollins SD, Powers CN, Bardales RH, Korourain S, et al. Basal cell (monomorphic) and minimally pleomorphic adenomas of the salivary glands. Distinction from the solid (anaplastic) type of adenoid cystic carcinoma in fine-needle aspiration. Am J Clin Pathol. 1996;106:35–41. doi: 10.1093/ajcp/106.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Satko I, Stanko P, Longauerová I. Salivary gland tumours treated in the stomatological clinics in Bratislava. J Craniomaxillofac Surg. 2000;28:56–61. doi: 10.1054/jcms.1999.0092. [DOI] [PubMed] [Google Scholar]
- 22.Lima SS, Soares AF, de Amorium RF, Frietas Rde A. Epidemiologic profile of salivary gland neoplasms: Analysis of 245 cases. Braz J Otorhinolaryngol. 2005;34:533–6. doi: 10.1016/S1808-8694(15)31332-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klijanienko J, Vielh P. Fine-needle sampling of salivary gland lesions. IV. Review of 50 cases of mucoepidermoid carcinoma with histologic correlation. Diagn Cytopathol. 1997;17:92–8. doi: 10.1002/(sici)1097-0339(199708)17:2<92::aid-dc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Rajwanshi A, Gupta K, Gupta N, Shukla R, Srinivasan R, Nijhawan R, et al. Fine-needle aspiration cytology of salivary glands: Diagnostic pitfalls – Revisited. Diagn Cytopathol. 2006;34:580–4. doi: 10.1002/dc.20353. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Dewan D, Kumar D, Suri J. Fine needle aspiration cytology of salivary gland lesions with histopathological correlation in a district hospital of Jammu region. Indian J Pathol Oncol. 2016;3:32–7. [Google Scholar]


