Abstract
Introduction and Objectives:
Angiogenesis is a complex event facilitated by angiogenic factors released from neoplastic and host immune cells. Among host immune cells, mast cells (MCs) may have greater significance in tumor progression through angiogenesis. The objectives of the study were to evaluate and correlate mast cell density (MCD) and microvessel density (MVD) in normal gingival tissue, leukoplakia with and without dysplasia and oral squamous cell carcinoma (OSCC) cases.
Materials and Methods:
Among eighty selected cases, twenty were of normal gingiva, twenty each of leukoplakia without and with dysplasia and twenty of OSCC. The slides were stained with CD34 and counterstained with 0.1% toluidine blue, followed by quantification of MCD and MVD per high-power field (×40) using Image-Pro Express software.
Statistical Analysis:
Chi-square test and correlation coefficient were used for statistical analysis.
Observation and Results:
A statistically significant difference in the values of MVD and MCD between normal gingival tissue, leukoplakia with and without dysplasia and OSCC (P = 0.000) was observed. MVD and MCD showed a positive correlation between the study groups.
Conclusion:
MVD and MCD increased significantly in cases of OSCC as compared to leukoplakia with and without dysplasia and normal gingival tissue. It was concluded that MCs may play a significant role in angiogenesis by releasing pro-angiogenic and angiogenic factors which may in turn favor the progression of premalignant lesion to a malignant one.
Keywords: Angiogenesis, CD34, mast cell density, mast cells, microvessel density, toluidine blue
INTRODUCTION
Mast cells (MCs) were first described by Paul Ehrlich in his 1878 doctoral thesis due to their unique staining characteristics and large granules.[1] Shortly after its discovery, MC's tendency to concentrate around blood vessels in inflammatory and neoplastic foci was identified. It was later demonstrated that they accumulate around tumors before the onset of tumor-associated angiogenesis.[2]
MCs are also a prime source of angiogenic factors. Under physiological conditions, they are particularly prominent in proximity of capillaries and lymphatic channels and also appear at the edges of invasive tumors where they facilitate angiogenesis by releasing preformed mediators or by triggering proteolytic release of extracellular matrix-bound angiogenic compound. They are also key host cells in tumor infiltrate with important consequence for tumor cell fate.[3]
Angiogenesis, the growth of new blood vessels from preexisting ones, is an essential phenotype of tumor formation.[4] Accumulation of MCs around tumor margins and the subsequent release of angiogenic factors possibly represent a tumor–host interaction which favors tumor progression.[5]
Stains which identify angiogenesis and MCs include CD34 and toluidine blue, respectively. Present in hematopoietic progenitor cells and endothelial cells, CD34 is an antigen composed of glycoprotein, making it more resistant to formalin fixation and is thus studied as a marker for vascular tumors. Anti-CD34 antibody is a highly sensitive marker for endothelial cell differentiation, which stains neoplastic endothelium a deeper shade than normal endothelium.[6] It has equal staining intensity for small and large blood vessels.[7] In addition, toluidine blue reveals MCs as large, purple, oval and highly granulated cells.[5]
MATERIALS AND METHODS
This retrospective study was conducted on tissue sections obtained from diagnosed cases of leukoplakia and oral squamous cell carcinoma (OSCC), retrieved from archives of the department.
The study group comprised a total of eighty cases, of which twenty cases were normal gingival tissue, twenty each of leukoplakia with and without dysplasia and twenty of OSCC.
Inclusion criteria
Normal gingival tissue obtained from patients undergoing tooth extraction for orthodontic purposes
All oral leukoplakia cases with and without dysplasia
Incisional and excisional biopsies of primary OSCC.
Informed consent was obtained from patients for normal gingival tissues.
Exclusion criteria
Secondary and metastatic OSCC cases and other white lesions of the oral cavity
Oral mucosa biopsy of patients with signs of inflammatory gingival and periodontal disease.
Monoclonal (1A4) mouse anti-human CD34 antibody (BioGenex) and Super Sensitive™ Polymer-HRP IHC Detection System HRP/DAB (BioGenex) were used for the study. Counterstaining was done with 0.1% toluidine blue (slides were quickly dipped 15 times in toluidine blue solution and washed in tap water to remove the excess chromogen. The slides were quickly dipped once in a solution containing 70% alcohol and 0.5% HCl). Presence of brown-colored precipitate at the site of target antigen indicated positive immunoreactivity. The overall slide background was clear without any extraneous deposits.
The microvessel density (MVD) and mast cell density (MCD) quantification was performed with a binocular light microscope under high-power magnification (×40) in ten consecutive high-power fields (×40) using Image-Pro Express software (Fiji is developed by contributors around the world, and funded from various sources. It is maintained by Curtis Rueden and the ImageJ development team at the Laboratory for Optical and Computational Instrumentation (LOCI) at the University of Wisconsin-Madison). The total count was divided by 10 (number of fields studied) to obtain the average MVD and MCD.
Statistical analysis
Chi-square (χ2) test was calculated to test the significance of MVD and MCD between the study groups, and correlation coefficient (r) was calculated to determine the interdependency of MVD and MCD.
The hypotheses assumed for χ2 testing are as follows:
H0 (null hypothesis): There is no association of MCs in angiogenesis and angiogenesis in progression from premalignant to malignant lesion
H1 (alternative hypothesis): There is an association between MCs in angiogenesis and angiogenesis in progression from premalignant to malignant lesion.
-
The hypotheses for testing correlation (r) are stated below:
- H0 (null hypothesis): The hypothesis assumed is that if the r value is 0, then there is no association between the groups
- H1 (alternative hypothesis): The alternate hypothesis to be taken is that if the r value is ≠ 0, there is association between the groups.
RESULTS
The study samples were categorized into four groups as Group 1 (normal gingival tissue), Group 2 (leukoplakia without dysplasia), Group 3 (leukoplakia with dysplasia) and Group 4 (OSCC). The tissue sections were stained with CD34 antibody and counterstained with 0.1% toluidine blue. MVD and MCD were quantified in 10 high-power field for each of the study group, following which the total and average values were calculated. Statistical tools were applied to the obtained values.
A statistically significant difference was observed in the χ2 values of MVD and MCD when compared between the study groups as shown in Tables 1 and 2. The χ2 test showed MVD to be significantly higher when compared between Groups 3 and 4 than between Groups 2 and 3 and Groups 1 and 2 (P = 0.000) [Tables 3–5]. Similarly, the χ2 value of MCD was significantly higher when compared between Groups 3 and 4 than between Groups 2 and 3 and Groups 1 and 2 (P = 0.000) [Tables 6–8]. MVD and MCD were positively correlated (P = 0.000) as shown in Tables 9 and 10.
Table 1.
Microvessel density

Table 2.
Mast cell density

Table 3.
Chi-square value of microvessel density between Groups 1 and 2
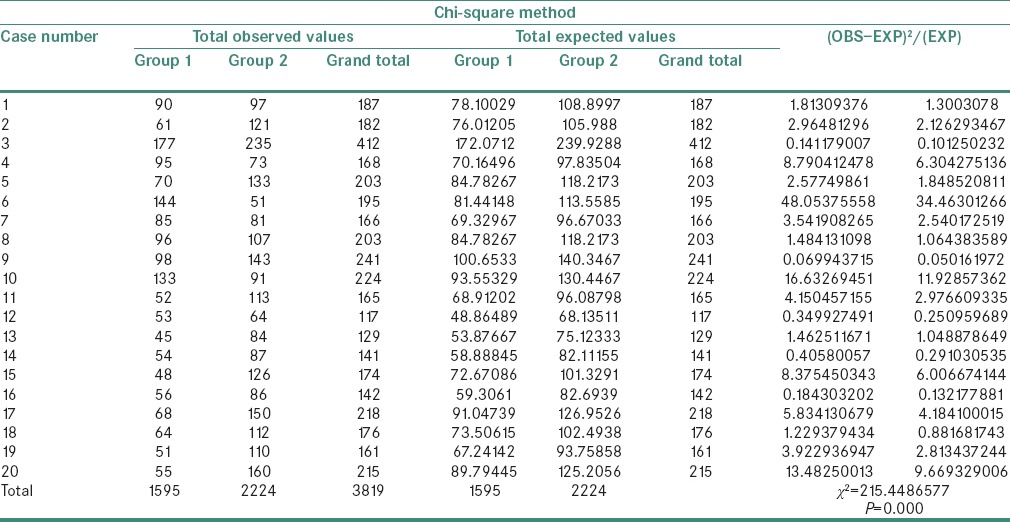
Table 5.
Chi-square value of microvessel density between Groups 3 and 4
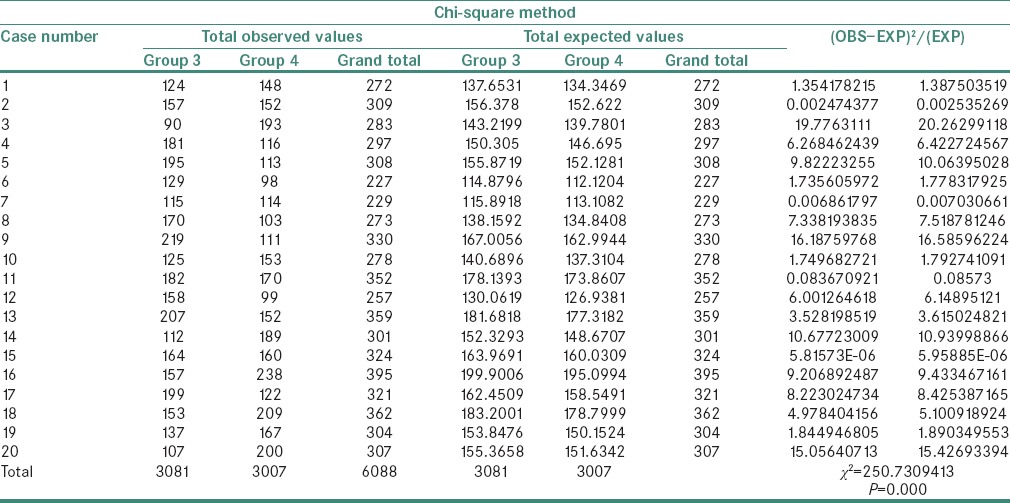
Table 6.
Chi-square value of mast cell density between Groups 1 and 2
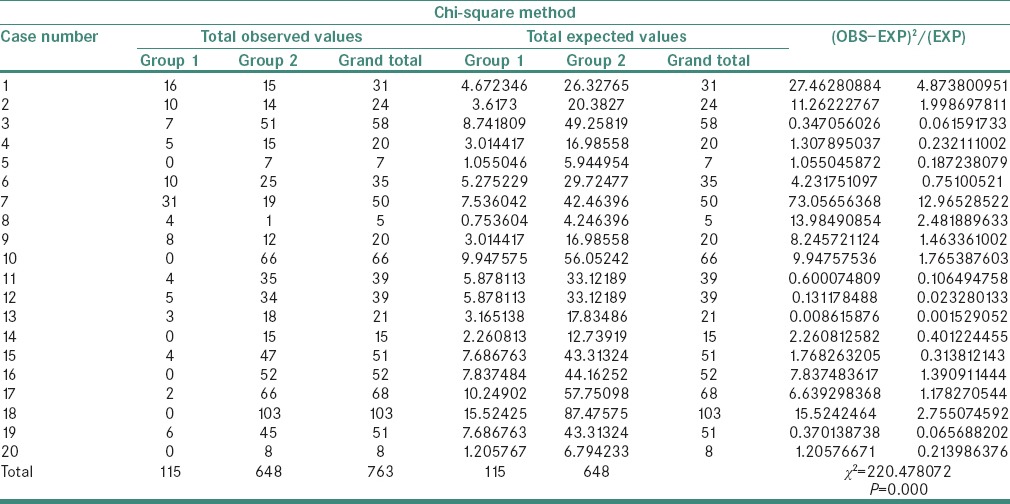
Table 8.
Chi-square value of mast cell density between Groups 3 and 4
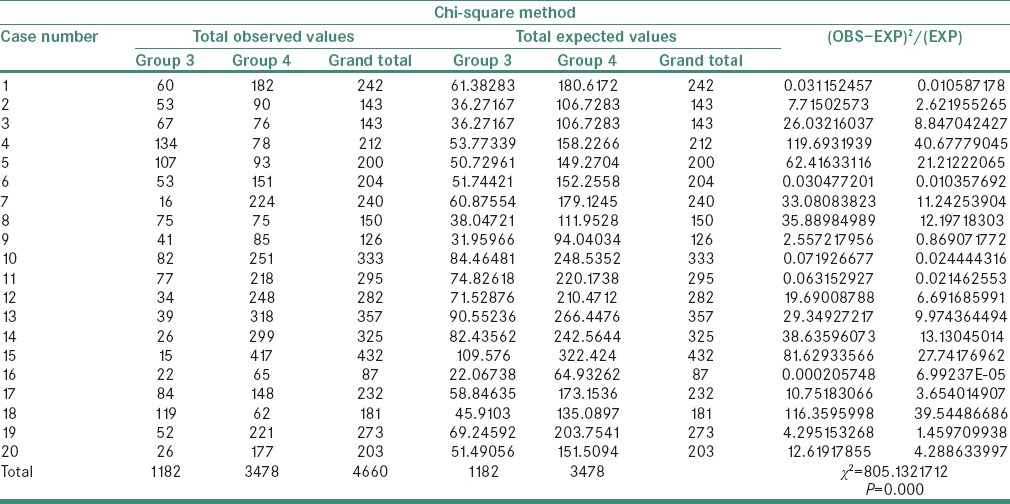
Table 9.
Correlation between microvessel density and mast cell density

Table 10.
Correlation coefficient between microvessel density and mast cell density
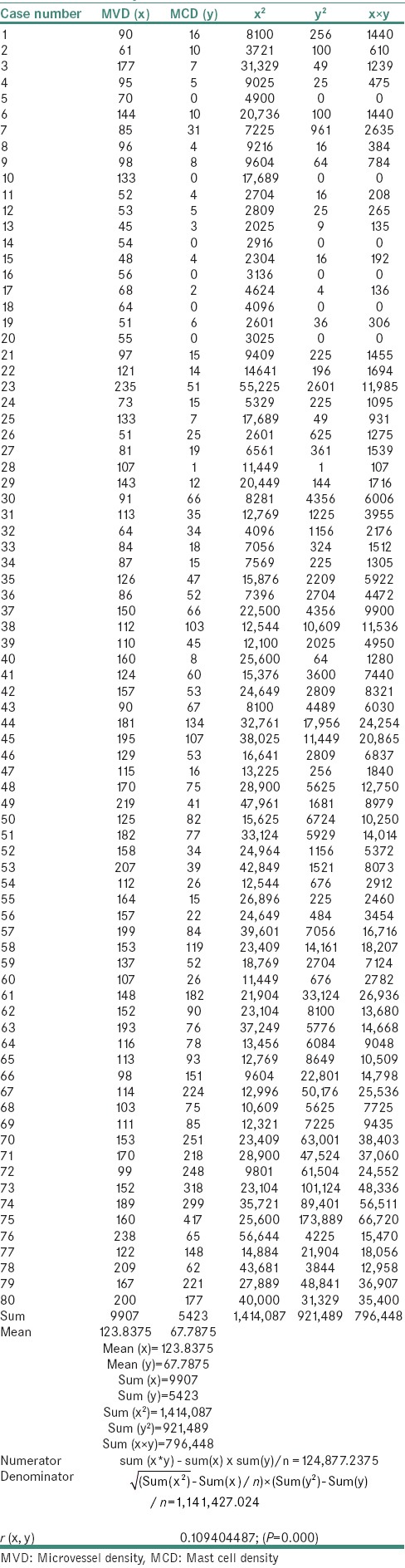
Table 4.
Chi-square value of microvessel density between Groups 2 and 3
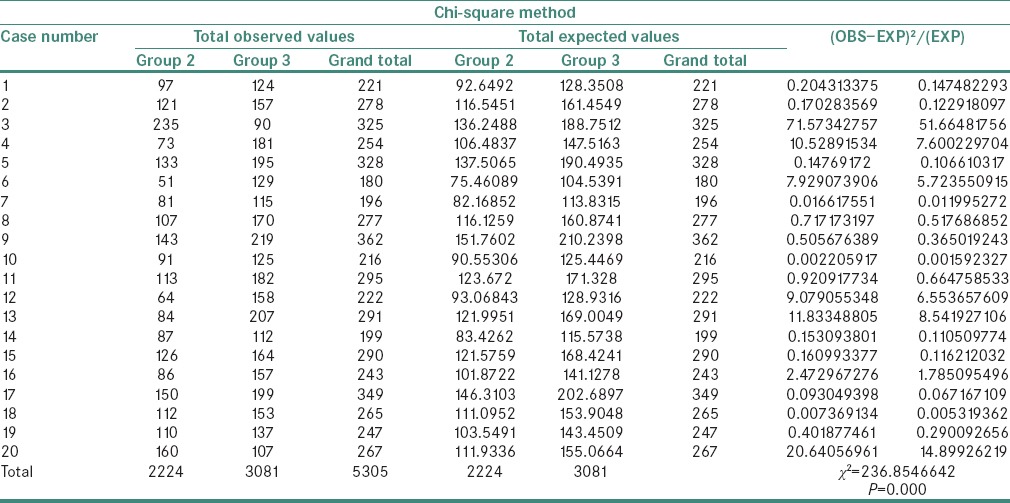
Table 7.
Chi-square value of mast cell density between Groups 2 and 3
After comparing the χ2 values of the different groups for 19 degrees of freedom, the null hypothesis was rejected at P < 0.01. Therefore, we conclude that there is a significant association between all the groups.
Correlation coefficient (r) is calculated to be 0.109404487. “r” value can range from −1 to +1; a positive r value indicates an increase of one variable when the other variable increases, i.e., the values are directly proportional. Hence, it is concluded that there is an association between MVD and MCD. This implies that the null hypothesis is invalid.
A significant difference was also observed in the average values of MVD [Table 11] between the study groups [Figure 1].
Table 11.
Mean or average values of microvessel density
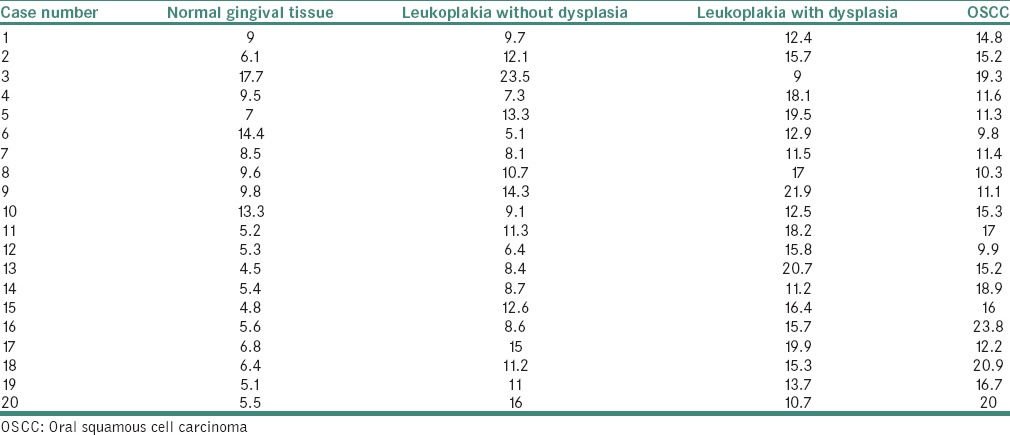
Figure 1.
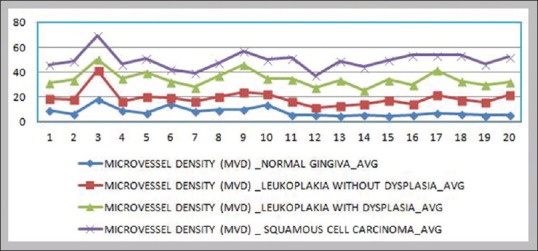
Comparison of microvessel density between the study groups
A significant difference was observed in the average values of MCD [Table 12] between the study groups [Figure 2].
Table 12.
Mean or average values of mast cell density
Figure 2.
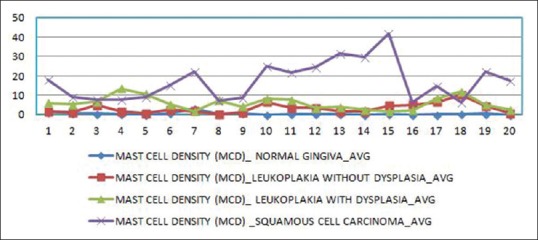
Comparison of mast cell density between the study groups
DISCUSSION
First described by Paul Ehrlich in his doctoral thesis, MCs are bone marrow-derived tissue-homing leukocytes.[8] They tend to concentrate around blood vessels in inflammatory and neoplastic foci and later accumulate near tumors before the onset of tumor-associated angiogenesis.[2] They also play an important function in the regulation of physiological and pathological neovascularization, mostly on the basis of histological observations.[9] Therefore, this study was undertaken to evaluate and correlate MCD and MVD in normal gingival tissue, leukoplakia with and without dysplasia and OSCC.
A correlational study of MVD and MCD by Sharma et al. revealed a linear increase in MVD and MCD, suggesting a positive correlation.[10] Further, Michailidou et al. observed a significant increase in MVD and MCD between the cases of normal mucosa, leukoplakia without dysplasia, leukoplakia with mild, moderate or severe dysplasia and OSCC. They concluded that an angiogenic switch seemed to be turned on in the early stages of epithelial malignant transformation.[5]
A study by Mohtasham et al., on MCD and angiogenesis in oral dysplastic epithelium and low- and high-grade OSCC using CD34 and counterstaining with Meyer's hematoxylin, found a significant correlation between MC count and MVD in agreement with the concept that MCs promote tumor progression through upregulation of angiogenesis. MC count and the degree of angiogenesis can be potentially used as an indicator of evolution of squamous cell carcinoma from epithelial dysplasia.[11]
The present study was conducted in agreement with previous studies conducted by Michailidou et al., Mohtashan et al. and Sharma et al.
MCs are a prime source of angiogenic factors. Under physiological conditions, they are particularly prominent near capillaries and lymphatic channels. In many inflammatory disorders characterized by profound vascular remodeling, the infiltrate exhibits numerous MCs which show structural features of degranulating elements. In various tumor models, MCs appear at the edges of invasive tumors to facilitate angiogenesis by releasing preformed mediators or by triggering proteolytic release of extracellular matrix-bound angiogenic compounds.[3] Angiogenesis refers to the formation of new blood vessels from preexisting vascular structures, i.e., capillaries and postcapillary venules. It is a critical process in tumor progression as vascular networks produced by the host are essential to allow neoplastic cell populations to form a clinically observable tumor.[3]
In 1971, Dr. Judah Folkman published a landmark paper in the New England Journal of Medicine, hypothesizing that solid tumor promoted angiogenesis in the tumor microenvironment by secreting pro-angiogenic factors.[12] Angiogenic factors are produced by tumor cells and accessory host cells such as macrophages, MCs and lymphocytes which may be attracted to the tumor.[13] Angiogenesis enhances entry of tumor cells into the circulation by providing an increased density of immature, highly permeable blood vessels which have little basement membrane and fewer intercellular junctional complexes than normal mature vessels.[13]
Associated with metastasis, intratumoral blood vessels are known to play an important role in cancer growth by supplying oxygen, nutrients and excreting metabolic products.[14] In 1945, Algire et al. first reported active neovascularization by the host to neoplastic tissues. Later, Folkman conducted a series of studies on cancer growth and neovascularization, and demonstrated that blood vessels in the host underwent angiogenesis and developed into intratumoral vessels that were closely related to tumor growth.[14] Williams et al. demonstrated a correlation between recurrence of OSCC and blood vessel count.[14] Moriyama et al. reported a correlation between metastasis and vessel density at the tip of infiltration when measured using CD31.[14]
Gruber et al. account that angiogenic factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and platelet-derived growth factor-AB stimulate MC migration. MCs might migrate into hypoxic areas chemotactically as a result of angiogenic factors released from cancer cells. Once in these hypoxic areas, MCs might produce angiogenic products that stimulate infiltration of more MCs.[15]
To grow to 2 mm3 or more, solid tumors require oxygen. This necessitates the formation of new microvasculature. Angiogenesis occurs due to imbalance of positive and negative angiogenic factors produced by tumor and host cells. Among the host cells, MCs produce and release considerable quantities of pro-angiogenic and angiogenic factors. The factors such as histamine, heparin, chymase, bFGF and VEGF promote the migration or proliferation of endothelial cells.[5]
To measure angiogenic activity in this study, a Pan-endothelial marker (CD34) was used to stain microvessels; counterstaining was performed with 0.1% toluidine blue to observe the possible angiogenic activity of MCs at the same optical field as microvessels [Figures 3–6].
Figure 3.
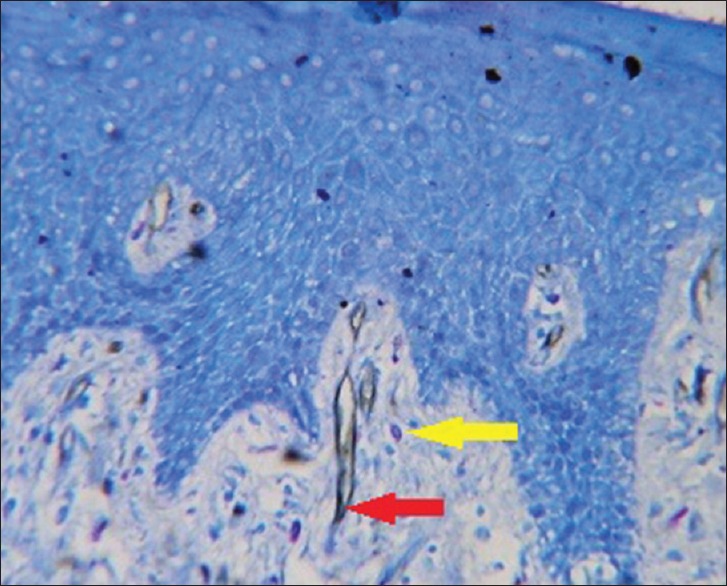
Photomicrograph of normal gingival tissue (red arrow - microvessel, yellow arrow - mast cell)
Figure 6.
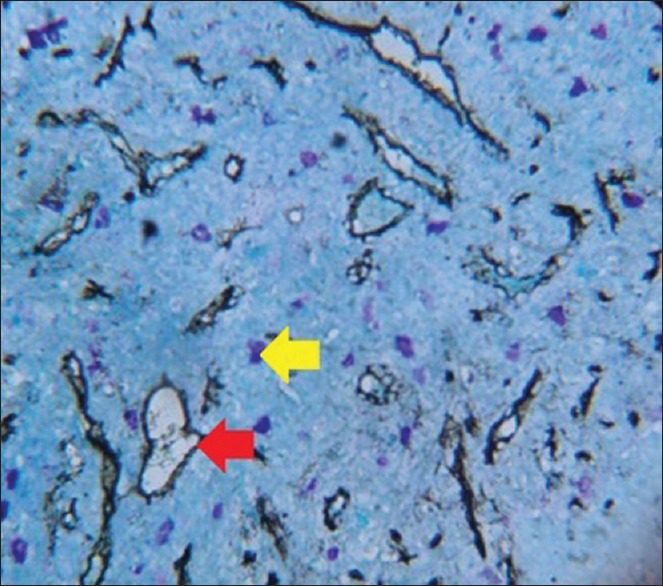
Photomicrograph of oral squamous cell carcinoma (red arrow - microvessel, yellow arrow - mast cell)
Figure 4.
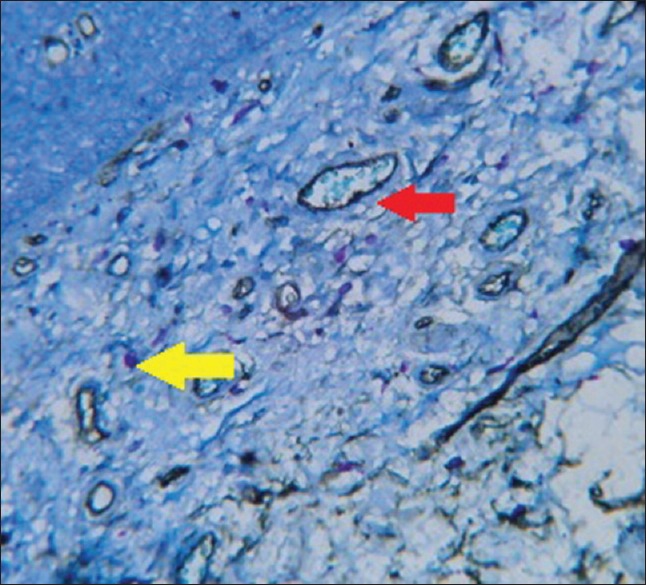
Photomicrograph of leukoplakia without dysplasia (red arrow - microvessel, yellow arrow - mast cell)
Figure 5.
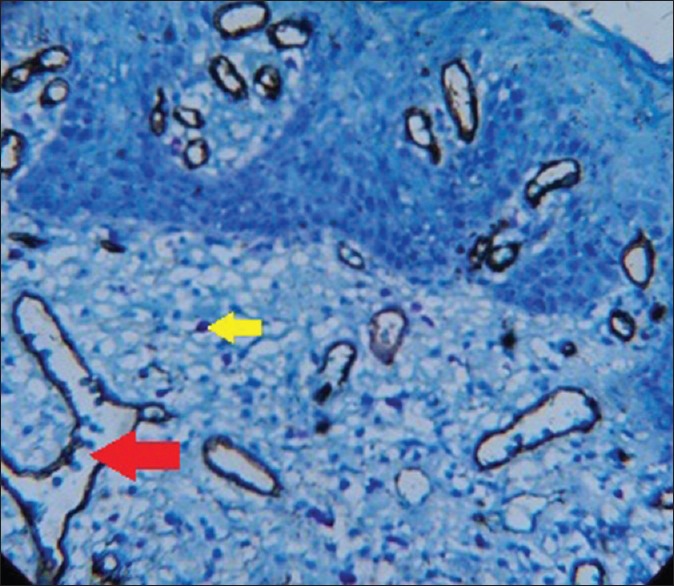
Photomicrograph of leukoplakia with dysplasia (red arrow - microvessel, yellow arrow - mast cell)
MCs surround various tumors and are sometimes detected in large numbers before the occurrence of neovascularization in some malignancies. MC-deficient mice exhibit less tumor angiogenesis when compared to mice with normal MC numbers. Moreover, they were found to induce neovascularization through carcinogenesis of squamous cells.[16]
Algire and Chalkley were the first to suggest that tumor growth is closely related to the development of an intrinsic vascular network. Angiogenesis is necessary to provide oxygen, nutrients and immune cells to the tumor microenvironment and also removes its waste products.[17]
In the early phase of hyperplasia and dysplasia, infiltrating MCs degranulate and activate dermal fibroblasts which intensify angiogenesis. They also activate progelatinase B (matrix metalloproteinase family) which is involved in extracellular remodeling and angiogenic regulation. MCs activate and progressively intensify angiogenesis by releasing sequestered angiogenic activators.[18] As the neoplastic sequence progresses, angiogenic growth factor gene expression is upregulated in cancer cells. This is the progression to the second cancer phase where tumor cells directly control their angiogenic phenotype instead of manipulating inflammatory cells to indirectly affect neovascularization.[18]
Studies show that growth of vessels adjacent to tumor cells increases the chances of tumor cells entering blood circulation. Alternatively, primary tumors containing a high proportion of angiogenic cells will seed into the circulation giving rise to additional metastatic deposits, thus amplifying its growth and dissemination. This is because newly proliferating capillaries have fragmented permeable basement membranes, making them more accessible to tumor cells than mature vessels.[19]
A study by Rakesh et al. found that MCs and their regulatory role in angiogenesis and inflammation by the release of mediators may play an important role in tumor progression, facilitating the transformation of oral leukoplakia into invasive carcinoma,[20] reinforcing the observation of this study.
Another supporting study by Mohtasham et al. found a significant correlation between MCD and MVD in OSCC, concluding that MCs may promote tumor progression by regulating angiogenesis.[11,21]
A pioneering study by Tomita et al. put forth two reasons for the conflicting reports on the role of MCs.[10] First, MC's cytotoxic function suppresses tumor activities initially when they infiltrate tumor tissue. However, after infiltration, tumor cells might instigate MC's angiogenic properties while suppressing their cytotoxic functions, thereby leading to tumor angiogenesis. Second, cell-mediated cytotoxic effects of MCs are reported when MC–tumor ratio is >20:1. Conversely, these effects are nullified when the MC–tumor ratio is increased from 10:1 to 1:100 leading to tumor progression. Hence, the effect of MCs against cancer cells might depend on the concentration of MC products in the microenvironment. Based on these findings, the researcher hypothesized that reversing this process, that is, enhancing the cytotoxic functions of MCs and suppressing their angiogenic functions, could lead to a new anticancer treatment strategy.[10,15]
CONCLUSION
In this study, the MVD and MCD increased significantly in the following cases; the increase was greater in cases of OSCC, followed by leukoplakia with dysplasia, leukoplakia without dysplasia and normal gingival tissue. Therefore, it is concluded that MCs may play a significant role in angiogenesis by releasing pro-angiogenic and angiogenic factors which may in turn favor the progression of premalignant lesion to a malignant one. A limitation of this study is that the role of MCs in angiogenesis and the role of angiogenesis in the evolution of squamous cell carcinoma from epithelial dysplasia need to be validated using a larger sample size and further follow-up studies. A specially stratified sample using differently graded cases of OSCC and leukoplakia will lead to even more conclusive results. Immunostaining of MCs with tryptase will render a specialized understanding of human MCs – thereby advancing cancer research and developing better treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to the principal of our college and technical staff of our department for their valuable contribution.
REFERENCES
- 1.Kinra M, Ramalingam K, Sarkar A, Rehman F, Girish KL. Study on comparison of mast cell count and mast cell density in normal mucosa, oral leukoplakia, oral lichen planus, oral submucous fibrosis and oral squamous cell carcinoma – A study on 50 cases. JPSI. 2012;1:4–11. [Google Scholar]
- 2.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: Cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003;92:485–92. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 3.Crivellato E, Nico B, Ribatti D. Mast cell contribution to tumor angiogenesis: A clinical approach. Eur Cytokine Netw. 2009;20:197–206. doi: 10.1684/ecn.2009.0167. [DOI] [PubMed] [Google Scholar]
- 4.Hasina R, Lingen MW. Angiogenesis in oral cancer. J Dent Educ. 2001;65:1282–90. [PubMed] [Google Scholar]
- 5.Michailidou EZ, Markopoulos AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;2:126–32. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira SC, Silva BB, Pinto GA, Vassallo J, Moraes NG, Santana JO, et al. CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol Res Pract. 2005;201:313–8. doi: 10.1016/j.prp.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.LI SH, Hung PH, Chou KC, Hsich SH, Shieh YS. Tumor angiogenesis in oral squamous cell carcinoma: The significance of endothelial markers and hotspot selection. J Med Sci. 2009;29:67–74. [Google Scholar]
- 8.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Crivellato E, Ribatti D. Involvement of mast cells in angiogenesis and chronic inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:9–11. doi: 10.2174/1568010053622876. [DOI] [PubMed] [Google Scholar]
- 10.Sharma B, Sriram G, Saraswathi TR, Sivapathasundharam B. Immunohistochemical evaluation of mast cells and angiogenesis in oral squamous cell carcinoma. Indian J Dent Res. 2010;21:260–5. doi: 10.4103/0970-9290.66655. [DOI] [PubMed] [Google Scholar]
- 11.Mohtasham N, Babakoohi S, Salehinejad J, Montaser-Kouhsari L, Shakeri MT, Shojaee S, et al. Mast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol Scand. 2010;68:300–4. doi: 10.3109/00016357.2010.494622. [DOI] [PubMed] [Google Scholar]
- 12.Cook KM, Figg WD. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J Clin. 2010;60:222–43. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–24. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 14.Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, Sugahara T, et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:70–6. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg. 2000;69:1686–90. doi: 10.1016/s0003-4975(00)01160-7. [DOI] [PubMed] [Google Scholar]
- 16.Elpek GO, Gelen T, Aksoy NH, Erdoğan A, Dertsiz L, Demircan A, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–4. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: An overview. Eur J Intern Med. 2009;20:663–71. doi: 10.1016/j.ejim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Anuradha A, Kiran Kumar Naik B, Vijay Srinivas G, Devi RS, Puneet HK. Incidence of mast cells in oral squamous cell carcinoma: A short study. J Oncol. 2014;2014:614291. doi: 10.1155/2014/614291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalra M, Rao N, Nanda K, Rehman F, Girish KL, Tippu S, et al. The role of mast cells on angiogenesis in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e190–6. doi: 10.4317/medoral.17395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakesh S, Vidya M, Janardhanan M, Vinod Kumar RB, Savithri V. Analysis of mast cell count in oral leukoplakia. Oral Maxillofac Pathol. 2012;3:181–5. [Google Scholar]
- 21.Ciurea R, Mărgăritescu C, Simionescu C, Stepan A, Ciurea M. VEGF and his R1 and R2 receptors expression in mast cells of oral squamous cells carcinomas and their involvement in tumoral angiogenesis. Rom J Morphol Embryol. 2011;52:1227–32. [PubMed] [Google Scholar]


