Abstract
Transformation of a normal cell into a cancerous phenotype is essentially backed by genetic mutations that trigger several oncogenic signaling pathways. These signaling pathways rewire the cellular metabolism to meet the bioenergetic and biomass requirement of proliferating cell, which is different from a quiescent cell. Although the change of metabolism in a cancer cell was observed and studied in the mid-20th century, it was not adequate to explain oncogenesis. Now, equipped with a revolution of oncogenes, we have a genetic basis to explain the transformation. Through several studies, it is clear now that such metabolic alterations not only promote cancer progression but also contribute to the chemoresistance of cancer. Targeting specific enzymes and combinations of enzymes can improve the efficacy of cancer therapy and help to overcome the therapeutic resistance.
Keywords: Cancer cell, metabolic alterations, therapeutics
INTRODUCTION
The relationship between cancer and altered cellular metabolism has been deciphered decades ago. However, the importance of tumor metabolism has dwindled over the past due to limited knowledge of tumorigenesis. It's only after the oncogenic revolution, the interest in tumor cell metabolism and signaling pathways is being renewed, and presently metabolic reprogramming is considered as a hallmark of cancer.[1]
The three basic requirements of cancer to sustain are rapid growth and proliferation of cancer cells, the capability of the tumor cells to evade the normal apoptotic pathways, thus favoring survival and unrestricted entry of the tumor cells into cell cycle progression even in the absence of growth signals. All these requirements are met by the tumor cell by acquiring a phenotype as a result of various host cell mutations that combine to alter the metabolic pathways. Many of these adaptations are also seen in rapidly proliferating normal cells, in which they represent appropriate response to physiological growth signals as opposed to constitutive cell autonomous adaptations.[2,3] In case of cancer, this adaptability should also work in a stressful microenvironment which is deficit in nutrients.[4]
Energy and biomass requirement of proliferating cells are more than that of normal cells. In tumor cells when nutrients are abundant, the requirements are met by reprogramming pathways to increase acquisition and utilization of nutrients, and in case of nutrient stress, the metabolism is rewired to compensate for the scarcity of one nutrient by filling the metabolite pool with another nutrient.[5,6,7]
In this review, we will discuss the alterations in cellular metabolism that the transforming cells undergo to sustain and proliferate in all nutrient conditions. Further, how these alterations can produce opportunity to target specific enzymes or combination of enzymes will also be discussed.
NUTRIENT SUFFICIENCY STATE
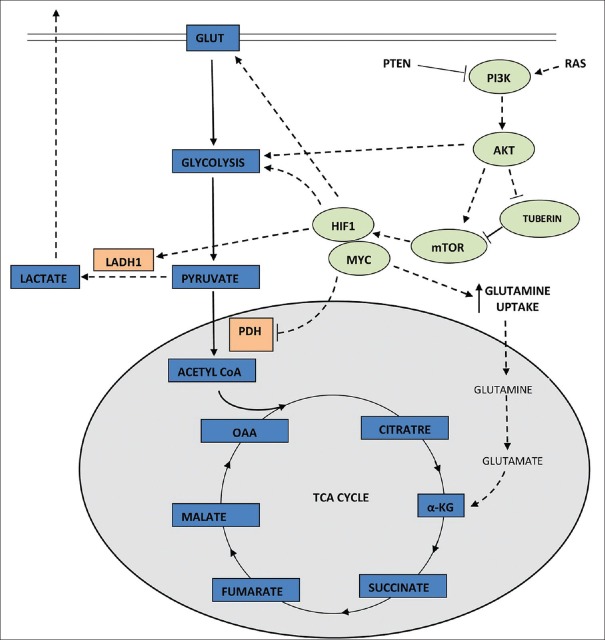
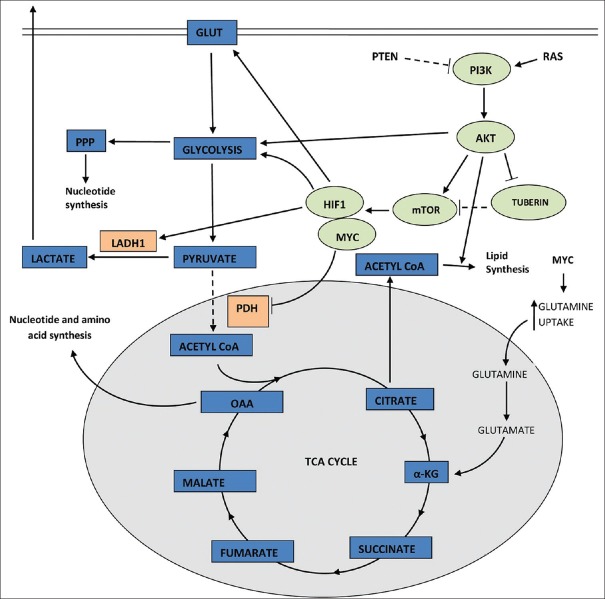
In the presence of abundant nutrient, oncogenic RAS stimulates the uptake and utilization of glucose[8,9] by activation of PI3K pathway [Figures 1 and 2].[10] PI3K pathway is one of the most commonly altered pathways in many human cancers. Apart from oncogenic RAS, this pathway is also activated by mutation in the tumor suppressor gene PTEN[11,12,13] or by aberrant signaling from receptor tyrosine kinase.[14] The activation of PIK3 in turn activates the downstream effectors AKT1 which strongly stimulates signaling through mammalian target of rapamycin (mTOR) by inhibiting tuberin.[15] At molecular level, mTOR indirectly causes metabolic changes by activating transcription factors such as hypoxia-inducible factor 1 (HIF1). HIF1 is a heterodimer that is stabilized in hypoxia.[16] However, it can also be activated in normal oxygen concentration by oncogenic signaling pathways such as PI3K.[17,18] HIF1, once activated, amplifies transcription of gene encoding glucose transporter (GLUT1) and most glycolytic enzymes, increasing influx of glucose into cell.[19]
Figure 1.
Glucose metabolism (represented by solid lines) in a quiescent cell
Figure 2.
Genetic mutations of gene such as RAS and PTEN can trigger several oncogenic signaling pathways such as PI3K and mammalian target of rapamycin that in turn upregulate glucose transporters and enzymes that catalyze various steps of glycolysis, thus favoring aerobic glycolysis
High expression of MYC collaborates with HIF1 inactivation of glucose receptors and glycolytic enzymes.[20,21] Increased MYC also enhances glutamine uptake and metabolism.[22,23] Glutamine has an added advantage of providing its two nitrogen atoms for biomass synthesis.[24]
In terms of adenosine triphosphate (ATP) generation, one of the most characteristic metabolic alterations occurring in tumor cells is shift from ATP generation through oxidative phosphorylation to ATP generation through glycolysis, even in the presence of normal oxygen concentration (Warburg effect).[25] Although the ATP generation by this pathway is faster, it is inefficient in terms of number of ATPs produced per glucose molecule. Earlier it was hypothesized that this shift might an adaptation to defective mitochondria.[26] However, it was later appreciated that mitochondrial defects are rare,[27,28,29] and oxygen consumption of tumor cells remains same as that of normal cell. The faster rate of ATP production by this pathway can justify such switch, but this is possible only in an environment where nutrient supply is surplus. The most recent theory regarding the switch believes that aerobic glycolysis provides a biosynthetic advantage for tumor cells by a high flux of substrate through glycolysis.[2,3]
AKT1, which is a downstream effector of PI3K activation, is an important driver of tumor glycolytic phenotype. It phosphorylates and activates glycolytic enzymes such as hexokinase and phosphofructokinase 2.[15,30]
The subsequent activation of HIF1 decreases the flow of glucose-derived pyruvate into tricarboxylic acid (TCA) cycle[31,32,33] by activation of pyruvate dehydrogenase kinase which inactivates mitochondrial pyruvate dehydrogenase complex. Oncogenic MYC activates lactate dehydrogenase A that catalyzes the conversion of pyruvate to lactate.[20,21] Recently, 13C-nuclear magnetic resonance spectroscopy measurements have shown that glioblastoma cells in culture convert as much as 90% of glucose and 60% of glutamine they acquire into lactate or alanine.[34]
ADAPTATION TO NUTRIENT STRESS
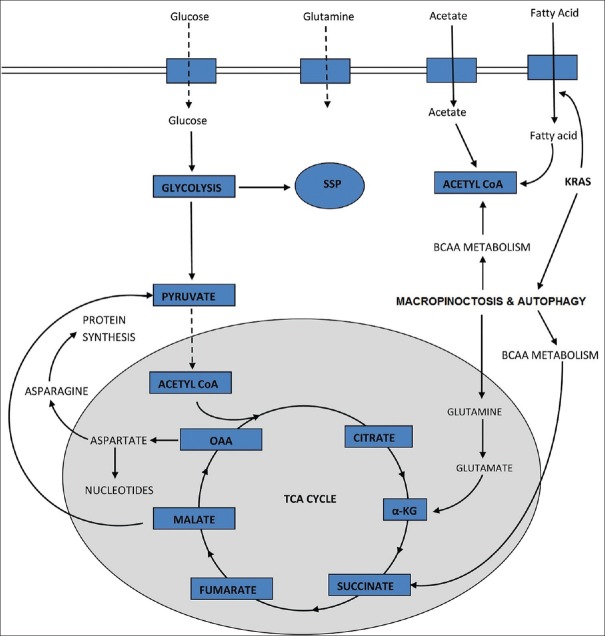
For most of the mammalian cells in culture, glucose and glutamine are the only two molecules that are catabolized appreciably to meet most of the energy and biomass requirement of cell.[2] Tumor cell deprived of these nutrients are supposed to die of starvation. On the contrary, nutrient deprivation has been correlated with poor survival of patients[35] suggesting that scarcity of nutrient makes the cancer cell stronger. This may be attributed to the biochemical alterations leading to acquisition of necessary plasticity of cancer cells that is required to reprogram metabolism [Figure 3] in response to different nutritional conditions.
Figure 3.
Reprogramming of metabolism in cancer cells, deprived of glucose and glutamine (reduced intake represented by dotted lines). Pool of amino acids and tricarboxylic acid cycle intermediates, required are maintained by activating pathways that promote autophagy, macropinocytosis and scavenging fatty acids
In most of the cancer cells, oxaloacetate (OAA) in TCA cycle is supplied by glutamine which compliment with acetyl-CoA from glucose. In case of glucose deprivation, carbon from glutamine has been seen to be rerouted to acetyl CoA in some cancers.[5] Similarly, glutamine deficiency can induce metabolic pathway changes. One of such changes is a loss of citrate synthase. Citrate synthase condenses OAA to acetyl-CoA to maintain TCA cycle.[35] However, in scarcity of glutamine, OAA is shunted toward asparagine formation to support cell survival.[36] Expression of asparagine synthetase has been seen to be associated with poor prognosis of glioma and neuroblastoma,[37] suggesting that maintenance of asparagine pool may provide an advantage to tumor cells.
In RAS expression, cancer, glutamine stimulates macropinocytosis.[38] In this process, extracellular matrix is captured and internalized. This allows starving cell to generate pools of glutamine and other amino acids supply to TCA cycle.[38] This process must be highly controlled as hyperactive macropinocytosis can lead to cell death in a process previously misidentified as autophagic cell death.
Along with scavenging on extracellular matrix, autophagic degradation of macromolecules is also active in cancer cells.[39,40,41,42] During autophagy, the organelles and the macromolecules are degenerated to produce small molecule nutrients to feed intermediary metabolism.[38,41,43] This process has been seen to be crucial in tumor growth and survival of cancer cells in some RAS driven tumor.[44]
In the presence of oxygen and abundant nutrients, cell synthesizes fatty acids de novo.[45,46] However, under nutrient stress, scavenging extracellular lipids become an adaptive mechanism for the cell.[47,48] Scavenging instead of synthesizing spares the cell from the need to supply carbon to pentose phosphate pathway (PPP) for NADPH production which is required for lipid synthesis. In case of ovarian cancer, a cooperative mechanism exists between stromal cell and cancer cell. The stromal cells have been found to provide fatty acid to tumor cells. When ovarian cancer cells were cocultured with adipocytes, the transfer of fatty acid from adipocytes to tumor cells triggered activation of adenosine monophosphate-activated protein kinase and fatty acid oxidation leading to enhanced cell proliferation.[49]
Deprivation of glucose or glutamine leads to activation of serine synthesis pathway (SSP) in tumor cells.[50] By this pathway, serine and glycine can be synthesized from glutamine by a process “reverse glycolysis.”[51] Serine driven one carbon metabolism produces reducing equivalent (NADPH) with a comparable importance to PPP.[52] Serine binding has also been related to activation of PKM2 enzymatic activation.[53] M2 is an isoform of pyruvate kinase which is present in self-renewing cells such as embryonic and adult stem cells.[54] It is found to be expressed in many tumor cells and therefore might be a useful biomarker for early detection of tumors.[4,55,56,57] Unlike the other isoform PKM1, it is usually found inactive and is inefficient at promoting glycolysis.[58,59,60] Due to its nature of inhibiting Warburg effect and ATP production, which is unfavorable for tumor progression, presence of PKM2 in cancer cells was ignored for several years. However, recent work has produced evidences that PKM2 exerts a regulatory contribution to SSP.[61] Further work is required to understand the regulatory cascade completely.
Acetate is one of the smallest molecules available as nutrient in mammals. It is converted to acetyl-CoA by acetyl-CoA synthetase. Although acetate has a very low concentration in circulating fluid,[62] it can be taken up by tumor cells and oxidized.[63] However, the primary role of acetate utilization is still to be evaluated.
Depending on the tumor microenvironment, the tumor cells rewire their metabolism to ultimately direct the available nutrient into the synthesis of new biomass while maintaining adequate level of ATP for survival.
THERAPEUTIC PROSPECTS
Over the past few decades, hundreds of genes have been identified that are mutated in cancer. Many of these genetic alterations that are known to promote cancer lead to a single converging metabolic phenotype that is characterized by reorganization of metabolic pathway in such a way that biosynthesis of macromolecules and ATP production to support cell survival are well balanced.[26,64,65] As all cancer cells are dependent on this alteration of metabolism, these altered pathways represent attractive therapeutic targets.[66,67] Further, effective agents targeting many of the common driver mutation in cancer are not available. For example, mutation of RAS or dysregulated expression of MYC is frequent events in human cancer, yet no specific therapies exist to treat cancers based on either genetic event, and many RAS-driven cancers are nonresponsive to existing therapies.[68,69] RAS-mutant cells are dependent on sufficient glucose uptake,[70] and MYC-dependent cells have a particular reliance on glutamine metabolism.[22,23,71] Small molecule inhibitors that disrupt glucose intake and metabolism has been found to decrease the growth of tumors that are derived from cells driven by these oncogenes in preclinical models.[70,72,73] Targeting metabolism may also be synergistic with many of the existing therapies such as kinase inhibitor and cytotoxic therapies which act by impairing glucose metabolism.[2,74,75]
Cancer development, progression and treatment outcomes are linked to whole body metabolism. Obesity, hyperglycemia and insulin resistance are all associated with an increased risk of developing cancer and worse clinical outcomes in patients suffering from cancer.[76,77,78,79,80] Insulin and insulin-like growth factor, which are capable of activating signaling pathways that drive cell growth, are increased in circulation of individuals suffering from obesity and insulin resistant. This suggests that obesity and insulin resistance promote cancer at least in part by activating pathways that drive cell growth.[77] These same signaling pathways also drive nutrient uptake into cells.[78] Further, elevated levels of glucose alone can promote increased glucose uptake in some cells, and lower glucose levels are seen to be associated with better cancer treatment outcomes.[81,82,83,84] Hence, antidiabetic drugs are being explored for anticancerous activity. Retrospective clinical studies have shown decreased cancer mortality rate in patients on metformin.[85,86] Metformin lowers level of glucose and insulin by inhibiting gluconeogenesis.[87] Other antidiabetic drugs which act by other mechanisms such as increasing the level of insulin in blood may worsen the clinical outcome in cancer.
Glutamine is an important nutrient source for cancer cell, and some cancers are addicted to glutamine. This increased reliance of some cancers on glutamine makes glutamine a prospective therapeutic target. Small molecules such as 2-amino-(2,2,1)-heptane-2-carboxylic acid that inhibits glutamine transporters have been shown to slow proliferation and tumor growth.[88,89] Another potential therapeutic target is glutaminase, the enzyme that catalyzes the conversion of glutamine to glutamate. The growth of transformed cells can be selectively inhibited by targeting glutaminase activity.[90,91] Molecules such as bis-2-(5-phenylacetamido-1,2,4-thiodiazol-2-yl) ethyl sulfide have been shown to successful in inhibiting glutaminase.[91,92]
Although biochemistry and metabolism of transforming cells are extensively studied for the past few decades, targeting cancer metabolism for therapy of cancer still remains as a challenge. As normal proliferating cells have same metabolic requirements as cancer cells, so finding a therapeutic window is difficult. Sometimes, it is assumed that a therapeutic window is obtained by chemotherapeutic agents because cancer cell proliferates more rapidly than normal cells. However, this is not always true. Proliferative cells of gut can have cell cycle as frequently as 10 h[93,94] and hematopoiesis in human can generate 2 million red blood precursors per second.[95] Like the cancer cells, rapidly proliferating cells of immune system rely on aerobic glycolysis[96,97,98] and glutamine metabolism.[99,100] Another challenge in targeting cancer metabolism is the metabolic flexibility of the transformed cell. Cancer cells often have a remarkable ability to shift fuel source when deprived of favored metabolic pathways.[101,102,103]
CONCLUSION
The present cancer therapeutics that target DNA synthesis are not found to be promising because instead of a single tumor-specific metabolism, several metabolic programming exists that promotes proliferation of cancer cell. A better understanding of how metabolism is altered in specific genetic contexts that lead to cancer will guide to formulate strategies to target specific enzyme or combination of enzyme in cancers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 4.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 5.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–21. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–93. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Matés JM, et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–9. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–5. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 12.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–7. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 13.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 14.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plas DR, Thompson CB. Akt-dependent transformation: There is more to growth than just surviving. Oncogene. 2005;24:7435–42. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL. HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–6. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–93. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–6. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 22.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBerardinis RJ, Cheng T. Q's next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–24. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 26.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 27.Frezza C, Gottlieb E. Mitochondria in cancer: Not just innocent bystanders. Semin Cancer Biol. 2009;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 30.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 31.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–14. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–14. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–18. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deberardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–80. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- 38.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–7. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25–57. doi: 10.1016/B978-0-12-416618-9.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: The interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–8. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 43.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: Cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: New players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–65. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 46.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–22. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 47.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–7. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L, et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 2013;27:1115–31. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L, Song L, Wan Q, Wu G, Li X, Wang Y, et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–44. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell. 2013;152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaneton B, Hillmann P, Zheng L, Martin AC, Maddocks OD, Chokkathukalam A, et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–62. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–8. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Schneider J, Neu K, Grimm H, Velcovsky HG, Weisse G, Eigenbrodt E. Tumor M2-pyruvate kinase in lung cancer patients: Immunohistochemical detection and disease monitoring. Anticancer Res. 2002;22:311–8. [PubMed] [Google Scholar]
- 56.Cerwenka H, Aigner R, Bacher H, Werkgartner G, el-Shabrawi A, Quehenberger F, et al. TUM2-PK (pyruvate kinase type tumor M2), CA19-9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. Anticancer Res. 1999;19:849–51. [PubMed] [Google Scholar]
- 57.Lüftner D, Mesterharm J, Akrivakis C, Geppert R, Petrides PE, Wernecke KD, et al. Tumor type M2 pyruvate kinase expression in advanced breast cancer. Anticancer Res. 2000;20:5077–82. [PubMed] [Google Scholar]
- 58.Mazurek S, Zwerschke W, Jansen-Dürr P, Eigenbrodt E. Metabolic cooperation between different oncogenes during cell transformation: Interaction between activated ras and HPV-16 E7. Oncogene. 2001;20:6891–8. doi: 10.1038/sj.onc.1204792. [DOI] [PubMed] [Google Scholar]
- 59.Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Dürr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96:1291–6. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–6. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 61.Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109:6904–9. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–14. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Locasale JW, Cantley LC, Vander Heiden MG. Cancer's insatiable appetite. Nat Biotechnol. 2009;27:916–7. doi: 10.1038/nbt1009-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell. 2009;136:823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–77. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 68.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–66. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 69.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–27. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 70.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–20. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 73.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou R, Vander Heiden MG, Rudin CM. Genotoxic exposure is associated with alterations in glucose uptake and metabolism. Cancer Res. 2002;62:3515–20. [PubMed] [Google Scholar]
- 76.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 77.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 78.Wellen KE, Thompson CB. Cellular metabolic stress: Considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–32. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 80.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 81.Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–85. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 82.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–40. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 83.Maestu I, Pastor M, Gómez-Codina J, Aparicio J, Oltra A, Herranz C, et al. Pretreatment prognostic factors for survival in small-cell lung cancer: A new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol. 1997;8:547–53. doi: 10.1023/a:1008212826956. [DOI] [PubMed] [Google Scholar]
- 84.Eschwège E, Balkau B. Hyperglycaemia: Link to excess mortality. Int J Clin Pract Suppl. 2001;123:3–6. [PubMed] [Google Scholar]
- 85.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29:1990–1. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 87.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaira K, Sunose Y, Ohshima Y, Ishioka NS, Arakawa K, Ogawa T, et al. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer. 2013;13:482. doi: 10.1186/1471-2407-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105:1463–73. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 90.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–7. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol. 2014;42:247–51. doi: 10.1016/j.exphem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 93.Potten CS, Kellett M, Rew DA, Roberts SA. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: Data for different sites, proximity to a tumour, and polyposis coli. Gut. 1992;33:524–9. doi: 10.1136/gut.33.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rew DA, Wilson GD. Cell production rates in human tissues and tumours and their significance. Part II: Clinical data. Eur J Surg Oncol. 2000;26:405–17. doi: 10.1053/ejso.1999.0907. [DOI] [PubMed] [Google Scholar]
- 95.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A. 2009;106:17413–8. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+T cell subsets. J Immunol. 2011;186:3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–36. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takenaka M, Noguchi T, Sadahiro S, Hirai H, Yamada K, Matsuda T, et al. Isolation and characterization of the human pyruvate kinase M gene. Eur J Biochem. 1991;198:101–6. doi: 10.1111/j.1432-1033.1991.tb15991.x. [DOI] [PubMed] [Google Scholar]
- 100.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 101.Raimundo N, Baysal BE, Shadel GS. Revisiting the TCA cycle: Signaling to tumor formation. Trends Mol Med. 2011;17:641–9. doi: 10.1016/j.molmed.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–99. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen V, Shtivelman E. CC3/TIP30 regulates metabolic adaptation of tumor cells to glucose limitation. Cell Cycle. 2010;9:4941–53. doi: 10.4161/cc.9.24.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]