Summary
Women with autoimmune diseases are more at risk for infertility and subfertility, menstrual irregularities and decreased parity due to multiple possible etiologies, including underlying inflammatory disease, gonadotoxic medications, and psychosocial issues related to living with chronic disease. An awareness of these issues, and the validation and support of patients going through fertility related issues, is important for providing comprehensive care to this patient population. In particular, an understanding of the expanding options for fertility preservation strategies during gonadotoxic medications is essential, including gonadotropin releasing hormone analogues (GnRH-a) co-therapy and oocyte cryopreservation. Referral to a reproductive endocrinology clinic is indicated in this patient population, in part to help manage symptoms of hypoestrogenism that may result from GnRH-a therapy.
Keywords: infertility, systemic autoimmune diseases, fertility preservation
Introduction
For women with rheumatic autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis and ankylosing spondylitis, the risk of subfertility and infertility is higher than in the general population. Given that early and mid-adulthood is a period of increasing risk for development of many autoimmune diseases 1,2, particular emphasis on reproductive and family planning issues is of upmost importance. For instance, lupus has often been referred to as a disease of women in their childbearing years; indeed, we have shown that incidence of SLE among black females peaks during ages 25-29 years (see Figure 1)3.
Figure 1.
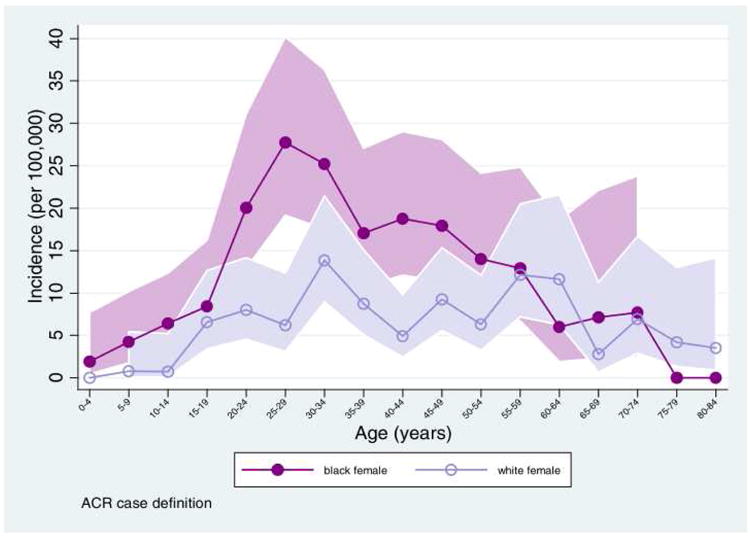
Age-specific SLE incidence for females. Data from the Michigan Lupus Epidemiology & Surveillance (MILES) Program 2,4. From Marder W., Vinet É., Somers EC. Rheumatic autoimmune diseases in women and midlife health. Women's Midlife Heal 2015;1(1):11. Doi: 10.1186/s40695-015-0012-9; with permission.
Underlying diminished ovarian reserve and lower parity rates have been observed in both rheumatic conditions and chronic diseases in general, and the use of alkylating agents for treatment of severe disease manifestations often causes ovarian damage. The increased recognition of broad health implications beyond fertility, related to ovarian damage, has heightened awareness of the need for ovarian protection during treatment with alkylating agents. In particular, the use of gonadotropin releasing hormone analogues (GnRH-a) during gonadotoxic therapy has become an acceptable option. A shift towards agents without gonadotoxicity, such as mycophenolate mofetil and rituximab, has also reduced the risk of medication-related ovarian damage in rheumatic disease patients. This review summarizes current research and practice relating to rheumatic autoimmune diseases and fertility in women, and outlines strategies for prevention of infertility. In males, autoimmune diseases such as lupus are rare and tend to have peak onset during late adulthood 5,6; thus, the topic of infertility among male rheumatic disease patients has not been thoroughly investigated. However, the reader is referred to a review elsewhere which covers male-specific considerations 7.
I. Primary ovarian insufficiency (POI)
The term “primary ovarian insufficiency” or POI is used when a woman less than 40 years old experiences amenorrhea for 4 months or more, with two serum follicle stimulating hormone (FSH) levels obtained at least one month apart in the menopausal range8. POI is most frequently considered to be idiopathic, affecting 1% of women in the general population under the age of 409. The term POI is preferred when discussing issues of subfertility, as opposed to terms such as “primary ovarian failure” or “premature menopause,” as POI better reflects a continuum of impaired ovarian function rather than a dichotomous state 10,11. This distinction is clinically important, because unlike natural menopause, which occurs at an average age of approximately 50 years, at least 50% of women with POI continue to experience variations in ovarian function, and anywhere between 5%–10% will conceive and deliver a child after a POI diagnosis 12,13.
Anti-Mullerian hormone (AMH) is perhaps the most widely used and reliable serum biomarker of ovarian reserve and surrogate predictor for POI. AMH has demonstrated utility not only in predicting time to natural menopause, with serum levels declining to very low and non-detectable levels 5 years prior to the last menstrual period, but for estimation of ovarian reserve in assisted reproductive therapy and after chemotherapy 14–16. AMH is produced in the granulosa cells of the ovarian follicles, and reflects the transition of resting primordial follicles into growing follicles. The ovarian pool of oocytes and their reproductive potential decline with age, and such decline is accelerated by exposure to chemotherapeutic agents. AMH is more highly correlated with the antral follicle count than are other reproductive hormones such as FSH, luteinizing hormone (LH), or estradiol 17. AMH furthermore varies less across the menstrual cycle as compared to other biomarkers of ovarian activity, and does not appear to be influenced by chronic underlying disease, making it an excellent biomarker for studies of ovarian function in women with rheumatic disease (RD)18–20.
In the absence of treatment with alkylating agents, some studies have observed no differences in POI prevalence between women with rheumatic autoimmune diseases and normal populations21–23. However, these studies have focused primarily on menstrual histories and cessation of menses as outcomes, and not on biologic markers of ovarian reserve. Other studies examining AMH levels reveal mixed results: studies of women with RDs including SLE, rheumatoid arthritis, spondyloarthropathy, Takayasu's arteritis and Behcet's disease without prior exposure to cytotoxic medications found significantly diminished levels of AMH among women with these diseases compared to age matched healthy controls 24–28. No correlation was observed between disease activity measures and AMH levels, although disease activity in these studies was generally mild.
What is unknown, however, is the extent to which depressed markers of ovarian reserve in these patients represents the long term effects of systemic inflammatory disease and the underlying mechanisms. Conversely, a case-control study of 80 premenopausal women with SLE and age matched controls revealed no differences in AMH levels between groups, and no associations between lupus disease activity or disease duration and AMH29.
II. Alterations in the HPG axis
Studies of women with rheumatic autoimmune diseases, primarily SLE, have revealed alterations in the functioning of the hypothalamic-pituitary-gonadal axis as well as menstrual irregularities. In a study of 30 juvenile SLE (JSLE) patients, the mean age of menarche was significantly older in JSLE than controls (13.1 vs 11.6 years), and significantly more menstrual disturbances (including higher FSH, and lower progesterone and LH levels) and longer length cycles were also observed30. There was no significant difference in the rate of menstrual irregularities between JSLE patients who had previously received CYC and those who had not. While these findings support the possibility of HPG axis dysfunction and diminished ovarian reserve, AMH levels, arguably a more accurate assessment of ovarian reserve, were not assessed. Another study of 298 female JSLE patients receiving CYC revealed amenorrhea in 11.7% and normal FSH and estradiol levels for their ages. The CYC exposure was comparable in patients with and without amenorrhea, however lupus diseases activity indices were significantly higher among those with amenorrhea, suggesting the possibility that disease activity and not HPG axis leads to menstrual irregularities in these patients31.
III. Parity in women with autoimmune diseases
Prior to advances in therapies and disease management, many women with RDs were discouraged from having children for multiple reasons, including concerns that disease flare during pregnancy may affect their health or their baby's health, and that disease related disability may impact their ability to care for a child32. This widely held sentiment is reflected in part in observed rates of decreased parity and smaller family size among women with SLE and different types of inflammatory arthritis33–36. A 2012 study of 852 women with RA and 165 women with SLE found that more than half of those who received their diagnoses prior to completing child-bearing had fewer children than they had originally planned35. Underlying factors cited by the subjects included: infertility rates among women with RA, pregnancy loss among women with SLE, and in both groups, concerns that their disease would adversely impact their offspring as well as family life. Supporting these observations is a large body of work describing concerns related to pregnancy and childcare that are common to many women with chronic diseases, and are well documented among women with inflammatory arthritis. Survey studies have revealed that issues surrounding family planning significantly impact women with rheumatic diseases, including disease related physical limitations, balancing antenatal medication exposure and disease control, and uncertainty about the physical, social and psychologic challenges that come with motherhood, all of which can lead to significant anxiety surrounding reproductive choices3,37–40.
Ultimately, these concerns may manifest in choices made by women with RA to have fewer children than they had planned, or remain childless all together38,41. A large Norwegian study found a higher proportion of women with chronic inflammatory arthritis (including RA and idiopathic juvenile arthritis) treated with disease modifying antirheumatic medications (DMARDs) or biologic agents were nulliparous compared to a randomly selected age matched reference population: 32.6% of inflammatory arthritis patients were nulliparous vs 26.4% in reference population (P<0.001)33. Furthermore, while adjusted relative fertility rates in patients with chronic inflammatory arthritis before their diagnoses were not reduced, after diagnosis the relative fertility rates were significantly lower in these populations.
A prolonged time to pregnancy (TTP) has also been observed among patients with RA prior to conception42,43. In a Dutch cohort of pregnant women with RA, 42% of the patients had a TTP exceeding 12 months, significantly longer than TTP among women in a comparable general Western European population, in which 50% of women have a TTP of 3 months and around 70% have TTP of 6 months after starting unprotected intercourse44. Age, nulliparity, disease activity and preconception use of NSAIDs and dose-dependent prednisone therapy (significantly longer if >7.5mg) were all independently associated with TTP. Autoantibodies, past DMARD use, smoking, and disease duration did not affect TTP.
Encouragingly, a recent study of national healthcare databases has revealed an increase in the numbers of children born to mothers with inflammatory arthritis. A 2016 study looked at trends in birth rates over time among women in Norway with inflammatory joint disease, and found higher parity rates particularly from 1990s onward 45; this trend may in part reflect the development of better and safer treatment options, in particular the use of anti-TNF agents in the 1990s.
IV. Effects of medications on ovarian function
Alkylating agents
Cyclophosphamide (CYC) and chlorambucil have been widely used in the treatment of severe autoimmune disease, particularly lupus nephritis46. While chlorambucil is used less frequently, CYC continues to have an important role for treating severe organ-threatening manifestations of systemic autoimmune diseases, although increasingly less so, as studies support the use of mycophenolate mofetil and lower dose “Euro-Lupus” CYC regimens for induction of remission in proliferative lupus nephritis47–50, and rituximab for use in ANCA-associated vasculitis51.
CYC-induced ovarian toxicities reported in animal models include DNA crosslinking of granulosa cells, reduced numbers of granulosa cells, ovarian fibrosis, and decreased progesterone and estrogen52,53. In humans, CYC has been shown to impair follicle maturation, and is associated with a dose dependent depletion of the primordial antral follicle pool54. In lupus, CYC-associated premature ovarian failure occurs in 16%-54% of patients 55–58, and is strongly associated with age at CYC initiation and cumulative dose 57,59. Gradations of ovarian insult have also been observed: among women (mean age 35 years) with granulomatosis with polyangiitis in a trial of daily oral CYC or methotrexate, AMH levels declined by 0.74 ng/mL for each 10 gm of CYC, highlighting the impact of oral CYC even for courses of less than 6 months 60.
V. Preservation of fertility in women receiving gonadotoxic therapies
Assisted reproductive technologies (ART)
Women facing the prospect of gonadotoxic therapies should have the opportunity to discuss their options with a reproductive endocrinologist. While embryo cryopreservation is a proven ART method, the requirements for an available partner/sperm and several weeks of preparation limit its utility in patients facing treatment for cancer or severe manifestations of autoimmune disease61. The 2013 guidelines issued by the Societies for Reproductive Medicine and Assisted Reproductive Technology state that given dramatic improvements in the success of oocyte cryopreservation, this technique should no longer be considered experimental and should be recommended, with appropriate counseling, in patients at risk of infertility due to chemotherapy or gonadotoxic therapies62.
There is no evidence that fertility success rates or the number of in vitro fertilization (IVF) cycles among women with RDs undergoing ART differ from the general population, however studies have suggested worse outcomes if disease is active in pregnancy63–66. Antiphospholipid antibody positivity also does not appear to influence IVF outcomes67. However, hormonal manipulations specific to particular ART strategies, and potential implications related to estrogen levels, disease flare, or thrombosis should be evaluated in the context of the individual patient's underlying disease and risk factors7.
Procedures involving ART generally require planning, for example, 12 days are allowed for maturation of oocytes prior to retrieval, so they are less attractive if treatment with cyclophosphamide is indicated urgently. Both procedures are associated with medication costs of controlled ovarian hyperstimulation, retrieval costs, thawing and transfer costs, and yearly storage fees that can run into tens of thousands of dollars. Experimental ART methods, such as ovarian tissue cryopreservation, may also warrant exploration in the patient population exposed to gonadotoxic drugs.
GnRH-analogue therapy
Given the numerous health concerns associated with POI, including increased risk of cardiovascular disease, bone loss, mental health comorbidities, and all-cause mortality 68, mitigation of ovarian damage associated with gonadotoxic therapy should be a treatment goal even among women who do not desire future pregnancy. Temporary ovarian suppression with GnRH-a therapy concurrent with chemotherapy administration is a promising strategy for ovarian protection. Although still considered investigational, it is attractive due to its non-invasive nature, and potential to provide protection against ovarian damage and forestall premature ovarian insufficiency. Aside from treatment regimens designed to limit cumulative exposure to gonadotoxic drugs (eg, “Euro-lupus”49), adjunctive GnRH-a therapy currently is the most promising strategy available for ovarian preservation rather than solely maintenance of reproductive capacity. It is also less costly and invasive than other ART procedures.
GnRH-a for ovarian preservation has been examined in both cancer and autoimmune populations undergoing various chemotherapeutic regimens. Our group was among the first to report a benefit of GnRH-a for protection against premature ovarian failure among women with severe lupus on a standard cyclophosphamide regimen 69. As shown by the time to event analysis in Figure 2, cumulative preservation of ovarian function following CYC therapy was significantly greater among women treated with adjunctive GnRH-a compared to controls matched by age and cumulative CYC dose. The endpoint of “premature ovarian failure” in this study was defined as amenorrhea of at least 12 months duration and an FSH level ≥40 mIU/ml.
Figure 2.
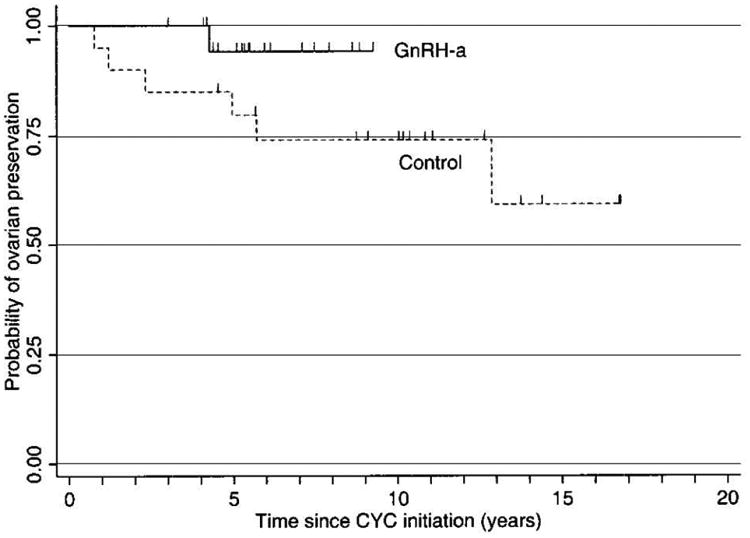
Kaplan-Meier survival estimates of time to premature ovarian failure (POF). Tick marks indicate censored observations (i.e., the final point of followup for patients who did not develop the end point of POF). GnRH-a = gonadotropin-releasing hormone analog; CYC = cyclophosphamide.
Somers EC., Marder W., Christman GM., et al. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum 2005;52:2761–7. Doi: 10.1002/art.21263; with permission.
GnRH-a may also provide gradations of benefit for ovarian health, as measured by biomarkers of ovarian function. Indeed, we found higher post-CYC levels of AMH among women who received adjunctive GnRH-a during CYC therapy 70. A review published in 2015 found that on aggregate GnRH-a was positively associated with benefit based on 20 studies inclusive of 2038 patients; benefit was not detected in 8 studies inclusive of 509 patients.71
The standard GnRH-a protocol is comprised of 3.75 mg depot leuprolide acetate (a GnRH-a agonist) injection once per month throughout CYC therapy 69. To reduce ovarian CYC exposure during the initial estrogen surge associated with GnRH-a, the injection is ideally timed to precede the subsequent monthly IVCYC bolus by ∼10-14 days. For CYC courses started on an urgent basis, initiation of GnRH-a is generally postponed until after the first monthly IVCYC (though exception to this may be contemplated for women in the luteal phase of their menstrual cycle, when an estrogen flare is less likely). In the clinical setting, patients tolerating the first 30 day GnRH-a dose may subsequently be switched to quarterly injection. To reduce symptoms of hormone withdrawal, transdermal estrogen may be considered, if not otherwise contraindicated (e.g. a hypercoagulable state such as antiphospholipid antibody syndrome), to maintain slightly less than physiologic estrogen (early follicular phase) levels.
It should be noted that the various strategies for preserving fertility outlined above are not mutually exclusive, and often multiple options may be integrated in an effort to optimize outcomes. Close collaboration with reproductive endocrinology specialists is essential in order to design an individualized plan taking into account patient goals and preferences.
Key Points.
Women with autoimmune diseases have elevated risk for primary ovarian insufficiency, likely resulting from the underlying inflammatory state, alterations of the HPG axis, and medication exposures
Increasingly, more options are available for ovarian preservation alongside gonadotoxic treatment regimens, including strategies for minimizing cumulative exposure of alkylating agents such as cyclophosphamide, and the use of adjunctive GnRH-analog therapy
Given that early- and mid-adulthood is a period of increasing risk for development of many autoimmune diseases, particular emphasis on reproductive and family planning issues in this population is of upmost importance.
Footnotes
disclosure statement: the authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3-4):197–207. doi: 10.1016/j.jaut.2009.09.008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers EC, Marder W, Cagnoli P, et al. Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan lupus epidemiology and surveillance program. Arthritis Rheumatol. 2014;66(2):369–78. doi: 10.1002/art.38238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marder W, Vinet É, Somers EC. Rheumatic autoimmune diseases in women and midlife health. Women's Midlife Heal. 2015;1(1):11. doi: 10.1186/s40695-015-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SS, Drenkard C, McCune WJ, et al. Population-based lupus registries: advancing our epidemiologic understanding. Arthritis Rheum. 2009;61(10):1462–6. doi: 10.1002/art.24835. [DOI] [PubMed] [Google Scholar]
- 5.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 6.Somers EC, Thomas SL, Smeeth L, et al. Incidence of systemic lupus erythematosus in the United Kingdom, 1990-1999. Arthritis Rheum. 2007;57(4):612–8. doi: 10.1002/art.22683. [DOI] [PubMed] [Google Scholar]
- 7.Hickman RA, Gordon C. Causes and management of infertility in systemic lupus erythematosus. Rheumatology (Oxford) 2011;50(9):1551–8. doi: 10.1093/rheumatology/ker105. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–14. doi: 10.1056/NEJMcp0808697. doi:360/6/606[pii]10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–6. [PubMed] [Google Scholar]
- 10.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalantaridou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann N Y Acad Sci. 2000;900:393–402. doi: 10.1111/j.1749-6632.2000.tb06251.x. [DOI] [PubMed] [Google Scholar]
- 12.van Noord PA, Dubas JS, Dorland M, et al. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68(1):95–102. doi: 10.1016/s0015-0282(97)81482-3. doi:S0015028297814823[pii] [DOI] [PubMed] [Google Scholar]
- 13.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113(6):1355–63. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 14.Majumder K, Gelbaya TA, Laing I, et al. European Journal of Obstetrics & Gynecology and Reproductive Biology ¨ llerian hormone and antral follicle count to predict the The use of anti-Mu potential of oocytes and embryos. Eur J Obstet Gynecol. 2010;150(2):166–70. doi: 10.1016/j.ejogrb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser JA, Schipper I, Laven JSE, et al. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- 17.Fanchin R, Schonäuer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 18.La Marca A, Grisendi V, Griesinger G. How Much Does AMH Really Vary in Normal Women? Int J Endocrinol. 2013;2013:1–8. doi: 10.1155/2013/959487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update nd. 16(2):113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 20.Visser JA, de Jong FH, Laven JS, et al. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 21.Pasoto SG, Mendonça BB, Bonfá E. Menstrual disturbances in patients with systemic lupus erythematosus without alkylating therapy: clinical, hormonal and therapeutic associations. Lupus. 2002;11(3):175–80. doi: 10.1191/0961203302lu163oa. [DOI] [PubMed] [Google Scholar]
- 22.Alpízar-Rodríguez D, Romero-Díaz J, Sánchez-Guerrero J, et al. Age at natural menopause among patients with systemic lupus erythematosus. Rheumatology (Oxford) 2014;53(11):2023–9. doi: 10.1093/rheumatology/keu222. [DOI] [PubMed] [Google Scholar]
- 23.Mayorga J, Alpízar-Rodríguez D, Prieto-Padilla J, et al. Prevalence of premature ovarian failure in patients with systemic lupus erythematosus. Lupus. 2015 doi: 10.1177/0961203315622824. [DOI] [PubMed] [Google Scholar]
- 24.Henes M, Froeschlin J, Taran F a, et al. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Beh??et's disease and spondyloarthritis on anti-M??llerian hormone levels. Rheumatology (Oxford) 2015;54(9):1709–12. doi: 10.1093/rheumatology/kev124. [DOI] [PubMed] [Google Scholar]
- 25.Wei W, Lin Q, Huang Q, et al. Impact of Systemic Lupus Erythematosus on Ovarian Reserve in Premenopausal Women before Receiving Cyclophosphamide Therapy: Evaluation Using Anti-Mɒllerian Hormone. Adv Reprod Sci. 2016;04(01):17–22. doi: 10.4236/arsci.2016.41003. [DOI] [Google Scholar]
- 26.Yamakami LYS, Serafini PC, de Araujo DB, et al. Ovarian reserve in women with primary antiphospholipid syndrome. Lupus. 2014;23(9):862–7. doi: 10.1177/0961203314529468. [DOI] [PubMed] [Google Scholar]
- 27.Mont'Alverne ARS, Pereira RMR, Yamakami LYS, et al. Reduced ovarian reserve in patients with Takayasu arteritis. J Rheumatol. 2014;41(10):2055–9. doi: 10.3899/jrheum.131360. [DOI] [PubMed] [Google Scholar]
- 28.Lawrenz B, Henes J, Henes M, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus. 2011;20(11):1193–7. doi: 10.1177/0961203311409272. [DOI] [PubMed] [Google Scholar]
- 29.Gasparin AA, Souza L, Siebert M, et al. Assessment of anti-Müllerian hormone levels in premenopausal patients with systemic lupus erythematosus. Lupus. 2016;25(3):227–32. doi: 10.1177/0961203315598246. [DOI] [PubMed] [Google Scholar]
- 30.Medeiros PB, Febrônio MV, Bonfá E, et al. Menstrual and hormonal alterations in juvenile systemic lupus erythematosus. Lupus. 2009;18(1):38–43. doi: 10.1177/0961203308094652. [DOI] [PubMed] [Google Scholar]
- 31.Silva C a, Yamakami LYS, Aikawa NE, et al. Autoimmune primary ovarian insufficiency. Autoimmun Rev. 2014;13(4-5):427–30. doi: 10.1016/j.autrev.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Ostensen M. Counselling women with rheumatic disease–how many children are desirable? Scand J Rheumatol. 1991;20(2):121–6. doi: 10.3109/03009749109165287. [DOI] [PubMed] [Google Scholar]
- 33.Wallenius M, Skomsvoll JF, Irgens LM, et al. Fertility in women with chronic inflammatory arthritides. Rheumatology. 2011;50(February):1162–7. doi: 10.1093/rheumatology/keq458. [DOI] [PubMed] [Google Scholar]
- 34.Skomsvoll JF, Ostensen M, Baste V, et al. Number of births, interpregnancy interval, and subsequent pregnancy rate after a diagnosis of inflammatory rheumatic disease in Norwegian women. J Rheumatol. 2001;28(10):2310–4. [PubMed] [Google Scholar]
- 35.Clowse MEB, Chakravarty E, Costenbader KH, et al. Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64(5):668–74. doi: 10.1002/acr.21593. [DOI] [PubMed] [Google Scholar]
- 36.Vinet E, Pineau C, Gordon C, et al. Systemic lupus erythematosus in women: Impact on family size. Arthritis Rheum. 2008;59(11):1656–60. doi: 10.1002/art.24203. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarty EF. Rheumatoid arthritis and pregnancy: beyond smaller and preterm babies. Arthritis Rheum. 2011;63(6):1469–71. doi: 10.1002/art.30206. [DOI] [PubMed] [Google Scholar]
- 38.Katz PP. Childbearing decisions and family size among women with rheumatoid arthritis. Arthritis Rheum. 2006;55(2):217–23. doi: 10.1002/art.21859. [DOI] [PubMed] [Google Scholar]
- 39.Del Junco DJ, Annegers JF, Coulam CB, et al. The relationship between rheumatoid arthritis and reproductive function. Br J Rheumatol. 1989;28(Suppl 1):33. doi: 10.1093/rheumatology/xxviii.suppl_1.33. discussion 42–5. [DOI] [PubMed] [Google Scholar]
- 40.Meade T, Sharpe L, Hallab L, et al. Navigating Motherhood Choices in the context of Rheumatoid Arthritis: Women's Stories. Musculoskeletal Care. 2013;11(2):73–82. doi: 10.1002/msc.1031. [DOI] [PubMed] [Google Scholar]
- 41.Ostensen M. Pregnancy in patients with a history of juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34(7):881–7. doi: 10.1002/art.1780340714. [DOI] [PubMed] [Google Scholar]
- 42.Jawaheer D, Zhu JL, Nohr EA, et al. Time to pregnancy among women with rheumatoid arthritis. Arthritis Rheum. 2011;63(6):1517–21. doi: 10.1002/art.30327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouwer J, Hazes JMW, Laven JSE, et al. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann Rheum Dis. 2015;74(10):1836–41. doi: 10.1136/annrheumdis-2014-205383. [DOI] [PubMed] [Google Scholar]
- 44.Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group Hum Reprod. 1999;14(5):1250–4. doi: 10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- 45.Wallenius M, Salvesen KÅ, Daltveit AK, et al. Reproductive trends in females with inflammatory joint disease. BMC Pregnancy Childbirth. 2016;16(1):123. doi: 10.1186/s12884-016-0919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marder W, McCune WJ. Advances in immunosuppressive therapy Semin. Respir Crit Care Med. 2007;28(4):398–417. doi: 10.1055/s-2007-985612. [DOI] [PubMed] [Google Scholar]
- 47.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–28. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 48.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houssiau FA, Vasconcelos C, D'Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–31. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 50.Houssiau FA, Vasconcelos C, D'Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69(1):61–4. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 51.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N Engl J Med. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slater CA, Liang MH, McCune JW, et al. Preserving ovarian function in patients receiving cyclophosphamide. Lupus. 1999;8(1):3–10. doi: 10.1191/096120399678847335. [DOI] [PubMed] [Google Scholar]
- 53.Ataya KM, Valeriote FA, Ramahi-Ataya AJ. Effect of cyclophosphamide on the immature rat ovary. Cancer Res. 1989;49(7):1660–4. [PubMed] [Google Scholar]
- 54.Warne GL, Fairley KF, Hobbs JB, et al. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–62. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 55.Clowse MEB, Behera MA, Anders CK, et al. Ovarian Preservation by GnRH Agonists during Chemotherapy: A Meta-Analysis. J Women's Heal. 2009;18(3):311–9. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park MC, Park YB, Jung SY, et al. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13(8):569–74. doi: 10.1191/0961203304lu1063oa. [DOI] [PubMed] [Google Scholar]
- 57.McDermott EM, Powell RJ. Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis. 1996;55(4):224–9. doi: 10.1136/ard.55.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boumpas DT, Austin HA, 3rd, Vaughan EM, et al. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119(5):366–9. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 59.Mok CC, Lau CS, Wong RW. Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum. 1998;41(5):831–7. doi: 10.1002/1529-0131(199805)41:5<831∷AID-ART9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Clowse MEB, Copland SC, Hsieh TC, et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (Wegener's) Arthritis Care Res (Hoboken) 2011;63(12):1777–81. doi: 10.1002/acr.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ASRM/ART PC. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2004;82(4):993–8. doi: 10.1016/j.fertnstert.2004.07.925. [DOI] [PubMed] [Google Scholar]
- 62.ASRM/ART PC. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 63.Guballa N, Sammaritano L, Schwartzman S, et al. Ovulation induction and in vitro fertilization in systemic lupus erythematosus and antiphospholipid syndrome. Arthritis Rheum. 2000;43(3):550–6. doi: 10.1002/1529-0131(200003)43:3<550∷AID-ANR10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 64.Di Nisio M, Rutjes AWS, Ferrante N, et al. Thrombophilia and outcomes of assisted reproduction technologies: a systematic review and meta-analysis. Blood. 2011;118(10):2670–8. doi: 10.1182/blood-2011-03-340216. [DOI] [PubMed] [Google Scholar]
- 65.Steinvil A, Raz R, Berliner S, et al. Association of common thrombophilias and antiphospholipid antibodies with success rate of in vitro fertilisation. Thromb Haemost. 2012;108(6):1192–7. doi: 10.1160/TH12-06-0381. [DOI] [PubMed] [Google Scholar]
- 66.Levine a B, Lockshin MD, aBL, et al. Assisted reproductive technology in SLE and APS. Lupus. 2014;23(12):1239–41. doi: 10.1177/0961203314527370. [DOI] [PubMed] [Google Scholar]
- 67.Chighizola CB, de Jesus GR. Antiphospholipid antibodies and infertility. Lupus. 2014;23(12):1232–8. doi: 10.1177/0961203314529171. [DOI] [PubMed] [Google Scholar]
- 68.Marder W, Fisseha S, Ganser MA, et al. Ovarian Damage During chemotherapy in Autoimmune Diseases: Broad Health Implications beyond Fertility. Clin Med Insights Reprod Heal. 2012;2012(6):9–18. doi: 10.4137/CMRH.S10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Somers EC, Marder W, Christman GM, et al. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761–7. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 70.Marder W, McCune WJ, Wang L, et al. Adjunctive GnRH-a treatment attenuates depletion of ovarian reserve associated with cyclophosphamide therapy in premenopausal SLE patients. Gynecol Endocrinol. 2012;28(8):624–7. doi: 10.3109/09513590.2011.650752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blumenfeld Z, Evron A. Preserving fertility when choosing chemotherapy regimens - the role of gonadotropin-releasing hormone agonists. Expert Opin Pharmacother. 2015;16(7):1009–20. doi: 10.1517/14656566.2015.1031654. [DOI] [PubMed] [Google Scholar]


