Abstract
Background
Buprenorphine has recently emerged as a safe and effective treatment option for pregnant women with opioid use disorder (OUD) and is associated with superior neonatal outcomes. This study characterized and compared patient populations who used buprenorphine versus methadone during pregnancy in an academic medical center.
Methods
Observational retrospective cohort evaluation of 791 pregnant women with OUD on opioid maintenance treatment from 2009 to 2012. Buprenorphine versus methadone use was defined as use after either a) conversion from illicit opioid use during pregnancy or b) ongoing pre-pregnancy use. Multivariable logistic regression was used to identify patient characteristics predictive of buprenorphine use.
Results
Among 791 pregnant women, 608 (76.9%) used methadone and 183 (23.1%) used buprenorphine. From 2009 to 2012, buprenorphine use during pregnancy increased from 10.1% to 33.2%. Pregnant women using buprenorphine were significantly more likely to be older, married, employed, have more education and have a history of prescription opioid use compared to women using methadone. In contrast, pregnant women using methadone were significantly more likely to have hepatitis C virus infection, use cocaine, benzodiazepines, marijuana and have a history of heroin and/or intravenous opioid use. In multivariable analysis, pregnant women who were older (OR 1.01; 95% CI 1.02, 1.11), employed (1.87; 1.20, 2.90) and who had a history of opioid maintenance treatment prior to pregnancy (2.68; 1.78, 4.02) were more likely to use buprenorphine during pregnancy. Pregnant women with a history of benzodiazepine use (0.48; 0.30, 0.77), who had children no longer in their legal custody (0.63; 0.40, 0.99) and who had a partner with a substance use history (0.37; 0.22, 0.63) were less likely to use buprenorphine during pregnancy.
Conclusions
Disparities exist among patients who use buprenorphine versus methadone during pregnancy and indicate the need to improve the availability and accessibility of buprenorphine for many pregnant women.
Keywords: Opioid use disorder, pregnancy, buprenorphine, methadone
Introduction
The incidence of opioid use disorder (OUD) in pregnancy is rising and associated with significant maternal and neonatal morbidity.1 Approximately 30% of pregnancies complicated by OUD end in preterm birth (<37 weeks), a rate 3 times the national average.2,3 Neonates chronically exposed to opioids in utero are more likely to be admitted to the neonatal intensive care unit and require prolonged treatment for neonatal abstinence syndrome (NAS).4,5 NAS, a drug withdrawal syndrome after birth, affects 45-94% of infants born to mothers with OUD.6-10 Average hospital costs for NAS infants are $53,400 compared to $9,500 for all other births.1
Opioid withdrawal is associated with recidivism and ongoing illicit drug use and is not recommended in pregnancy.11,12 Instead, conversion from illicit opioid use to opioid maintenance treatment decreases maternal and neonatal morbidity by providing a stable dosing regimen, reducing risk-taking behavior and decreasing the spread of hepatitis C virus (HCV) and HIV.13,14 Methadone, a full mu opioid receptor agonist, is the recommended, standard treatment for pregnant women with OUD.15 However, in 2002, the Food and Drug Administration approved buprenorphine, a partial mu opioid receptor agonist, for the treatment of opioid addiction. Randomized controlled trials have established buprenorphine's safety in pregnancy and demonstrated superior neonatal outcomes compared to methadone. 16-19 In 2010, an international, multi-site, double-blind, double-dummy randomized clinical trial demonstrated that infants exposed to buprenorphine had a shorter treatment duration for NAS, required less morphine for NAS symptoms and had a shorter hospital stay than infants exposed to methadone.20
Buprenorphine's association with decreased NAS severity and improved neonatal outcomes has provided patients with a safe and effective alternative to methadone that has many maternal as well as neonatal advantages. Methadone is dispensed from tightly regulated, federally licensed treatment programs which require patients to return each day for supervised dosing. In contrast, due to a decreased risk of overdose and an enhanced safety profile, buprenorphine is dispensed in office-based settings, by a variety of providers and with less regulatory oversight than methadone, which may eliminate many treatment barriers for women with work or childcare responsibilities.21-25 However, buprenorphine's partial agonist activity may inadequately mitigate cravings and be less effective for patients with greater addiction severity. 28,29
The use of buprenorphine among pregnant patients with OUD has important maternal and neonatal implications and has the potential to significantly reduce healthcare costs associated with NAS. However, the impact of buprenorphine's emergence as a safe and effective treatment alternative to methadone on patterns of opioid maintenance treatment utilization in pregnancy has not been evaluated. Therefore, the purpose of this study is to a) characterize and compare patient populations who initiated buprenorphine versus methadone maintenance treatment during pregnancy in a large tertiary care, academic medical center and b) identify patient characteristics predictive of buprenorphine use during pregnancy.
Methods
Study sample and setting
This study was performed at a large, tertiary care, university-affiliated academic medical center with approximately 10,000 deliveries per year. Prenatal and postpartum care is provided to approximately 300 pregnant women with OUD each year. All of the patients in our sample received opioid maintenance treatment (either methadone or buprenorphine) during their pregnancy. Patients using methadone received their treatment from a federally licensed facility and patients using buprenorphine received their treatment in an office-based setting from a variety of buprenorphine providers in the metropolitan area.
Dataset
This was a retrospective cohort study of all pregnant women with OUD who delivered an infant at our institution between 2009 and 2012. International Classification of Diseases, Ninth Revision (ICD-9) codes for opioid dependence (304.0x) were used to identify the sample (ICD-10 equivalent codes F11.2x). Variables including maternal demographics, substance use history, maternal medical co-morbidities and maternal and paternal psychosocial risk factors were extracted were extracted from provider progress notes and laboratory data in the electronic medical record. Extracted data extracted was validated through a double entry process. Researchers experienced in medical record extraction individually and separately extracted each variable from the medical record into two separate databases. These two datasets were then merged and analyzed for discrepancies between the entered data. Each discrepancy was reviewed and resolved by the authors (EK, SD) through a third review of the medical record. This study was approved by the University Institutional Review Board, IRB # PRO14030301.
Patient characteristics
Maternal demographics, parity, medical co-morbidities and prenatal care provider information were extracted from each patient's medical record. HCV infection was defined as documentation of either an anti-HCV antibody test or a provider discussion regarding a known HCV positive diagnosis during pregnancy. Hepatitis B virus infection was defined as documentation of a positive Hepatitis B antigen test. No patients in our sample had HIV which was defined as a positive anti-HIV antibody test during pregnancy. Prenatal care providers in the academic teaching service included obstetrics and gynecology resident physicians and certified nurse midwives who are supervised by attending obstetrics and gynecology physicians.
Substance use and treatment history
All of the patients in our sample had a history of illicit opiate use and the diagnosis of OUD according to DSM-V criteria. Opioid type and route were self-reported. Buprenorphine versus methadone use during pregnancy was defined by the type of maintenance treatment initiated at the beginning of pregnancy after either a) conversion from illicit opioid use to buprenorphine or methadone during the first (if more than one conversion documented in pregnancy) or only opioid maintenance treatment conversion during pregnancy or b) the continuation of buprenorphine or methadone from ongoing pre-pregnancy use. Tobacco use was defined as use at any time during pregnancy. Alcohol abuse was defined as a self-reported problem with alcohol use prior to pregnancy. Amphetamine/methamphetamine, barbiturate, cocaine, non-prescription use of benzodiazepines and/or marijuana use was defined as ≥ one positive urinary toxicology screen for these substances at any time during pregnancy and/or a self-reported history of use. Pre-pregnancy opioid maintenance treatment was defined as the use of either buprenorphine or methadone prior to pregnancy. A history of inpatient treatment rehabilitation was defined as the history of at least one inpatient admission or hospitalization for the treatment of a substance use disorder.
Psychosocial risk factors
Maternal psychiatric risk factors were either self-reported and extracted from provider progress notes or documented directly in the electronic medical record (i.e. evidence of an inpatient hospital admission for a psychiatric disorder). Maternal psychiatric disorder was defined as the diagnosis of a psychiatric disorder by a physician at any time either before or during pregnancy. History of suicide attempt was defined as the history of at least one suicide attempt or attempt to self-harm (i.e. cutting) prior to or during pregnancy. History of an inpatient admission for a psychiatric disorder was the history of at least one inpatient hospital admission for a psychiatric disorder either prior to or during pregnancy. Maternal social risk factors were self-reported and included: history of or current prostitution, legal problems, homelessness, abuse and children not in maternal custody. Legal problems were defined as a history of or current probation, arrest and/or incarceration. Abuse was defined as history of or current intimate partner violence or sexual abuse. Partner with a substance use history was self-reported by patients without partner confirmation. Paternal support was defined as documentation in provider progress notes that the patient's partner was supportive of the pregnancy and/or living with the patient during the pregnancy. Family history of drug use was defined as a history of or current illicit drug use by a first degree relative (mother, father and/or sibling).
Maternal treatment outcomes
Treatment discontinuation was defined as the discontinuation of buprenorphine or methadone maintenance treatment after use in pregnancy. Illicit opioid use was defined as the documentation of ≥ one positive urine drug screen for illicit opioids after initiation of opioid maintenance treatment in pregnancy. Illicit drug use was evaluated as both a composite variable and as individual variables. The composite illicit drug use variable was defined as the documentation of ≥ one positive urine drug screen for amphetamine/methamphetamines, barbiturates, benzodiazepines, cocaine and/or marijuana after initiation of opioid maintenance treatment in pregnancy.
Data Analysis and Model Development
Descriptive statistics were used to describe patient characteristics among women who used buprenorphine versus methadone during pregnancy. Chi square and t-tests were used to test for each variable's association with buprenorphine versus methadone use in pregnancy. Variables included in our multivariate model were selected after evaluating for statistical significance in bivariate and univariate logistic regression analyses (p-values < 0.10), collinearity as determined by the variance inflation factor (VIF) and clinical relevance.30 Stepwise logistic regression with backward elimination based on the p-values of the coefficient estimates (p values <0.10) was used to develop the model.
The predictive performance of the model was assessed by calculating the Bayesian Information Criterion (BIC), the Akaike Information Criterion (AIC) and by constructing a receiver-operating characteristic (ROC) curve.31 To evaluate the generalizability of our model, a 10-fold cross-validation procedure was used to simulate a situation where we apply our model to a new cohort of patients that we did not have the data for at the time of model development.32 Subjects with missing values were excluded from the analysis and a p-value of <0.05 was considered statistically significant. All analyses were conducted with STATA® 13 (StataCorp, College Station, TX) and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sample characteristics
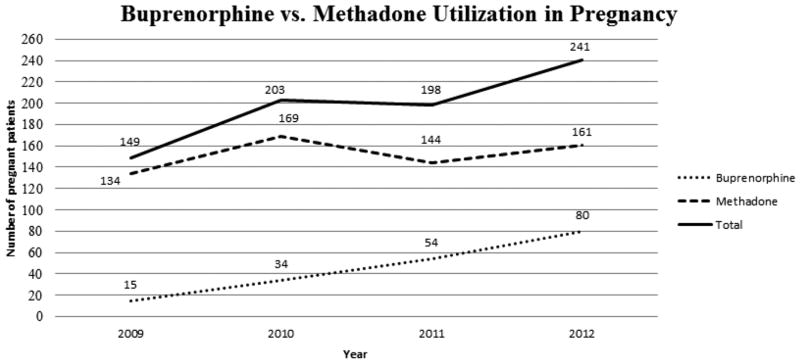
The total sample consisted of 791 pregnant women on opioid maintenance treatment (either methadone or buprenorphine) who delivered an infant at our institution between 2009 and 2012 (Table 1). During this time period, the rate of buprenorphine use during pregnancy at our institution increased from 10.1% to 33.2% (Figure 1). Overall, 183 (23.1%) patients used buprenorphine and 608 (76.9%) patients used methadone in pregnancy. The mean age of patients in our sample was 27 years old and the majority of patients were Caucasian, single, unemployed, had Medicaid insurance and had a high school education. Approximately two-thirds of patients received their prenatal care from the academic teaching service compared to private practice providers. Over 80% of patients smoked tobacco and approximately one fourth of patients had a history of cocaine, benzodiazepine or marijuana use. Prior to conversion to opioid maintenance therapy, the majority of patients reported a history of heroin use compared to prescription opioid use and the majority of patients disclosed a history of IV opioid use. Over 70% of patients had a diagnosis of a psychiatric disorder either before or during pregnancy and many patients had multiple social risk factors. Approximately one-fourth of patients reported a history of or current legal problems, abuse (intimate partner violence and/or sexual abuse), had a partner with a history of substance use and/or had children who were no longer in their legal custody. Approximately 5% of patients reported a history of or current prostitution and/or homelessness.
Table 1. Characteristics of pregnant women who used buprenorphine versus methadone during pregnancy, n=791a.
| All n=791 |
Buprenorphine n=183 |
Methadone n=608 |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age [years; mean (±SD)] | 27.3 (±4.7) | 28.4 (±4.9) | 27.0 (±4.6) | <0.01 |
| Race | ||||
| Caucasian | 760 (96.9) | 179 (98.4) | 581 (96.5) | 0.21 |
| Other | 24 (3.1) | 3 (1.7) | 21 (3.5) | |
| Married | 99 (12.7) | 33 (18.0) | 66 (11.0) | 0.01 |
| Employed | 134 (16.9) | 47 (25.7) | 87 (14.3) | <0.01 |
| Education | ||||
| ≤HS/GED | 384 (65.6) | 66 (55.5) | 318 (68.2) | 0.03 |
| Some college/Associates degree | 167 (28.6) | 45 (37.8) | 122 (26.2) | |
| ≥Bachelor's | 34 (5.8) | 8 (6.7) | 26 (5.6) | |
| Medicaid | 757 (95.7) | 169 (92.4) | 588 (96.7) | 0.01 |
| Primaparous | 271 (34.3) | 66 (36.1) | 205 (33.7) | 0.56 |
| Prenatal care provider type | ||||
| Academic Teaching Service | 505 (63.8) | 82 (44.8) | 423 (69.6) | <0.01 |
| Private Practice | 286 (36.2) | 101 (55.2) | 185 (30.4) | |
| Medical Co-morbidities | ||||
| Hepatitis C virus infection | 369 (46.7) | 70 (38.3) | 299 (49.2) | <0.01 |
| HIV | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Hepatitis B virus infection | 8 (1.0) | 1 (0.6) | 7 (1.2) | 0.47 |
| Substance Use Historyb | ||||
| Tobacco | 657 (83.3) | 145 (79.2) | 512 (84.5) | 0.10 |
| Alcohol abuse | 53 (6.7) | 16 (8.7) | 37 (6.1) | 0.21 |
| Amphetamine/methamphetamines | 11 (1.4) | 5 (2.8) | 6 (1.0) | 0.08 |
| Barbiturates | 11 (1.4) | 4 (2.2) | 7 (1.2) | 0.29 |
| Cocaine | 165 (20.9) | 19 (10.4) | 146 (24.0) | <0.01 |
| Benzodiazepines | 209 (26.7) | 28 (15.5) | 181 (30.0) | <0.01 |
| Marijuana | 197 (25.0) | 32 (17.5) | 165 (27.2) | <0.01 |
| Opioid type | ||||
| Heroin use | 522 (66.0) | 104 (56.8) | 418 (68.8) | <0.01 |
| Prescription opioid use | 265 (33.5) | 78 (42.6) | 187 (30.8) | <0.01 |
| Opioid route | ||||
| Intravenous (IV) opioid use | 498 (62.9) | 98 (53.6) | 400 (65.8) | <0.01 |
| Substance Use Treatment History | ||||
| History of opioid maintenance treatment prior to pregnancy | 470 (59.4) | 144 (78.7) | 326 (53.6) | <0.01 |
| History of inpatient rehabilitation | 181 (22.9) | 29 (15.9) | 152 (25.0) | 0.01 |
| Psychiatric Risk Factors | ||||
| Maternal psychiatric disorderc | 576 (72.8) | 119 (65.0) | 457 (75.2) | <0.01 |
| History of suicide attempt/self-harm | 71 (9.1) | 11 (6.1) | 60 (10.0) | 0.11 |
| History of inpatient admission for a psychiatric disorder | 84 (10.6) | 16 (8.7) | 68 (11.2) | 0.35 |
| Social Risk Factors | ||||
| Maternal | ||||
| Prostitution | 42 (5.3) | 6 (3.3) | 36 (5.9) | 0.16 |
| Legal problemsd | 215 (27.2) | 27 (14.8) | 188 (30.9) | <0.01 |
| Homelessness | 32 (4.1) | 2 (1.1) | 30 (5.0) | 0.02 |
| Abusee | 172 (21.7) | 34 (18.6) | 138 (22.7) | 0.24 |
| Children not in maternal custody | 221 (28.1) | 35 (19.1) | 186 (30.7) | <0.01 |
| Paternal/partner | ||||
| Partner with substance use history | 189 (23.9) | 19 (10.4) | 170 (28.0) | <0.01 |
| Paternal support | 636 (80.4) | 154 (84.2) | 482 (79.3) | 0.15 |
| Partner legal problemsd | 86 (10.9) | 15 (8.2) | 71 (11.7) | 0.19 |
| Family | ||||
| Substance use in family | 106 (13.4) | 9 (4.9) | 97 (16.0) | <0.01 |
n (%) unless otherwise indicated; less than 1.0% missing data for all variables except education with 26.0% missing data
≥ one positive urine drug screen for these substances at any time during pregnancy and/or a self-reported history of use
Diagnosis by a physician at any time either before or during pregnancy
History of or current probation, arrest and/or incarceration
History of or current intimate partner violence and/or sexual abuse
Figure 1.
Buprenorphine vs. methadone utilization in pregnancy.
Patient characteristics associated with buprenorphine versus methadone use in pregnancy: Bivariate analysis
Maternal demographic data, substance use history and maternal psychosocial risk factors for pregnant patients who used buprenorphine versus methadone during pregnancy were compared (Table 1). Pregnant women who used buprenorphine were significantly more likely to be older (28.4% vs. 27.0%; p<0.01), married (18.0% vs. 11.0%; p=0.01), employed (25.7% vs. 14.3%; p<0.01), have some college or an associate's degree (37.8% vs. 26.2%; p=0.03), be cared for by private practice prenatal care providers (55.2% vs. 30.4%; p<0.01) and have a history of prescription opioid use (42.6% vs. 30.8%; p<0.01) compared to pregnant women who used methadone. In contrast, pregnant women who used methadone were significantly more likely to have HCV (49.2% vs. 38.3%; p<0.01), use cocaine (24.0% vs. 10.4%; p<0.01), benzodiazepines (30.0% vs. 15.5%; p<0.01), marijuana (27.2% vs. 17.5%; p<0.01) and have a history of heroin (68.8% vs. 56.8%; p<0.01) and intravenous opioid use (65.8% vs. 53.6%; p<0.01). Pregnant women using methadone were also significantly more likely to have psychosocial risk factors such as a psychiatric disorder (75.2% vs. 65.0%; p<0.01), legal problems (30.9% vs. 14.8%; p<0.01), homelessness (5.0% vs. 1.1%; p=o.02), children not in their custody (30.7% vs. 19.1%; p<0.01), a partner with a substance use history (28.0% vs. 10.4%; p<0.01) and a family history of substance abuse (16.0% vs.4.9%; p<0.01) than pregnant women who used buprenorphine.
Multivariable analysis
Univariable and multivariable logistic regression models were developed to identify patient characteristics predictive of buprenorphine use in pregnancy (Table 2). After evaluating for multicollinearity, “IV” and “heroin” were found to be collinear (VIF > 3.5) and only “heroin” was used in multivariable analyses. The variables “race” (p=0.22), “parity” (p=0.56), “prenatal care provider” (p=0.23), “barbiturates” (p=0.29), “alcohol abuse” (p=0.21), “suicide attempt” (p=0.11), “inpatient admission for a psychiatric disorder” (p=0.35), “hepatitis B” (p=0.49), “prostitution” (p=0.17), “abuse” (p=0.24), “paternal support” (p=0.15) and “partner legal problems” (p=0.19) were also excluded from multivariate analyses due to p-values less than 0.10 in univariable analyses. Finally, the variable “education” was excluded from multivariable analyses due to 26% missing data.
Table 2. Patient characteristics predictive of buprenorphine use in pregnancy, n=791a.
| Unadjusted OR (95% CI) |
Adjusted ORb (95% CI) |
|
|---|---|---|
| Demographics | ||
| Age [years; mean (±SD)] | 1.06 (1.03, 1.10) | 1.01 (1.02, 1.11) |
| Race | ||
| Caucasian | ref | -- |
| Other | 0.46 (0.14, 1.57) | -- |
| Married | 1.78 (1.13, 2.80) | -- |
| Employed | 2.07 (1.38, 3.09) | 1.87 (1.20, 2.90) |
| Education | -- | |
| ≤HS/GED | ref | -- |
| Some college/Associates degree | 1.78 (1.15, 2.74) | -- |
| ≥Bachelor's | 1.48 (0.64, 3.42) | -- |
| Medicaid | 0.41 (0.20, 0.83) | -- |
| Primaparous | 1.11 (0.79, 1.57) | -- |
| Prenatal care provider | ||
| Academic Teaching Service | ref | ref |
| Private Practice | 1.15 (0.91, 1.45) | -- |
| Medical Co-morbidities | ||
| Hepatitis C | 0.64 (0.46, 0.90) | -- |
| HIV | -- | -- |
| Hepatitis B | 0.47 (0.06, 3.86) | -- |
| Substance Use Historyc | ||
| Tobacco | 0.70 (0.46, 1.07) | -- |
| Alcohol abuse | 1.48 (0.80, 2.72) | -- |
| Amphetamine/methamphetamines | 2.84 (0.86, 9.43) | -- |
| Barbiturates | 1.94 (0.56, 6.71) | -- |
| Cocaine | 0.37 (0.22, 0.61) | 0.59 (0.34, 1.04) |
| Benzodiazepines | 0.43 (0.28, 0.66) | 0.48 (0.30, 0.77) |
| Marijuana | 0.57 (0.37, 0.87) | -- |
| Opioid Type | ||
| Heroin use | 0.60 (0.43, 0.84) | -- |
| Prescription opioid use | 1.67 (1.19, 2.35) | -- |
| Opioid Route | ||
| Intravenous (IV) opioid use | 0.60 (0.43, 0.84) | -- |
| Substance Use Treatment History | ||
| Pre-pregnancy opioid maintenance treatment | 3.19 (2.17, 4.71) | 2.68 (1.78, 4.02) |
| History of inpatient rehabilitation | 0.56 (0.36, 0.87) | -- |
| Psychiatric Risk Factors | ||
| Maternal psychiatric disorderd | 0.61 (0.43, 0.88) | -- |
| History of suicide attempt | 0.58 (0.30, 1.13) | -- |
| History of inpatient admission for a psychiatric disorder | 0.76 (0.43, 1.35) | -- |
| Social Risk Factors | ||
| Maternal | ||
| Prostitution | 0.54 (0.22, 1.30) | -- |
| Legal problemse | 0.39 (0.25, 0.60) | -- |
| Homelessness | 0.22 (0.05, 0.91) | -- |
| Abusef | 0.78 (0.51, 1.18) | -- |
| Children not in maternal custody | 0.53 (0.35, 0.80) | 0.63 (0.40, 0.99) |
| Paternal/partner | ||
| Partner with substance use history | 0.30 (0.18, 0.50) | 0.37 (0.22, 0.63) |
| Paternal support | 1.39 (0.89, 2.16) | -- |
| Partner legal problemse | 0.68 (0.38, 1.21) | -- |
| Family | ||
| Substance use in family | 0.27 (0.13, 0.55) | -- |
OR=odds ratio; 95% CI=95% confidence interval
Values representative of the final multivariable model
≥ one positive urine drug screen for these substances at any time during pregnancy and/or a self-reported history of use
Diagnosis by a physician at any time either before or during pregnancy
History of or current probation, arrest and/or incarceration
History of or current intimate partner violence and/or sexual abuse
In the final multivariable model, pregnant women who were older (OR 1.01; 95% CI 1.02, 1.11), employed (1.87; 1.20, 2.90), used opioid maintenance treatment prior to pregnancy (2.68; 1.78, 4.02) were more likely to use buprenorphine during pregnancy. Pregnant women with a history of benzodiazepine use (0.48; 0.30, 0.77), who had children not in their custody (0.63; 0.40, 0.99) and who had a partner with a substance use history (0.37; 0.22, 0.63) were less likely to use buprenorphine during pregnancy.
Model performance
Our final model had a BIC=793.49 and an AIC=756.22 and the area under the curve (AUC) was 0.74. A 10-fold cross-validation analysis was performed on the final model to calculate an average misclassification rate of 0.21 and an average AUC of 0.74.
Maternal treatment outcomes
Maternal treatment outcomes were also compared among women who used buprenorphine versus methadone during pregnancy (Table 3). Among 183 pregnant women who used buprenorphine, the median dose of buprenorphine at the time of delivery was 16 mg/day. Among 608 pregnant women who used methadone, the median dose at the time of delivery was 93.5 mg/day. Women who used buprenorphine were more likely to discontinue treatment than women who used methadone (13.1% vs. 1%; p<0.01). In contrast, women who used methadone were more likely to use illicit opioids (24.9% vs. 14.8%; p<0.01) and other illicit drugs (35.4% vs. 23.5%; p<0.01) during pregnancy than women who used buprenorphine.
Table 3. Maternal treatment outcomes following buprenorphine versus methadone use in pregnancy, n=791a.
| Buprenorphine n=183 |
Methadone n=608 |
p-value | |
|---|---|---|---|
| Dose at delivery, median [5%, 95%] | 16 mg [2, 24] | 93.5mg [35, 185] | - |
| Treatment discontinuationb | 24 (13.1) | 1 (0.2) | <0.01 |
| Illicit opioid usec | 27 (14.8) | 151 (24.9) | <0.01 |
| Illicit drug used | 43 (23.5) | 215 (35.4) | <0.01 |
| Amphetamine/methamphetamines | 2 (1.1) | 3 (0.5) | 0.37 |
| Barbiturates | 0 (0) | 1 (0.2) | 0.58 |
| Benzodiazepines | 10 (5.5) | 78 (12.8) | <0.01 |
| Cocaine | 15 (8.2) | 100 (16.5) | <0.01 |
| Marijuana | 25 (13.7) | 123 (20.2) | 0.05 |
n (%) unless otherwise indicated
discontinuation of buprenorphine or methadone maintenance treatment after use in pregnancy
≥ one positive urine drug screen for illicit opioids after initiation of opioid maintenance treatment in pregnancy
Composite illicit drug use variable defined as ≥ one positive urine drug screen for amphetamine/methamphetamines, barbiturates, benzodiazepines, cocaine and/or marijuana after initiation of opioid maintenance treatment in pregnancy
Discussion
This evaluation of opioid maintenance treatment utilization at a tertiary care, academic medical center found an increase in the prevalence of buprenorphine use during pregnancy. However, despite increasing use, we found stark differences in the demographic characteristics, drug use patterns and psychosocial risk factors of pregnant women using buprenorphine compared to methadone. Our findings indicate that women with more education, resource availability and social support are more likely to use buprenorphine during pregnancy. Previous evaluations of buprenorphine waiver programs indicate similar socioeconomic differences among non-pregnant patients. In an analysis of variation in buprenorphine and methadone use patterns by racial, ethnic and income characteristics in New York City, Hansen et al. found that buprenorphine use is concentrated in higher income areas and in areas with a higher percentage of white residents.33
Disparities in use patterns have unique implications during pregnancy and important maternal and neonatal consequences.16-19 In pregnancy, buprenorphine is associated with superior neonatal outcomes compared to methadone including a shorter duration of treatment for NAS, less opioid medication required for NAS treatment and shorter hospitalizations for NAS treatment and observation.20 Less severe NAS has a significant impact on health care costs and potentially the long-term health of NAS infants.1 Moreover, buprenorphine's office-based availability may eliminate treatment barriers for women who often have limited transportation, work or childcare responsibilities.34
Differences in buprenorphine versus methadone use patterns have been attributed to limited buprenorphine availability and accessibility. Many buprenorphine providers often have strict entry program criteria, waiting lists, fail to offer services to low-income patients who lack adequate health insurance coverage and often feel more comfortable prescribing buprenorphine to patients with a history of prescription opiate use versus heroin and/or intravenous opioid use.33 Moreover, busy office-based providers may not have the time or infrastructure to adequately address the complex psychosocial needs of patients including screening for intimate partner violence, sexual abuse and psychiatric co-morbidities.34
Provider concerns related to diversion, treatment dropout and ongoing illicit drug use in patients with a greater addiction severity may also contribute to disparities in buprenorphine use patterns. A systematic review of 31 clinical trials conducted in non-pregnant patients concluded that buprenorphine was less effective for treatment retention.35 Buprenorphine's partial agonist activity may result in greater withdrawal severity and/or precipitate withdrawal during induction.36 Even after induction, buprenorphine may ineffectively alleviate cravings28 and offer less euphoria29 than methadone.26,27 In pregnancy, buprenorphine has also been associated with greater treatment dropout and illicit opioid use compared to methadone.20,37 In our analysis, we found a greater rate of treatment discontinuation in women who used buprenorphine, but a greater rate of illicit opioid and other drug use in women who used methadone during pregnancy.
This study must be interpreted in light of certain limitations. This study represents the utilization patterns found in a large university-affiliated, tertiary care, academic health system and may not be generalizable to community hospital settings. Although consistent with previous demographic descriptions of opioid-using populations, our sample is also predominantly Caucasian, which may limit the generalizability of our findings to minority populations.38,39 Our data was extracted from documentation by clinical providers and laboratory data available in the medical record which may have inadequately captured maternal and paternal characteristics, substance use history and psychosocial risk factors. Specifically, our definition of illicit drug use, defined as at least one positive urinary toxicology screen during pregnancy, may have inaccurately captured and/or overestimated the true rate of illicit drug use during pregnancy. All of the pregnant women in our cohort received their opioid maintenance treatment from either federally licensed methadone treatment facilities or outpatient buprenorphine providers that do not share the same medical record system as providers in our health system. As a result, the medical records from these treatment providers were not available for review and may have contained additional information regarding ongoing illicit drug use and treatment discontinuation. Significant differences in the demographic and patient-level characteristics of women who used buprenorphine versus methadone in pregnancy may also limit the ability to draw definitive conclusions from findings related to treatment outcomes. Finally, observational study designs are characteristically susceptible to selection bias and the possibility of confounding remains despite the statistical measures we used to control for these factors in our analysis.
Methadone and buprenorphine are two clinically appropriate treatments in pregnancy. However, the vast majority of pregnant women use methadone and significant disparities exist among women who use buprenorphine versus methadone in pregnancy. Because the use of buprenorphine during pregnancy has important maternal and neonatal implications, our results indicate the need to improve the availability and accessibility of buprenorphine for many pregnant women. Importantly, the decision to pursue a particular treatment option should always incorporate a particular patient's previous treatment experiences and the clinical judgement of their treatment provider. Previous research evaluating the impact of buprenorphine versus methadone on neonatal outcomes has been limited to NAS and the neonatal period. As a result, future research should be dedicated to evaluating the long-term health and developmental outcomes of children exposed to buprenorphine during pregnancy as well as to the development of clinical guidelines and patient educational materials to guide patient and provider decision-making regarding the most effective treatment for a particular patient during pregnancy.
Acknowledgments
Funding: This study was funded in full by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000146 (Dr. Krans). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
All authors report no conflicts of interest.
This study was conducted at the University of Pittsburgh Medical Center located in Pittsburgh, PA.
Author Contributions: EEK contributed to the research conception, design, analysis, interpretation of the results and writing of the manuscript. DB, GR and ND contributed to the research conception, design and writing of the manuscript. SYP contributed to the analysis, interpretation of the results and writing of the manuscript. SLD contributed to the collection of data and writing of the manuscript.
References
- 1.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA : the journal of the American Medical Association. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 2.Almario CV, Seligman NS, Dysart KC, Berghella V, Baxter JK. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. American Journal of Obstetrics and Gynecology. 2009;201(3):326.e321–326.e326. doi: 10.1016/j.ajog.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 3.Cleary BJ, Donnelly JM, Strawbridge JD, et al. Methadone and perinatal outcomes: a retrospective cohort study. American Journal of Obstetrics and Gynecology. 2011;204(2):139.e131–139.e139. doi: 10.1016/j.ajog.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive diseases. 1975;2(1-2):141–158. [PubMed] [Google Scholar]
- 5.Logan BA, Brown MS, Hayes MJ. Neonatal abstinence syndrome: treatment and pediatric outcomes. Clin Obstet Gynecol. 2013;56(1):186–192. doi: 10.1097/GRF.0b013e31827feea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dryden C, Young D, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG: An International Journal of Obstetrics & Gynaecology. 2009;116(5):665–671. doi: 10.1111/j.1471-0528.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 7.Madden JD, Chappel JN, Zuspan F, Gumpel J, Mejia A, Davis R. Observation and treatment of neonatal narcotic withdrawal. Am J Obstet Gynecol. 1977;127(2):199–201. doi: 10.1016/s0002-9378(16)33250-1. [DOI] [PubMed] [Google Scholar]
- 8.Burns L, Conroy E, Mattick RP. Infant mortality among women on a methadone program during pregnancy. Drug and Alcohol Review. 2010;29(5):551–556. doi: 10.1111/j.1465-3362.2010.00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Bandstra ES, Morrow CE, Mansoor E, Accornero VH. Prenatal Drug Exposure: Infant and Toddler Outcomes. Journal of Addictive Diseases. 2010;29(2):245–258. doi: 10.1080/10550881003684871. [DOI] [PubMed] [Google Scholar]
- 10.Creanga AA, Sabel JC, Ko JY, et al. Maternal Drug Use and Its Effect on Neonates A Population-Based Study in Washington State. Obstetrics and gynecology. 2012;119(5):924–933. doi: 10.1097/AOG.0b013e31824ea276. [DOI] [PubMed] [Google Scholar]
- 11.Dashe JS, Jackson GL, Olscher DA, Zane EH, Wendel GD., Jr Opioid detoxification in pregnancy. Obstetrics and gynecology. 1998;92(5):854–858. doi: 10.1016/s0029-7844(98)00312-3. [DOI] [PubMed] [Google Scholar]
- 12.Stewart RD, Nelson DB, Adhikari EH, et al. The obstetrical and neonatal impact of maternal opioid detoxification in pregnancy. Am J Obstet Gynecol. 2013;209(3):267 e261–265. doi: 10.1016/j.ajog.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Jones H, Finnegan L, Kaltenbach K. Methadone and Buprenorphine for the Management of Opioid Dependence in Pregnancy. Drugs. 2012;72(6):747–757. doi: 10.2165/11632820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Jones HE, Deppen K, Hudak ML, et al. Clinical care for opioid-using pregnant and postpartum women: the role of obstetric providers. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. National Institutes of Health. Consensus Development Conference Statement. Effective medical treatment of opiate addiction. 1997 Nov 17-19; [Google Scholar]
- 16.Metz V, Jagsch R, Ebner N, et al. Impact of treatment approach on maternal and neonatal outcome in pregnant opioid-maintained women. Human Psychopharmacology: Clinical and Experimental. 2011;26(6):412–421. doi: 10.1002/hup.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: Comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug and Alcohol Dependence. 2008;96(1–2):69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix I, Berrebi A, Garipuy D, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur J Clin Pharmacol. 2011;67(10):1053–1059. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- 20.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England journal of medicine. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O'Connor PG. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA internal medicine. 2014;174(12):1947–1954. doi: 10.1001/jamainternmed.2014.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones ES, Moore BA, Sindelar JL, O'Connor PG, Schottenfeld RS, Fiellin DA. Cost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend. 2009;99(1-3):132–140. doi: 10.1016/j.drugalcdep.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiellin DA, Kleber H, Trumble-Hejduk JG, McLellan AT, Kosten TR. Consensus statement on office-based treatment of opioid dependence using buprenorphine. J Subst Abuse Treat. 2004;27(2):153–159. doi: 10.1016/j.jsat.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Barry DT, Irwin KS, Jones ES, et al. Integrating Buprenorphine Treatment into Office-based Practice: a Qualitative Study. J Gen Intern Med. 2009;24(2):218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teruya C, Schwartz RP, Mitchell SG, et al. Patient perspectives on buprenorphine/naloxone: a qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. Journal of psychoactive drugs. 2014;46(5):412–426. doi: 10.1080/02791072.2014.921743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potter JS, Marino EN, Hillhouse MP, et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START) Journal of studies on alcohol and drugs. 2013;74(4):605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. The Cochrane database of systematic reviews. 2008;(2) doi: 10.1002/14651858.CD002207.pub3. CD002207. [DOI] [PubMed] [Google Scholar]
- 29.Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94(9):1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 31.Box GEP, Draper NR. Empirical Model-Building and Response Surfaces. John Wiley & Sons; 1987. [Google Scholar]
- 32.Steyerberg EW. Clinical Prediction Models. Springer; New York, NT: 2009. [Google Scholar]
- 33.Hansen H, Siegel C, Case B, Bertollo D, DiRocco D, Galanter M. Variation in Use of Buprenorphine and Methadone Treatment by Racial, Ethnic, and Income Characteristics of Residential Social Areas in New York City. J Behav Health Serv Res. 2013;40(3):367–377. doi: 10.1007/s11414-013-9341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones ES, Fiellin DA. Women and opioid dependence treatment: office-based versus opioid treatment program-based care? Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2007;28(2):3–8. doi: 10.1300/J465v28n02_02. [DOI] [PubMed] [Google Scholar]
- 35.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. The Cochrane database of systematic reviews. 2014;2 doi: 10.1002/14651858.CD002207.pub4. CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitley SD, Sohler NL, Kunins HV, et al. Factors associated with complicated buprenorphine inductions. J Subst Abuse Treat. 2010;39(1):51–57. doi: 10.1016/j.jsat.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107(Suppl 1):5–27. doi: 10.1111/j.1360-0443.2012.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The Changing Face of Heroin Use in the United States A Retrospective Analysis of the Past 50 Years. Jama Psychiat. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 39.Terplan M, Smith EJ, Glavin SH. Trends in Injection Drug Use Among Pregnant Women Admitted into Drug Treatment: 1994-2006. J Womens Health. 2010;19(3):499–505. doi: 10.1089/jwh.2009.1562. [DOI] [PubMed] [Google Scholar]