Abstract
Background
Attentional biases, particularly difficulty inhibiting attention to negative stimuli, are implicated in risk for major depressive disorder (MDD). The current study examined a neural measure of attentional bias using a continuous index of visuocortical engagement (steady-state visual evoked potentials [SSVEPs]) before and after a negative mood induction in a population at high-risk for MDD recurrence due to a recently remitted MDD (rMDD) episode. Additionally, we examined working memory (WM) capacity as a potential moderator of the link between rMDD and visuocortical responses.
Methods
Our sample consisted of 27 women with rMDD and 28 never-depressed women. To assess attentional inhibition to emotional stimuli, we measured frequency-tagged SSVEPs evoked from spatially superimposed task-relevant stimuli and emotional distractors (facial displays of emotion) oscillating at distinct frequencies. WM capacity was assessed during a visuospatial memory task.
Results
Women with rMDD, relative to never-depressed women, displayed difficulty inhibiting attention to all emotional distractors before a negative mood induction, with the strongest effect for negative distractors (sad faces). Following the mood induction, rMDD women’s attention to emotional distractors remained largely unchanged. Among women with rMDD, lower WM capacity predicted greater difficulty inhibiting attention to negative and neutral distractors.
Conclusions
By exploiting the phenomenon of oscillatory resonance in the visual cortex, we tracked competition in neural responses for spatially superimposed stimuli differing in valence. Results demonstrated that women with rMDD display impaired attentional inhibition of emotional distractors independent of state mood and that this bias is strongest among those with lower WM capacity.
Keywords: Major Depressive Disorder, Risk and Resilience, Steady-State Visual Evoked Potentials, Visuocortical Competition, Working Memory, Attentional Bias
Introduction
The human visual system is exposed to more information than it can process given constraints on bandwidth and representational capacity (1). To enable adaptive behavior in complex environments, individuals display selective attention to information relevant to a certain task while inhibiting attention to task-irrelevant information (2). However, even while engrossed in a primary task (e.g., reading in a subway car), our attention can be readily captured by salient emotional distractors (e.g., the loud argument of a co-passenger) (3). The neural circuitry involved in this involuntary (“bottom-up”) capture of attention consists of several cortico-limbic regions that send projections into sensory systems. Under some circumstances, the extent of bottom-up capture of attention, particularly for negative emotional content, can become excessive, outcompeting “top-down” regulatory inputs from fronto-parietal systems that maintain task-focused attention (2). Chronic biases in attention to distracting emotional information are central to theories of emotional disorders such as major depressive disorder (MDD) (4).
There is considerable evidence that individuals with MDD display attentional biases to depression-relevant information (5). This trait persists following remission as individuals with remitted MDD (rMDD) exhibit selective attention to negative stimuli (6–10), which predicts prospective increases in depressive symptoms (11–13) and MDD recurrence (10). Despite the strengths of previous research in this area, the majority has used behavioral protocols such as the dot-probe task, which uses reaction time (RT) indices to examine static “snapshots” of attentional deployment. Recent research has highlighted problems with the reliability of RT-based measures of attention (14–16), leading to the use of eye-tracking indices with greater reliability and precision (14). Despite the utility of having a continuous index of visual processing, eye-tracking still relies on overt behavior and cannot capture covert shifts of attention (17). Additionally, neither RT nor eye-tracking indices can discriminate the focus of attention when stimuli overlap in space and time, which precludes a direct examination of attention under conditions of maximal competition (18). Depending on the amount of spatial separation between stimuli, the actual competition for neural responses may be considerably weakened or, in some situations, eliminated altogether if the visual receptive fields are non-overlapping (1,19). Therefore, although behavioral measures have provided evidence of attentional biases (5–10) and inhibition deficits in MDD (20), there is scant evidence to specifically link difficulty inhibiting attention from emotionally-salient distractors relative to concurrent task-relevant stimuli in MDD. This is concerning as attentional biases in MDD are thought to be specific to difficulties inhibiting attention to distracting depression-relevant information relative to goal-relevant information (4). To address these limitations, we utilized an electrophysiological measure – steady-state visual evoked potentials (SSVEPs) - that provides a continuous index of the degree to which attention is allocated to irrelevant vs. goal-relevant stimuli.
In brief, SSVEPs are large-scale oscillatory field responses of the visual cortex evoked in response to rhythmic luminance modulation of a visual stimulus at a fixed frequency. The SSVEP has the same temporal frequency as the driving stimulus and its amplitude tracks fluctuations in attention to the driving stimulus (21,22). Of particular interest here, is the possibility of “frequency-tagging” SSVEPs to discriminate neural responses to items that are spatio-temporally superimposed in the visual field by assigning a distinct frequency to each stimulus (23). This is particular advantageous since, as mentioned above, competition effects are maximal in situations that involve overlap of visual receptive fields as happens with spatio-temporal superposition of stimuli (3,24,25).
The primary aim of the current study, therefore, was to use SSVEPs to evaluate how the visual system resolves the trade-off between task-relevant neutral stimuli and emotional distractors in women known to be at high risk for MDD recurrence compared to never-depressed women. This high-risk population (i.e., women who had a fully remitted episode of MDD within the past five years) is at risk for two key reasons: (i) women have twice the rate of MDD compared to men (26–28) and (ii) over 60% of individuals who develop MDD will experience at least one recurrent episode within 5 years of remission (29). Based on previous research using behavioral measures of attention (6–10), we hypothesized that rMDD women, compared to their never-depressed counterparts, would display difficulty inhibiting attention to negative distractors. Given some evidence that attentional biases in individuals with rMDD may remain latent until primed by a negative mood induction (30,31; but see 32), our secondary aim was to determine whether women’s attention to emotional distractors changed from before to after a sad mood induction.
Finally, although there is a clear link between attention and depression, there is also a well-known heterogeneity of cognitive profiles in MDD (33). Therefore, when seeking to identify moderators of attentional bias, it is helpful to focus on neuropsychological factors known to affect the visual system. With regard to attentional inhibition, visuospatial working memory (WM) may be important because of its role in an individual’s ability to filter relevant from irrelevant visual information. Research has demonstrated that WM capacity predicts activity in the visual cortex when suppressing distracting information and that individuals with lower WM capacity have difficulty inhibiting attention to task-irrelevant information (34), an effect exacerbated by dysphoria (35). Additionally, there is a well-established link between MDD and WM deficits (36), and evidence that WM training can improve dysphoric individuals’ ability to filter irrelevant information from visual attention (37). Notably, WM deficits in MDD are not typically resolved with intervention or remission (38), so the identification of cognitive biases linked to WM deficits may provide novel avenues for intervention. In the current study, we predicted that rMDD women with lower WM capacity would have the greatest difficulty inhibiting attention to task-irrelevant negative distractors.
Methods and Materials
Participants
Participants were 55 women recruited from the community. Twenty-seven women had a history of MDD currently in full remission (rMDD) and 28 women had no lifetime history of any depressive disorder. Women in the never-depressed group had no history of any Axis I DSM-IV disorder (39). rMDD women could not have any current Axis I diagnosis, history of alcohol or substance dependence or abuse within the last six months, or a lifetime history of schizophrenia or bipolar disorder, and their MDD must have fully remitted within the last five years. Women with a lifetime history of seizures were excluded from the study given concerns that the luminance modulation used during the change-detection task could induce a seizure in these women. Similar to previous research (32), we excluded participants who experienced no change or decreases in sadness from before to after the negative mood induction (n = 4) to ensure that the mood induction was successful. The average age of women in the sample was 31.80 years (SD = 6.23), 76% were Caucasian, and the median annual family income was $45,001 to $50,000.
Measures
The Structured Clinical Interview for DSM-IV Disorders (40) was used to assess for lifetime histories of psychiatric disorders. Women’s current depression symptoms in the past two weeks were assessed using the Beck Depression Inventory-II (BDI-II) (41).
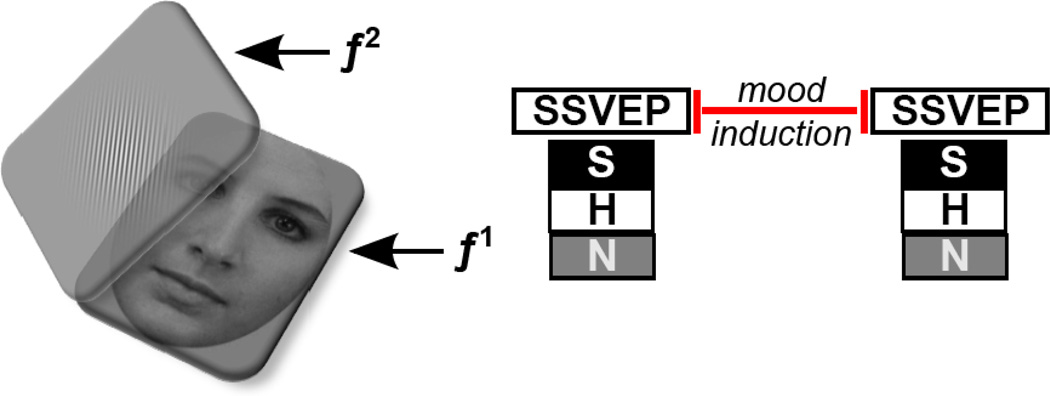
Women’s attentional inhibition to emotional distractors relative to task-relevant stimuli was assessed both before and after the negative mood induction using a simple change-detection paradigm (adapted from 25; see Figure 1). Participants sat 65 cm away from a 27” ASUS LCD monitor with a refresh rate of 60 Hz. On each trial, a facial stimulus was presented at the center of the screen for 5000 ms. Faces were flickered on/off (40% active duty cycle) at a frequency of 12 Hz (f1) to evoke SSVEPs frequency-tagged to the face. A semi-transparent 90° oriented Gabor patch was superimposed over the face, flickering on/off (50% active duty cycle) at 15 Hz (f2) to evoke SSVEPs frequency-tagged to the Gabor. Trials ended with a variable (2000 – 4000 ms) intertrial interval. On a random 25% of trials, the Gabor orientation was rotated clockwise 25° during the trial, and participants indicated if the Gabor shifted orientation by clicking the mouse button (task instructions are presented in the Supplement). Stimuli for the task were randomly selected without replacement for each trial and consisted of 90 pictures of 30 actors (15 males and 15 females) selected from the Karolinska Directed Emotional Faces stimulus set (KDEF) (42). Each actor was shown in a happy, neutral, or sad expression gazing directly at the camera. Pictures were converted to grayscale, trimmed of all non-facial features (i.e. hair and neck), cropped into an oval, and displayed against a gray background. The compound Gabor/face stimuli subtended of 10.5° visual angle vertically and 10.9° horizontally.
Figure 1.
A schematic layout of the change detection task, employing the frequency-tagging technique. Faces (distractors) were luminance modulated (i.e., on/off flickered) at a constant rate of 12 Hz (f1). The Gabor patch was fully spatially super-imposed over the face as a second, translucent layer, flickering at a distinct rate of 15 Hz (f2); individuals were tasked with monitoring the Gabor patch layer for changes in orientation. Subjectively, participants perceived a fused percept of face plus Gabor, however, the frequency-tagging approach allowed us to analyze visuocortical responses separately. The distractor faces contained sad (S), happy (H), or neutral (N) expressions on any given trial. The same SSVEP task was administered twice: once prior to and once following a negative mood induction procedure.
Continuous EEG was recorded during both change-detection tasks using a BioSemi ActiveTwo system (Biosemi B.V., Amsterdam, Netherlands), and recordings were taken from 34 scalp electrodes based on the 10/20 system (see Supplement for additional information). Off-line EEG analysis was performed using EEGLAB software and in-house scripts in MATLAB (Mathworks, Inc., Natick, MA) (43). Data were band-pass filtered with cutoffs of 0.01 Hz and 40 Hz and re-referenced to the average of the left and right mastoid electrodes. Large and stereotypical ocular components were identified and removed using independent component analysis (ICA) scalp maps (44). Epochs were then extracted from raw EEG and included 1000 ms pre-stimulus onset and 5000 ms post-stimulus onset. Epochs with large artifacts (greater than 200 µV) were excluded from analysis. Participants’ trial rejection did not exceed 30%. The artifact-free EEG trials were then averaged in the time domain, separately for the various viewing conditions and groups and subjected to linear detrending (removing the straight-line fit) prior to calculation of frequency spectra. Finally, the separate time domain averages were transformed into the frequency domain using a fast Fourier transform (FFT) at the Oz electrode site for segments extracted from 1000 ms to 5000 ms post-stimulus onset, resulting in a frequency resolution of 0.25 Hz. This epoch was chosen to exclude the initial non-stationary components of steady-state entrainment from calculation of the power spectrum. Mean SSVEP amplitudes were extracted as the magnitude of complex Fourier coefficients, normalized by the number of sample points submitted for the FFT analysis. The split-half reliabilities of the SSVEP indices to the Gabor and faces were excellent (Guttman split-half coefficients ranged from .92 to .99; see Supplemental Table S1).
To directly examine neural competition between the overlapping stimuli, a standardized SSVEP competition score was created to reflect the relative visual cortical activity evoked by each facial expression relative to the concurrent Gabor. Consistent with previous research (25), SSVEP amplitudes were T-transformed for the entire sample across the two time and three emotion conditions by first Z-transforming individual SSVEP amplitudes in response to faces and the Gabor separately and then multiplying values by 10 and adding a constant of 50. Competition scores were calculated by dividing T-transformed SSVEP amplitudes in response to the face by the sum of the T-transformed amplitudes to both the face and the concurrent Gabor: TSSVEPface/(TSSVEPface + TSSVEPGabor). Scores above .50 reflect greater attention to the emotional face relative to the Gabor, whereas scores below .50 indicate greater attention to the Gabor relative to the emotional face.1
During the visuospatial WM task (adapted from 45), a series of blue circles was presented at different locations on a computer screen, one at a time, and participants were asked to remember their locations. The different locations were selected randomly from a 16 × 16 grid. Each circle was displayed for 500 ms with no delay between circle presentations. Trials included an array of either four or six circles and array size was selected randomly for each trial. The memory probe was a blue circle presented at one location on the grid, and participants were asked to indicate whether the probe matched one of the locations of the array. Individual estimates of visuospatial WM capacity were calculated using Cowan’s K estimate (37, 38) separately for the four- and six-item arrays.
Procedure
Potential participants were screened over the telephone to determine eligibility. Upon arrival in the laboratory, participants completed informed consent and were administered questionnaires and the change-detection and visuospatial WM tasks. Next, they completed a standardized negative mood induction by watching a 3-minute scene from The Champ (48; see Supplement for additional information). Then, the change-detection task was re-administered. Finally, diagnoses were confirmed using diagnostic interviews. Participants were compensated $50 for their time. All study procedures were approved by the University’s Institutional Review Board.
Results
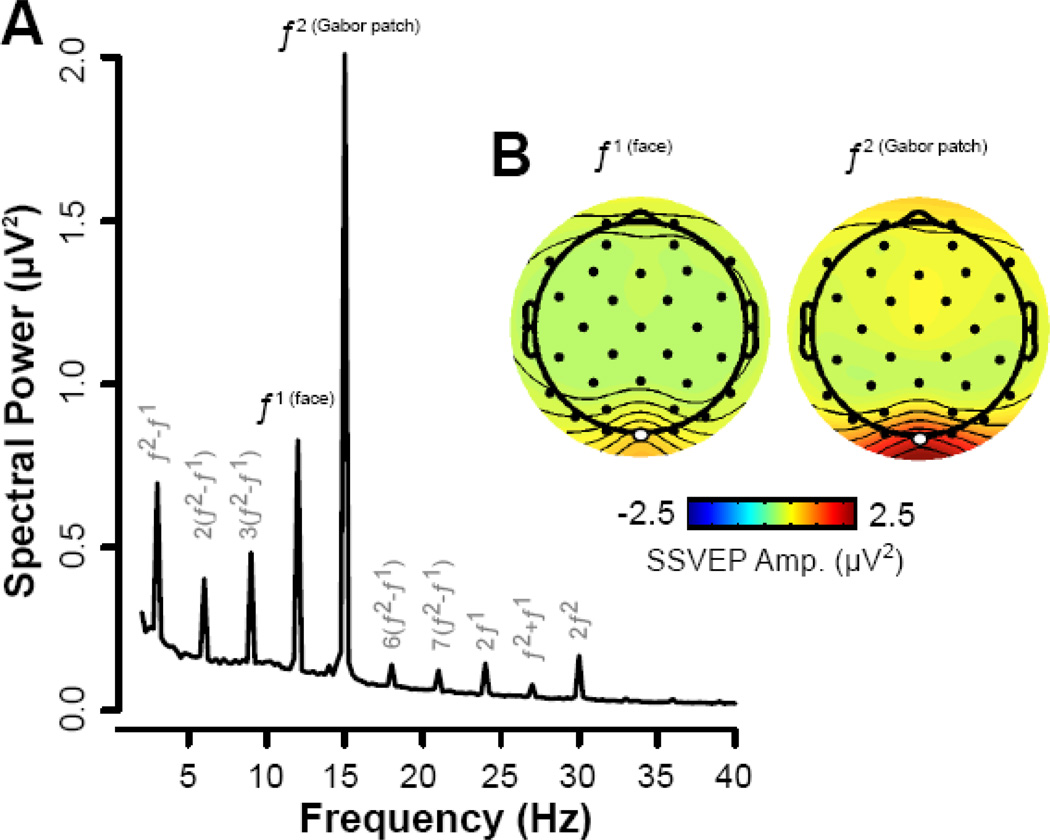
Demographic and clinical characteristics of the sample are provided in Supplemental Tables S3 and S4. SSVEP frequency power spectrum and corresponding scalp topographies are illustrated in Figure 2.
Figure 2.
The average power spectrum from the Oz electrode is depicted in (A), collapsing across all emotional expressions and pre- and post- assessments. Note that in addition to the discrete peaks matching the input frequency of the face (f1: 12 Hz) and the Gabor patch (f2: 15 Hz) there are also various intermodulation frequencies and higher harmonics. Both subtractive (f2 − f1) and additive (f1 + f2) intermodulation frequencies are evoked. The spatial topography corresponding to the frequency of the face and Gabor patch, collapsing across experimental groups, is depicted in (B) with electrode Oz highlighted in white. Note that SSVEP amplitudes were overall considerably stronger for the task-relevant Gabor patch compared to the face distractors. Note also that this figure provides an average of the spatial topography to both the face and Gabor patch separately (collapsed across all emotion conditions and time points). Information regarding changes in the power spectrum across emotion, time, and group conditions can be found in the Results and the Supplement.
Do rMDD women exhibit difficulty inhibiting attention to emotional distractors relative to never-depressed women in the absence of a mood manipulation?
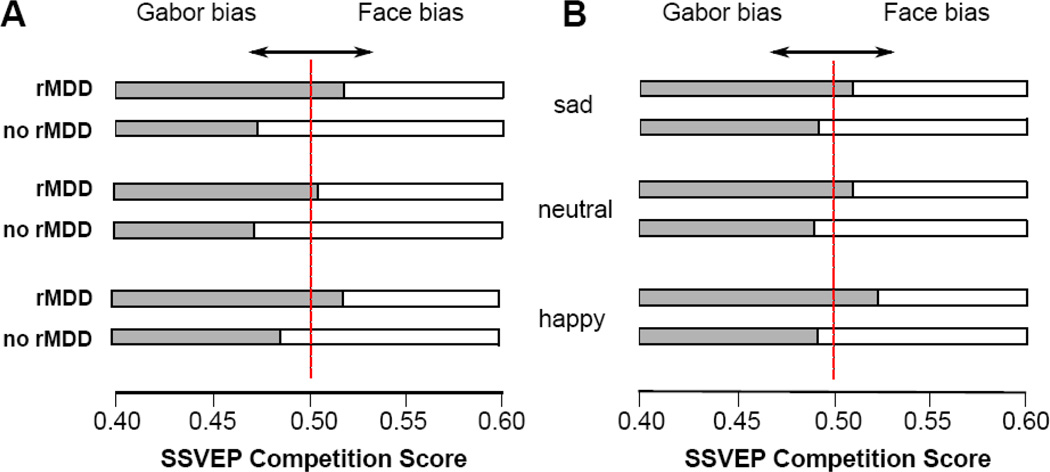
To examine the impact of rMDD history on attentional inhibition to emotional distractors before the negative mood induction, we conducted a 2 (Group: rMDD, no MDD) × 3 (Emotion: sad, happy, neutral) mixed-model ANOVA with SSVEP competition scores serving as the dependent variable (as noted above, scores above .50 reflect greater attention to the emotional distractor relative to the Gabor). Mauchly’s test indicated that the assumption of sphericity had been violated (χ2(2) = 7.11, p = .03) for the emotion condition; therefore, degrees of freedom and p-values for emotion effects and interactions were corrected using Greenhouse-Geisser corrections. Results revealed a Group × Emotion interaction, F(1.77, 53) = 3.55, p = .04, ηp2 = .06, shown in Figure 3a. To determine the form of this interaction, we examined the group difference for each emotion separately. Women with rMDD displayed more difficulty inhibiting attention to sad, F(1, 53) = 16.68, p < .001, ηp2 = .24, happy, F(1, 53) = 7.19, p = .01, ηp2 = .12, and neutral faces, F(1, 53) = 8.79, p = .01, ηp2 = .14, than never-depressed women. Notably, although there were group differences for each emotion, the group difference was stronger for sad faces than for happy (z = 2.53, p = .006) or neutral (z = 2.52, p = .006) faces, with the latter two not differing significantly (z = 0.55, p = .29). To examine the robustness of these group differences, we repeated the analyses statistically controlling for women’s current levels of depressive symptoms, state sadness, and history of anxiety disorders and each of the findings was maintained (all ps < .05).
Figure 3.
SSVEP competition scores as a function of Emotion condition (sad, neutral, happy) and Group (rMDD, no rMDD) prior to the negative mood induction are depicted in (A). SSVEP competition scores following the negative mood induction are depicted in (B). Please note, competition scores above .50 reflect greater selective attention toward the emotional face relative to the Gabor, whereas scores below .50 indicate greater selective attention toward the Gabor relative to the emotional face.
Next, we conducted one-sample t-tests to determine whether either group’s competition scores differed significantly from .50, thereby indicating the presence of a true attentional bias to emotional distractors. For the rMDD group, the SSVEP competition bias scores were significantly greater than .50 for both sad, t(26) = 3.30, p = .003, Cohen’s dz = .65 and happy, t(26)= 3.32, p = .003, Cohen’s dz = .65, faces but not for neutral faces, t(26)= 0.95, p = .35, Cohen’s dz = .19, suggesting that rMDD women display difficulty inhibiting attention to distracting sad and happy faces relative to the Gabor. In contrast, for the never-depressed group, the competition scores were significantly less than .50 for sad, t(27) = −2.85, p = .01, Cohen’s dz = .55, and neutral, t(27) = −2.93, p = .01, Cohen’s dz = .56, faces but not for happy faces, t(27)= −1.25, p = .22, Cohen’s dz = .24, indicating that never-depressed women were allocating attention to the task-relevant information (i.e., the Gabor) relative to distracting sad and neutral faces.
Does attentional inhibition to emotional distractors change from before to after a negative mood induction and is this moderated by MDD history?
To examine changes in attentional inhibition to emotional distractors from before to after the negative mood induction, we conducted a 2 (Group) × 2 (Time: pre, post) × 3 (Emotion) mixed-model ANOVA with SSVEP competition scores serving as the dependent variable, shown in Figure 3. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(2) = 22.59, p < .001, for the emotion condition; therefore, degrees of freedom and p-values for emotion effects and interactions were corrected using Greenhouse-Geisser corrections.
Importantly, there was a significant Group × Time × Emotion interaction, F(1.93, 53) = 6.78, p = .002, ηp2 = .11. To examine the form of this interaction, we examined the Group × Time interaction separately for each of the three Emotion conditions (see Table 1). For sad faces, although the main effect of Time was nonsignificant, there was a significant Group × Time interaction. Examining the two groups separately, there was an effect of Time for never-depressed women, F(1, 27) = 11.41, p = .002, ηp2 = .30, where difficulty inhibiting attention to sad faces increased from before (M = .47, SD = .05) to after (M = .49, SD = .04) the negative mood induction. In contrast, for rMDD women, the main effect of Time was not significant for sad faces, F(1, 26) = 3.43, p = .08, ηp2 = .12. For happy faces, the main effect of Time and the Group × Time interaction were both nonsignificant. For neutral faces, there was a significant main effect of Time indicating that attention to neutral faces increased for all women from before (M = .48, SD = .04) to after (M = .51, SD = .04) the negative mood induction. In contrast, the Group × Time interaction was nonsignificant.2
Table 1.
Mixed-model ANOVA results examining group differences in changes in attentional inhibition to emotional distractors from before to after the negative mood induction for each emotion condition separately
| Group F (ηp2) |
Time F (ηp2) |
Group × Time F (ηp2) |
|
|---|---|---|---|
| Sad | 9.53 (.15)** | 2.17 (.04) | 14.27 (.21)*** |
| Happy | 7.78 (.13)* | 2.15 (.04) | 0.07 (< .001) |
| Neutral | 7.01 (.12)* | 8.48 (.14)** | 2.83 (.05) |
p ≤ .05,
p ≤ .01,
p ≤ .001
Does WM capacity moderate the link between MDD history and attentional inhibition to emotional distractors?
To determine whether WM capacity moderated the above analyses, we conducted two separate 2 (Group) × 2 (Time) × 3 (Emotion) general linear models with the 4- and 6-item K scores added as a continuous predictor variable, respectively. The 4-item K score did not moderate any of the previous analyses (lowest p = .22). However, for the 6-item K score, there was a three-way Group × Memory × Emotion interaction, F(1.51,51) = 4.17, p = .03, ηp2 = .08. To examine the form of this interaction, we examined the Memory × Emotion interaction separately for each group. For rMDD women, there was a significant Memory × Emotion interaction, F(1.49,25) = 4.02, p = .04, ηp2 = .14, such that lower WM capacity during 6-item trials was associated with greater difficulty inhibiting attention to sad, r = −.51, p = .01, and neutral, r = −.58, p = .001, but not happy, r = −.33, p = .10, faces. In contrast, for never-depressed women, the Memory × Emotion interaction was not significant, F(1.54,26) = 0.99, p = .36, ηp2 = .04. Notably, the Group × Memory × Emotion × Time interaction was also nonsignificant, F(1.93, 51) = 0.46, p = .63, ηp2 = .01, suggesting that the above effects were not moderated by the mood induction.
Discussion
The primary aim of this study was to test the hypothesis that women with rMDD versus those with no depression history would exhibit difficulty inhibiting attention to negative emotional distractors relative to a task-relevant neutral stimulus using a direct neural measure of visuocortical competition (SSVEPs). Women with rMDD, compared to never-depressed women, displayed difficulty inhibiting attention to all distracting facial expressions of emotion before a negative mood induction and this effect was strongest for negative distractors (i.e., sad facial expressions). Notably, following the negative mood induction, rMDD women’s attentional inhibition to emotional distractors remained largely unchanged. Finally, results indicated that WM moderated the link between MDD history and attentional inhibition to distractors, such that rMDD women with lower WM capacity displayed even greater difficulty inhibiting attention to negative and neutral distractors.
The current findings demonstrate that individuals with rMDD, compared to those with no lifetime history of depression, display greater preferential processing in the visual cortex for emotional distractors, which suggests that emotional facial expressions confer a bottom-up competitive advantage in rMDD, especially for sad faces. This is consistent with research demonstrating that biased processing of facial expressions is a vulnerability for MDD (49), though a meta-analysis suggested that attentional biases in MDD are not different for facial expressions versus other emotional stimuli (5). The results of the current study are also noteworthy as the majority of past behavioral research has shown an attentional bias only for negative stimuli (6–10). This discrepancy could be due to the reduced reliability of behavioral measures of attention-bias (14–16) and/or the inability of behavioral measures to directly examine competition between spatio-temporally imposed stimuli. Notably, studies examining attentional bias in MDD using other neurophysiological measures have also shown a bias toward emotional stimuli in general (50), though these previous studies also did not test attention toward competing stimuli. Taken together, the current study and others suggest that individuals susceptible to MDD display biased visuocortical processing of emotional stimuli and that this bias may be strongest for negative stimuli and enhanced in the context of competition.
Importantly, the findings also suggest that attentional biases in rMDD are exacerbated by deficits in WM, such that rMDD women with lower WM capacity exhibit the greatest difficulty inhibiting attention to emotional distractors. These findings have important clinical implications. First, they could aid in the identification of subgroups most likely to display attentional biases. Identification of high-risk subgroups may be essential for reliably assessing and manipulating attentional biases in psychiatric disorders as it reduces the noise associated with the heterogeneity of disorders (33). Second, given that WM training improves dysphoric individuals’ ability to filter irrelevant information (37), WM training may be an important component of translational research to ameliorate attentional biases in MDD.
In a similar vein, since an attentional bias for negative stimuli is a risk factor for MDD recurrence (10), there is considerable interest in modifying attentional biases to treat or prevent MDD (51). However, large-scale reviews examining the efficacy of attention bias modifications (ABM) for emotional disorders such as depression and anxiety have provided mixed results (52,53), with researchers suggesting that the unreliability of the measures used to assess the biases may be to blame (54). Since the vast majority of ABM programs utilize behavioral measures of attentional bias, future studies of ABM may be better served by SSVEP indices, which exhibit excellent reliability (55). Furthermore, behavioral ABM programs seek to “retrain” attention by diverting attention away from negative and toward positive information, assuming MDD is only characterized by a bias toward negative stimuli, whereas SSVEP paradigms would instead provide opportunities to retrain attention more generally by training attention away from “task-irrelevant” emotional distractors and toward more neutral “task-relevant” stimuli. Finally, SSVEPs might be used to implement a brain-based version of attention training incorporating targeted neurofeedback similar to interventions using real-time fMRI neurofeedback to retrain attentional biases in depression (56). Importantly, SSVEPs could improve neurofeedback paradigms by providing almost instantaneous brain-based feedback to participants and drastically reducing the cost of neurofeedback implementation for clinicians and researchers.
In conclusion, the current study provides evidence that women with rMDD are more likely than their never-depressed counterparts to exhibit difficulties inhibiting attention to emotional distractors, compared to task-relevant neutral stimuli, both before and after a negative mood induction. By recording frequency-tagged SSVEPs, we were able to evaluate this perceptual competition at the level of neuronal populations in the visual cortex. This approach may be particularly helpful in the examination of attentional biases in MDD and other emotional disorders since it permits the examination of attention to concurrent, competing stimuli involving full overlap of visual receptive fields. Furthermore, our findings and others suggest that attentional biases for emotional stimuli in rMDD are not mood-state dependent and can be detected even in the absence of a sad mood induction (32). However, care must be taken in the interpretation of this finding given research suggesting the limited effectiveness of mood inductions on affective and physiological response (57). Finally, these findings complement and extend the findings of prior studies showing attentional biases in rMDD via behavioral measures (6–10), while also supporting the role of WM in attentional biases in MDD. However, the sample size of the current study was fairly modest and consisted of predominately white women, thus future studies are needed to determine if the findings would replicate and extend to a more representative population. A nuanced understanding of how attentional bias to emotional stimuli plays a role in MDD recurrence and for whom is key for individualized models of risk essential for intervention efforts.
Supplementary Material
Acknowledgments
This research was supported by the American Psychological Foundation/Council of Graduate Departments of Psychology Ruth G. and Joseph Matarazzo Scholarship, Sigma Xi Grant-in-Aid of Research (Grant ID: G20141015665524), Society for a Science of Clinical Psychology Dissertation Grant Award, and Binghamton University Dissertation Fellowship awarded to M. L. Woody and National Institute of Mental Health grant MH098060 awarded to B. E. Gibb. We would like to thank Drs. Gregory Strauss, Christopher Sears, and Katie Burkhouse for their feedback on the project and Allegra Anderson, Samantha Birk, Samantha Fradkin, Jigar Gosalia, and Emma Lecarie for their help in conducting assessments for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Results based on raw SSVEP amplitudes can be found in the Supplement.
Of note, the exclusion of participants who experienced no change or decreases in sadness from before to after the negative mood induction did not impact the analyses examining changes in attentional inhibition to emotional faces; the significant three-way Group × Time × Emotion interaction from the 2 (Group) × 2 (Time) × 3 (Emotion) mixed-model ANOVA was maintained when including the excluded participants (p = .002). In addition, all subsequent significant follow-up analyses from this interaction were maintained when these participants were included (all ps < .05).
References
- 1.Franconeri SL, Alvarez GA, Cavanagh P. Flexible cognitive resources: Competitive content maps for attention and memory. Trends Cogn Sci. 2013;17:134–141. doi: 10.1016/j.tics.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore T. The neurobiology of visual attention: Finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Müller MM, Andersen SK, Keil A. Time course of competition for visual processing resources between emotional pictures and foreground task. Cereb Cortex. 2008;18:1892–1899. doi: 10.1093/cercor/bhm215. [DOI] [PubMed] [Google Scholar]
- 4.Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. Am J Med Genet Part B Neuropsychiatr Genet. 2016;171:65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 6.Fritzsche A, Dahme B, Gotlib IH, et al. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol Med. 2012;29:997–1003. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Sears CR, Newman KR, Ference JD, Thomas CL. Attention to emotional images in previously depressed individuals: An eye-tracking study. Cognit Ther Res. 2011;35:517–528. [Google Scholar]
- 9.Soltani S, Newman K, Quigley L, Fernandez A, Dobson K, Sears C. Temporal changes in attention to sad and happy faces distinguish currently and remitted depressed individuals from never depressed individuals. Psychiatry Res. 2015;230:454–463. doi: 10.1016/j.psychres.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Woody ML, Owens M, Burkhouse KL, Gibb BE. Selective Attention Toward Angry Faces and Risk for Major Depressive Disorder in Women: Converging Evidence From Retrospective and Prospective Analyses. Clin Psychol Sci. 2016;4:206–215. doi: 10.1177/2167702615581580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognit Ther Res. 2003;27:619–637. [Google Scholar]
- 12.Beevers CG, Lee HG, Wells TT, Ellis AJ, Telch MJ. Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. Am J Psychiatry. 2011;168:735–741. doi: 10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- 13.Disner SG, Shumake JD, Beevers CG. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cogn Emot. 2016 doi: 10.1080/02699931.2016.1146123. [DOI] [PubMed] [Google Scholar]
- 14.Price RB, Kuckertz JM, Siegle GJ, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychol Assess. 2015;27:365–376. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmukle SC. Unreliability of the dot probe task. Eur J Pers. 2005;19:595–605. [Google Scholar]
- 16.Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychol Sci Q. 2009;51:339–350. [Google Scholar]
- 17.Kappenman ES, MacNamara A, Proudfit GH. Electrocorticol evidence for rapid allocation of attention to threat in the dot-probe task. Soc Cogn Affect Neurosci. 2015;4:577–583. doi: 10.1093/scan/nsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 19.Hindi Attar C, Andersen SK, Müller MM. Time course of affective bias in visual attention: Convergent evidence from steady-state visual evoked potentials and behavioral data. Neuroimage. 2010;53:1326–1333. doi: 10.1016/j.neuroimage.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 20.Joormann J, Arditte K. Cognitive aspects of depression. In: Gotlib IH, Hammen C, editors. Handbook of Depression. 3rd. New York, NY: Guilford Press; 2014. pp. 298–321. [Google Scholar]
- 21.Muller MM, Teder-Salejarvi WA, Hillyard S. The time course of cortical facilitatin during cued shifts of spatial attention. Nat Neurosci. 1998;1:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- 22.Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. 1989 [Google Scholar]
- 23.Wieser MJ, Miskovic V, Keil A. Steady-state visual evoked potentials as a research tool in social affective neuroscience. Psychophysiology. 2016 doi: 10.1111/psyp.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTeague LM, Shumen JR, Wieser MJ, Lang PJ, Keil A. Social vision: Sustained perceptual enhancement of affective facial cues in social anxiety. Neuroimage. 2011;54:1615–1624. doi: 10.1016/j.neuroimage.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieser MJ, McTeague LM, Keil A. Competition effects of threatening faces in social anxiety. Emotion. 2012;12:1050–1060. doi: 10.1037/a0027069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspect Psychol Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, McGonagle Ka, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC, Berglund P, Demler O, et al. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 29.Kessing LV, Andersen PK. Predictive effects of previous episodes on the risk of recurrence in depressive and bipolar disorders. Curr Psychiatry Rep. 2005;7:413–420. doi: 10.1007/s11920-005-0061-0. [DOI] [PubMed] [Google Scholar]
- 30.McCabe SB, Gotlib IH, Martin RA. Cognitive Vulnerability for depression: Deployment of attention as a function of history of depression and current mood state. Cognit Ther Res. 2000;24:427–444. [Google Scholar]
- 31.Vrijsen JN, Van Oostrom I, Isaac L, Becker ES, Speckens A. Coherence between attentional and memory biases in sad and formerly depressed individuals. Cognit Ther Res. 2014;38:334–342. [Google Scholar]
- 32.Newman KR, Sears CR. Eye Gaze Tracking Reveals Different Effects of a Sad Mood Induction on the Attention of Previously Depressed and Never Depressed Women. Cognit Ther Res. 2015;39:292–306. [Google Scholar]
- 33.Rose DT, Abramson LY, Hodulik CJ, Halberstadt L, Leff G. Heterogeneity of cognitive style among depressed inpatients. J Abnorm Psychol. 1994;103:419–429. doi: 10.1037//0021-843x.103.3.419. [DOI] [PubMed] [Google Scholar]
- 34.Gaspar JM, Christie GJ, Prime DJ, Jolicœur P, McDonald JJ. Inability to suppress salient distractors predicts low visual working memory capacity. Proc Natl Acad Sci. 2016;113:3696–3698. doi: 10.1073/pnas.1523471113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owens M, Koster EHW, Derakshan N. Impaired filtering of irrelevant information in dysphoria: An ERP study. Soc Cogn Affect Neurosci. 2012;7:752–763. doi: 10.1093/scan/nsr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder H. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owens M, Koster EHW, Derakshan N. Improving attention control in dysphoria through cognitive training: Transfer effects on working memory capacity and filtering efficiency. Psychophysiology. 2013;50:297–307. doi: 10.1111/psyp.12010. [DOI] [PubMed] [Google Scholar]
- 38.Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust N Z J Psychiatry. 2009;43:1105–1117. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- 39.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C.: 2000. [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for Axis I DSM-IV disorders. Patient Ed. 1994 [Google Scholar]
- 41.Beck A, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX, 78204-2498: 1996. [Google Scholar]
- 42.Lundqvist D, Anders F, Ohman A. The Karolinska Directed Emotional Faces (KDEF) [Accessed July 8, 2016];CD ROM from Dep Clin Neurosci Psychol Sect Karolinska Institutet. 1998 :91–630. http://www.emotionlab.se/ [Google Scholar]
- 43.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Jung T-P, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Analysis and visualization of single-trial event-related potentials. Hum Brain Mapp. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Aidroos N, Emrich SM, Ferber S, Pratt J. Visual working memory supports the inhibition of previously processed information: Evidence from preview search. J Exp Psychol Hum Percept Perform. 2012;38:643–663. doi: 10.1037/a0025707. [DOI] [PubMed] [Google Scholar]
- 46.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 47.Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- 48.Zeffirelli F. The Champ. USA: MGM; 1979. [Google Scholar]
- 49.Bistricky SL, Ingram RE, Atchley RA. Facial affect processing and depression susceptibility: Cognitive biases and cognitive neuroscience. Psychol Bull. 2011;137:998–1028. doi: 10.1037/a0025348. [DOI] [PubMed] [Google Scholar]
- 50.Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN. Depression and Event-related Potentials: Emotional disengagement and reward insensitivity. Curr Opin Psychol. 2015;4:110–113. doi: 10.1016/j.copsyc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Browning M, Holmes Ea, Charles M, Cowen PJ, Harmer CJ. Using attentional bias modification as a cognitive vaccine against depression. Biol Psychiatry. 2012;72:572–579. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke PJF, Notebaert L, MacLeod C. Absence of evidence or evidence of absence: Reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry. 2014;14 doi: 10.1186/1471-244X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mogoase C, David D, Koster EHW. Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. J Clin Psychol. 2014;70:1133–1157. doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- 54.Rodebaugh TL, Scullin RB, Langer JK, et al. Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. J Abnorm Psychol. 2016 doi: 10.1037/abn0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keil A, Smith JC, Wangelin BC, Sabatinelli D, Bradley MM, Lang PJ. Electrocortical and electrodermal responses covary as a function of emotional arousal: A single-trial analysis. Psychophysiology. 2008;45:516–523. doi: 10.1111/j.1469-8986.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 56.Schnyer DM, Beevers CG, DeBettencourt MT, et al. Neurocognitive therapeutics: From concept to application in the treatment of negative attention bias. Biol Mood Anxiety Disord. 2015 doi: 10.1186/s13587-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuijsters A, Redi J, de Ruyter B, Heynderickx I. Inducing sadness and anxiousness through visual media: Measurement techniques and persistence. Front Psychol. 2016;7 doi: 10.3389/fpsyg.2016.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.