Abstract
Background
Transcatheter aortic valve replacement (TAVR) represents a disruptive technology that is rapidly expanding in use. We evaluated the impact on surgical aortic valve replacement (SAVR) patient selection, outcomes, volume and cost.
Methods
A total of 11,565 patients who underwent SAVR with or without coronary artery bypass grafting (2002–2015) were evaluated from the Virginia Cardiac Services Quality Initiative database. Patients were stratified by surgical era: pre-TAVR era (2002–2008, n=5,113), early-TAVR era (2009–2011, n=2,709), and commercial-TAVR era (2012–2015, n=3,743). Patient characteristics, outcomes and resource utilization were analyzed by univariate analyses.
Results
Throughout the study period, statewide SAVR volumes increased with median volumes of pre-TAVR: 722 cases/year, early-TAVR: 892 cases/year, and commercial-TAVR: 940 cases/year (p = 0.005). Implementation of TAVR was associated with declining STS predicted risk of mortality among SAVR patients (3.7%, 2.6%, 2.4%; p<0.0001), despite increasing rates of comorbid disease. Mortality was lowest in the current commercial-TAVR era (3.9%, 4.3%, 3.2%; p=0.05) while major morbidity decreased throughout the time period (21.2%, 20.5%, 15.2%; p<0.0001). The lowest observed to expected ratios for both occurred in the commercial-TAVR era (0.9 and 0.9 respectively). Resource utilization increased generally, including total cost increases from $42,835 to $51,923 to $54,710 (p<0.0001).
Conclusions
At present SAVR volumes have not been affected by the introduction of TAVR. The outcomes for SAVR continue to improve, potentially due to availability of transcatheter options for high-risk patients. Despite rising costs for SAVR, open approaches still provide a significant cost advantage over TAVR.
There are three main forces with tremendous potential to increase the number of patients who are eligible for aortic valve replacement. The aging population in the United States is expected to expand the number of Americans over age 65 from 44.7 million in 2013 to 98 million by 2060 [1]. The prevalence of aortic stenosis in this population ranges from 1.3% to 9.8%, increasing with age [2]. Yet due to perceived risk, 30 to 40% of symptomatic patients are not referred for surgery [3]. Finally, there is increasing evidence regarding the poor outcomes of patients with asymptomatic aortic stenosis managed medically that may benefit from expanded surgical indications for replacement [4].
Transcatheter aortic valve replacement (TAVR) has revolutionized the treatment of aortic stenosis, providing a safe and less invasive alternative, albeit with limited mid- and long-term follow-up. In addition to being the standard of care for inoperable patients with a reasonable life expectancy, TAVR is now approved for both moderate and high-risk patients due to noninferiority compared to surgery [5]. There is conflicting evidence regarding outcomes in low risk patients and multiple industry-sponsored trials are underway to address this population [6–9]. More mature transcatheter markets suggest that future increases in aortic valve replacement volume may be entirely treated by transcatheter procedures, directly competing with surgical aortic valve replacement (SAVR) [10].
Although recent data has suggested that surgical volume continues to increase with the introduction of TAVR, contemporary and large regional data are limited [11, 12]. There continue to be many unknowns regarding TAVR including how adoption will proceed at smaller institutions, future cost changes, and long-term durability. With the rapidly changing landscape, our study aims to identify the impact that commercialization of TAVR has had on SAVR volume, patient risk profiles, and outcomes.
PATIENTS AND METHODS
Patient Data
The Virginia Cardiac Services Quality Initiative (VCSQI) currently includes 18 hospitals and cardiac surgical practices in the Commonwealth of Virginia that captures approximately 99% of adult cardiac surgery cases in the Commonwealth. VCSQI clinical and cost data acquisition and matching has been described previously [13, 14]. Clinical data is collected from each participating institution using Society of Thoracic Surgeons (STS) data entry forms. The VCSQI database pairs STS clinical data with hospital patient discharge information. Clinical variables utilize standard STS definitions including for operative mortality (in-hospital and 30-day) as well as major morbidity (permanent stroke, renal failure, prolonged ventilation, deep sternal wound infection, and reoperation).
Patient records for isolated SAVR and SAVR with coronary artery bypass grafting (CABG; n=11,565) were identified from January 1, 2002 through June 30, 2016, excluding patients with endocarditis. Patients were stratified for analysis by surgical era: pre-TAVR era (2002–2008, n=5,113), early-TAVR era (2009–2011, n=2,709), and commercial-TAVR era (2012–2015, n=3,743). Hospitals were classified as TAVR performing if they performed the procedure in the early-TAVR era. Six hospitals met this criterion, and only since 2015 have other hospitals begun performing TAVR. For cost analyses patients were excluded for missing or zero total cost. Cases in the first six months of 2016 (n=478) were included only in the case volume analysis after extrapolating for the rest of the year. The Institutional Review Board for the University of Virginia granted exemption due to lack of Health Insurance Portability and Accountability Act patient identifiers (IRB#19457).
Cost Data
STS clinical data is matched with Uniform Billing (UB)-04 files, and prior to 2013 was matched with UB-92 files, with a successful matching rate of 99%. These files are used to calculate charges that are classified by International Classification of Diseases, ninth revision (ICD-9)– based revenue codes. Estimated costs are derived from publicly available cost-to-charge ratios submitted to the Center for Medicare and Medicaid Services (CMS) by each institution. The cost and charge data is then categorized by phase of care for analysis (total stay, diagnostics, interventions, general care, other) as demonstrated in Supplemental Table 1. To account for medical specific inflation, cost data was adjusted to 2015 dollars using the market basket for the CMS Inpatient Prospective Payment System.
Statistical Analysis
Baseline characteristics, operative trends, and short-term outcomes were analyzed by univariate analysis. The Chi-Squared test was utilized for categorical variables and the Kruskal-Wallis test for continuous variables. Categorical data was summarized by proportions and continuous data by median and interquartile range due to skewedness, except for cost data, which was presented as mean and standard deviation. Hierarchic logistic regression analyzed operative mortality and major morbidity while adjusting for risk using STS predicted risk of mortality (PROM) and accounting for clustering at the hospital level. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Significance was determined as alpha less than 0.05.
RESULTS
Impact on Surgical Volume
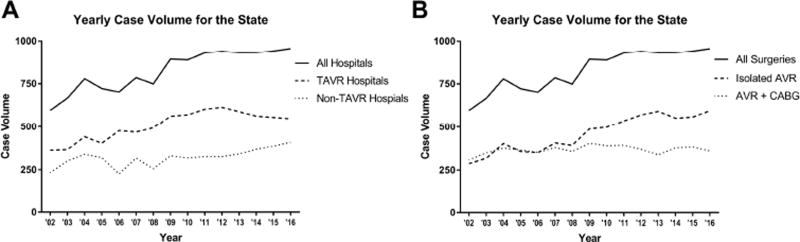
Throughout the study period, SAVR volumes in our statewide database increased with median volumes of 722 cases/year (pre-TAVR), 892 cases/year (early-TAVR) and 940 cases/year (commercial-TAVR; p = 0.003; Figure 1). On a per-hospital basis, the volume increased from a median of 46 cases/year (pre-TAVR) to 52 cases/year (early-TAVR) and stayed at 52 cases/year in the commercial-TAVR era (p=0.005). For all TAVR hospitals combined, median annual volume increased from 440 cases/year pre-TAVR to 570 cases/year (early-TAVR) before a slight decrease to 563 cases/year (commercial-TAVR; p=0.005; Figure 1A), which per hospital equates to 79, 95 and 94 cases/year (p=0.007). Meanwhile, the combined non-TAVR hospital volume increased from 301 cases/year pre-TAVR to 326 cases/year (early-TAVR) and 368 cases/year (commercial-TAVR; p=0.01), which on a per hospital basis is 29, 30 and 31 cases/year (p=0.51). Isolated SAVR volume increased over every era from a median of 357 to 498 to 565 cases per year (p=0.004; Figure 1B). This trend did not hold for combined SAVR and CABG, which demonstrated the highest median volume in the early-TAVR era (357 vs 393 vs 373 cases per year; p=0.03).
Figure 1.
(A) The yearly total volume for the state is plotted over time with two subcategories for TAVR hospitals and non-TAVR hospitals. (B) The yearly volume is plotted over time, with subcategories for isolated SAVR and SAVR with CABG.
Changing Risk profile
Implementation of TAVR was associated with a significant trend of declining STS PROM among SAVR patients (3.7%, 2.6%, 2.4%, p<0.0001). Driving the lower risk profile were declining rates of concomitant CABG, fewer reoperative surgeries, and more elective operations (Table 1). However, trends in patient co-morbid disease increased over surgical eras including more frequent diabetes, chronic lung disease, prior myocardial infarction, and recent heart failure (all p<0.0001; Table 1), among others. Interestingly, the severity of the aortic valve disease appears to have decreased slightly with the median maximum aortic gradient decreasing (48mmHg, 46mmHg, 45mmHg, p<0.0001).
Table 1.
Surgical aortic valve replacement patient characteristics
| TAVR Era | ||||
|---|---|---|---|---|
|
|
||||
| Baseline Characteristics | Pre-TAVR (n = 5,113) |
Early-TAVR (n = 2,709) |
Commercial-TAVR (n = 3,743) |
p value |
| Age (y, median, IQR) | 72 (63–78) | 72 (63–79) | 71 (63–78) | 0.005 |
| BMI (kg/m2, median, IQR) | 27.8 (24.5–32.0) | 28.7 (25.1–32.7) | 29.0 (25.4–33.3) | <0.0001 |
| Female | 1924 (37.6%) | 1012 (37.4%) | 1304 (34.8%) | 0.02 |
| CLD (moderate/severe) | 429 (8.5%) | 304 (11.2%) | 434 (11.8%) | < 0.0001 |
| Prior cerebrovascular accident | 336 (6.7%) | 95 (3.9%) | 288 (7.7%) | < 0.0001 |
| Diabetes | 1534 (30.0%) | 895 (33.0%) | 1372 (36.7%) | < 0.0001 |
| Dialysis dependent renal failure | 103 (2.1%) | 73 (2.7%) | 67 (1.8%) | 0.04 |
| Hypertension | 3739 (73.1%) | 2218 (81.9%) | 3091 (82.6%) | < 0.0001 |
| Peripheral arterial disease | 622 (12.2%) | 324 (12.0%) | 451 (12.1%) | 0.96 |
| Coronary artery disease | 2913 (57.2%) | 1522 (56.2%) | 2032 (55.0%) | 0.11 |
| Prior myocardial infarction | 709 (14.2%) | 482 (17.8%) | 680 (18.2%) | < 0.0001 |
| Heart failure within 2 weeks | 1628 (31.8%) | 1073 (39.6%) | 1664 (44.5%) | < 0.0001 |
| LVEF (median, IQR) | 55 (45–60) | 60 (50–63) | 60 (53–63) | < 0.0001 |
| AI (moderate/severe) | 1388 (27.4%) | 608 (22.5%) | 967 (26.0%) | < 0.0001 |
| MR (moderate/severe) | 300 (6.0%) | 284 (11.3%) | 457 (15.4%) | < 0.0001 |
| Maximum Mean Aortic Valve Gradient (mmHg, median, IQR) | 48 (36–60) | 46 (36–59) | 45 (36–55) | < 0.0001 |
| Prior valve surgery | 202 (4.0%) | 91 (3.4%) | 178 (4.8%) | 0.02 |
| Prior CABG | 503 (9.8%) | 257 (9.5%) | 254 (6.8%) | < 0.0001 |
| Previous Cardiac Intervention | 1285 (25.1%) | 730 (27.0%) | 1054 (28.2%) | 0.005 |
| STS PROM (median, IQR) | 3.7% (2.2–6.4%) | 2.6% (1.4–4.7%) | 2.4% (1.4–4.2%) | <0.0001 |
| STS PROMM (median, IQR) | 18.2% (12.5–27.1%) | 18.7% (12.6–27.1%) | 17.6% (12.5–25.4%) | 0.003 |
| Operative Characteristics | ||||
|
| ||||
| SAVR+CABG | 2555 (50.0%) | 1187 (43.8%) | 1468 (39.2%) | < 0.0001 |
| Reoperative Surgery | 494 (13.1%) | 344 (12.7%) | 395 (10.6%) | 0.002 |
| Annular enlargement | 170 (5.3%) | 109 (4.0%) | 157 (4.2%) | 0.03 |
| Elective | 3778 (74.0%) | 2011 (74.3%) | 2949 (78.8%) | <0.0001 |
| Bioprosthetic valve | 4228 (83.2%) | 2452 (91.0%) | 3462 (94.1%) | < 0.0001 |
| Cross clamp time (min; median, IQR) | 93 (73–121) | 86 (67–114) | 86 (66–112) | < 0.0001 |
| CPB time (min; median, IQR) | 125 (99–160) | 117 (93–151) | 117 (93–150) | < 0.0001 |
IQR = interquartile range; BMI = body mass index; CLD = chronic lung disease; LVEF = left ventricular ejection fraction; AI = aortic insufficiency; MR = mitral regurgitations; CABG = coronary artery bypass grafting; STS = Society of Thoracic Surgeons; PROM = predicted risk of mortality; PROMM = predicted risk of morbidity or mortality; SAVR = surgical aortic valve replacement; CPB = cardiopulmonary bypass
Within isolated SAVR, STS PROM also significantly declined from a median of 2.8% in the Pre-TAVR era to 2.0% and finally 1.8% (p<0.0001). The median PROM was similar between TAVR and non-TAVR hospitals for the pre-TAVR era (3.7% vs 3.8%, p=0.15) and the early-TAVR era (2.6% vs 2.7%, p=0.32), but was lower in TAVR sites in the commercial-TAVR era (2.3% vs 2.5%, p=0.0003).
Operative Trends and Outcomes
As described above patients underwent declining rates of concomitant CABG, reoperative surgery, and urgent or emergent surgery. In addition, the rate of bioprosthetic valve use increased significantly (83%, 91%, 94%, p<0.0001), while the rates of annular enlargement were slightly more common in the pre-TAVR era (5%, 4%, 4%; p=0.03). Similarly, cross clamp times (93min, 86min, 86min; P<0.0001) and cardiopulmonary bypass (CPB) times were longest in the pre-TAVR era (125min, 117min, 117min; p<0.0001).
With increasing utilization of TAVR came largely declining rates of operative mortality (3.9%, 4.3%, 3.2%; p=0.05; Table 2). More strikingly, rates of major morbidity decreased as well (21.2%, 20.5%, 15.2%, p<0.0001). In fact, all components of major morbidity had the lowest rate of complication in the current commercial-TAVR era (Table 2). Rates of transfusion significantly decreased throughout the study period (52%, 47%, 38%, p<0.0001), while rates for reoperation due to bleeding also decreased from the pre-TAVR era (4.4%, 3.3%, 3.4%, p=0.02). The only complication to increase over time was the rate of postoperative atrial fibrillation (24%, 28%, 30%, p<0.0001).
Table 2.
Surgical aortic valve replacement outcomes
| TAVR Era | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | Pre-TAVR (n = 5,113) |
Early-TAVR (n = 2,709) |
Commercial-TAVR (n = 3,743) |
p value |
| Operative mortality | 201 (3.9%) | 115 (4.3%) | 118 (3.2%) | 0.048 |
| Major morbidity† | 1081 (21.2%) | 554 (20.5%) | 570 (15.2%) | <0.0001 |
| Permanent stroke | 89 (1.8%) | 61 (2.3%) | 42 (1.1%) | 0.0017 |
| Cardiac arrest | 124 (2.4%) | 64 (2.4%) | 89 (2.4%) | 0.98 |
| Atrial fibrillation | 1238 (24.3%) | 756 (28.0%) | 1139 (30.4%) | < 0.0001 |
| Pneumonia | 210 (4.1%) | 101 (3.7%) | 105 (2.8%) | 0.004 |
| Prolonged ventilation | 647 (12.7%) | 388 (14.3%) | 399 (10.7%) | <0.0001 |
| Renal failure requiring dialysis | 115 (2.3%) | 56 (2.1%) | 70 (1.9%) | 0.44 |
| Renal failure | 328 (6.4%) | 146 (5.4%) | 109 (2.9%) | < 0.0001 |
| Deep sternal wound infection | 23 (0.5%) | 8 (0.3%) | 7 (0.2%) | 0.10 |
| Transfusion | 2646 (52.2%) | 1282 (47.4%) | 1432 (38.3%) | < 0.0001 |
| Transfusion (pRBC) | 1602 (38.4%) | 1196 (44.2%) | 1252 (33.5%) | <0.0001 |
| Reoperation for any reason | 453 (8.9%) | 188 (7.0%) | 216 (5.8%) | < 0.0001 |
| Reoperation for bleeding | 223 (4.4%) | 90 (3.3%) | 127 (3.4%) | 0.02 |
| Readmission | 486 (9.8%) | 278 (10.6%) | 364 (10.3%) | 0.57 |
| Discharge to facility | 836 (18.2%) | 716 (27.3%) | 1081 (29.6%) | < 0.0001 |
| Total cost (mean) | $40,745 | $51,923 | $54,710 | < 0.0001 |
| Length of stay (d; median, IQR) | 6 (5–9) | 6 (5–9) | 6 (5–8) | 0.12 |
| ICU stay (hr; median, IQR) | 48 (25.5–95) | 48 (25.8–93.8) | 49.5 (27.4–95) | 0.006 |
| Operative mortality O:E | 0.76 | 1.12 | 0.92 | |
| Morbidity and mortality O:E | ‡ | 1.0 | 0.80 | |
Major morbidity includes: permanent stroke, cardiac arrest, renal failure, deep sternal wound infection, prolonged ventilation, reoperation for any reason
STS PROMM not available for all years
IQR = interquartile range; ICU = intensive care unit; O:E = observed to expected ratio
After risk adjustment, operative era remains a significant predictor of morbidity and mortality (Table 3). The early-TAVR era was independently associated with increased risk for operative mortality and major morbidity, while for isolated SAVR only mortality was increased in this era. The commercial-TAVR era was independently associated with lower rates of major morbidity. TAVR performance was not independently associated with outcomes (all p>0.05).
Table 3.
Risk-adjusted surgical aortic valve replacement outcomes
| All Surgeries | Isolated SAVR | |||
|---|---|---|---|---|
|
| ||||
| Operative mortality | OR (CI) | p-value | OR (CI) | p-value |
| Early-TAVR vs Pre-TAVR era | 1.37 (1.07–1.75) | 0.01 | 1.47 (1.02–2.12) | 0.04 |
| Commercial-TAVR vs Pre-TAVR era | 1.05 (0.82–1.33) | 0.73 | 0.97 (0.67–1.41) | 0.88 |
| Major Morbidity | ||||
|
| ||||
| Early-TAVR vs Pre-TAVR era | 1.15 (1.02–1.29) | 0.03 | 1.11 (0.93–1.32) | 0.25 |
| Commercial-TAVR vs Pre-TAVR era | 0.86 (0.77–0.97) | 0.01 | 0.84 (0.71–0.996) | 0.045 |
SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement
Resource utilization
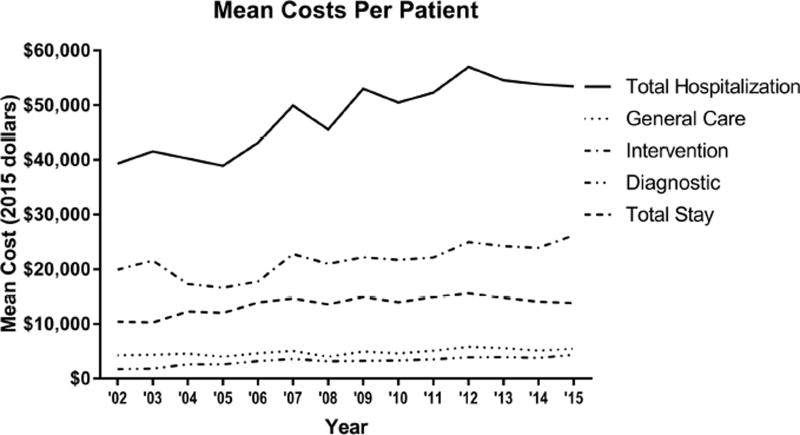
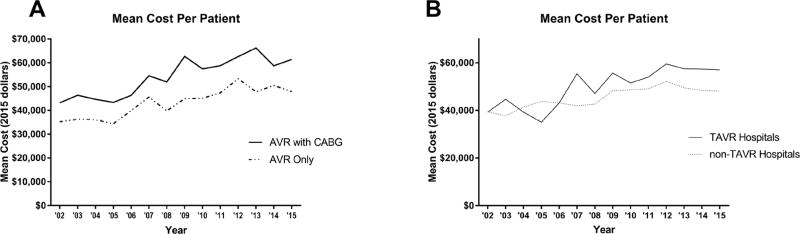
The improvement in outcomes was accompanied by a general increase in resource utilization (Table 2). This included higher frequency of discharge to a facility (18%, 27%, 30%, p<0.0001). While length of stay from surgery to discharge did not change (6d, 6d, 6d, p=0.23), ICU stay statistically increased, although this may not be clinically relevant change (48hr, 48hr, 49.8hr, p=0.006). Finally, average total hospital cost increased from $42,835 to $51,923 and $54,710 (p<0.0001) and the component costs by phase of care are displayed in Figure 2. The costs of isolated AVR increased over time (Figure 3A) with increasing mean cost ($38,410 vs $45,837 vs $49,878; p<0.0001). Interestingly, TAVR hospitals had higher mean total costs compared to non-TAVR hospitals ($50,880 vs $45,606; p<0.0001) with the yearly trends displayed in Figure 3B.
Figure 2.
The average total cost per patient is graphed over time with four subcategories of costs included.
Figure 3.
(A) The average cost per patient is graphed over time for SAVR with CABG and for isolated SAVR. (B) The average cost per patient is plotted for both TAVR hospitals and non-TAVR hospitals.
COMMENT
This study evaluates the impact of TAVR over three eras of implementation on SAVR outcomes, volume and cost. SAVR is unquestionably undergoing significant changes, but competition from TAVR has not resulted in declining surgical volume, which is instead continuing to increase. Additional changes include which patients are selected for surgery, leading to a generally declining risk profile. Outcomes, in particular major morbidity, are improving with the lowest rates of most complications in the current era. This contrasts with increasing resource utilization including length of stay and hospital cost.
Contrary to what many surgeons fear, declining SAVR volume due to competition from TAVR was not borne out in this analysis. TAVR volume experienced dramatically increased volume in 2015 [15]. Yet the annual statewide volume increased over every era and demonstrated similar results to recently published literature [11, 12]. Furthermore, the growth was more pronounced for TAVR hospitals during the early-TAVR phase, while for non-TAVR hospitals the growth was slower but continued steadily throughout the study (Figure 1A). On a per hospital basis volume increased between pre- and early-TAVR eras, but not from the early- to commercial-TAVR era. Volume for early adopters of TAVR appears to have stagnated around 52 cases/hospital/year, while non-TAVR hospitals have continued growth. Some of the non-TAVR hospitals in the final year of the study began performing TAVR and new heart teams and outreach likely increased their volume [12]. An overall increase in referrals is an unlikely explanation since similar risk profiles of patients in the pre- and early-TAVR era would argue against non-TAVR hospitals taking on more complex patients. However, as some of these hospitals began to perform TAVR in the commercial-TAVR era the “halo” effect brought in additional higher risk patients. In summary, SAVR continues to experience growth and currently is most predominant at hospitals that were late adopters of TAVR.
The high rate of concomitant coronary artery disease and stenting during TAVR prompted the inclusion of SAVR with CABG [16, 17]. However, the same trends in terms of patient risk, increased risk-adjusted odds of mortality during the early-TAVR period, and increasing resource utilization are seen with isolated SAVR. We hypothesize that the inclusion of extreme and high-risk patients in the TAVR clinical trials is the reason for these results. Many of these patients were deemed extreme risk for reasons not included in the STS risk model, where in the PARTNER B trial 32% of inoperable patients had severe frailty and 15% porcelain aorta [18].
The implementation of TAVR also appears to be changing both patient selection and the operations performed. The complexity of operations is decreasing with less concomitant CABG, non-elective cases, and prior cardiac surgery. Additionally, use of bioprosthetic valves is increasing, likely due to the availability of valve-in-valve transcatheter options if required in the future. In combination with patient factors, such as better left ventricular ejection fraction (LVEF) and less advanced valve disease, these changes have resulted in lower risk profiles of patients undergoing SAVR, potentially due to higher risk patients being selected for TAVR. Paradoxically, the decreased risk comes despite increasing comorbidities nearly across the board. It is clear that with TAVR as an alternative patient selection is driving the predicted risk of mortality lower.
Changing patients and operative trends has also translated to improved outcomes. Unadjusted mortality and major morbidity have improved with current rates of operative mortality at 3.2% and major morbidity at 15%. Interestingly, the observed to expected ratios (O:E) were highest during the early-TAVR era as clinical trials were underway in high-risk patients, demonstrating some likely unaccounted for risk during this time period. The O:E has since improved to 0.92 for operative mortality and 0.80 for morbidity and mortality.
The improving outcomes have come at the price of increased resource utilization, both in hospital with longer ICU stays, and post-acute care with increasing discharges to a facility. Hospital costs increased throughout the study period (Figure 2) and no single subcomponent appears to be the clear culprit. For comparison, estimates of cost for TAVR in the state of Virginia were recently published as $81,638, although costs vary widely [13]. On the lower end, costs were recently estimated for Medicare patients at $55,700, while the CoreValve Pivotal Trial estimated costs were $69,592 [19, 20]. The average cost overall of isolated SAVR compares favorably at $44,321, but the average cost in the current commercial-TAVR era was $49,878.
The average cost for SAVR with CABG was $54,396 and in the commercial-TAVR era was $62,130. This equates to a cost differential of approximately $10,000 compared to isolated SAVR. For comparison, the average cost of percutaneous coronary intervention (PCI) is $17,543, although there is large cost variation [21, 22]. Although cost trajectories for TAVR and PCI are not well established, the trend of increasing costs for SAVR has the potential to quickly erode this advantage. This is particularly important as TAVR devices improve with smaller devices increasing the ability to perform transfemoral deployment, which in one series had an economic advantage to SAVR in high-risk patients.[23]
This study is a retrospective review and therefore susceptible to selection bias. In addition, contemporary TAVR and PCI cost data at participating institutions was not widely available and had to be inferred using previously reported results and therefore formal cost-effectiveness modeling was not able to be performed. Any database research is inherently limited by errors in the database, although the STS database is a model for clinical databases with 97% concordance with chart abstraction [24].
The introduction of TAVR has been a significant force that is reshaping SAVR in the United States. The structural changes leading to multidisciplinary valve teams and changing referral patterns resulted in increased volume for early adopters. This is shifting as TAVR becomes more widely distributed. Additionally, SAVR patient risk continues to decrease in large part from decreased surgical complexity, despite patients having more comborbid disease. SAVR outcomes continue to improve with respect to both morbidity and mortality. Finally, despite increasing costs for SAVR, there appears to still be significant cost savings compared to TAVR. However, the rising costs of SAVR will require careful attention to maintain an advantage.
Supplementary Material
ABBREVIATIONS
- AI
aortic insufficiency
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CCU
coronary care unit
- CLD
chronic lung disease
- CMS
Centers for Medicare and Medicaid Services
- CPB
cardiopulmonary bypass
- ICU
intensive care unit
- IPPS
Inpatient Prospective Payment Sample
- IQR
interquartile range
- LVEF
left ventricular ejection fraction
- MR
mitral regurgitation
- O:E
observed to expected ratio
- OT
occupational therapy
- PT
physical therapy
- R2
coefficient of determination
- SAVR
surgical aortic valve replacement
- STS
Society of Thoracic Surgeons
- TAVR
transcatheter aortic valve replacement
- PROM
predicted risk of mortality
- PROMM
predicted risk of morbidity and mortality
- VCSQI
Virginia Cardiac Services Quality Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.2016 Aging statistics. [Accessed 2016 May 23, 2016]; Available at http://www.aoa.acl.gov/aging_statistics/index.aspx.
- 2.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The tromso study. Heart. 2013;99(6):396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: Why are so many denied surgery? Eur Heart J. 2005;26(24):2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T, Morimoto T, Shiomi H, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66(25):2827–2838. doi: 10.1016/j.jacc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 6.Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers notion randomized clinical trial. J Am Coll Cardiol. 2015;65(20):2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 7.2016 Medtronic transcatheter aortic valve replacement in low risk patients. [Accessed 2016 May 23, 2016]; Available at https://clinicaltrials.gov/ct2/show/NCT02701283.
- 8.2016 The safety and effectiveness of the sapien 3 transcatheter heart valve in low risk patients with aortic stenosis (partner 3) [Accessed 2016 May 23, 2016]; Available at https://clinicaltrials.gov/ct2/show/NCT02675114.
- 9.Rosato S, Santini F, Barbanti M, et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. 2016;9(5):e003326. doi: 10.1161/CIRCINTERVENTIONS.115.003326. [DOI] [PubMed] [Google Scholar]
- 10.Reinohl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373(25):2438–2447. doi: 10.1056/NEJMoa1500893. [DOI] [PubMed] [Google Scholar]
- 11.Brennan JM, Holmes DR, Sherwood MW, et al. The association of transcatheter aortic valve replacement availability and hospital aortic valve replacement volume and mortality in the united states. Ann Thorac Surg. 2014;98(6):2016–2022. doi: 10.1016/j.athoracsur.2014.07.051. discussion 2022. [DOI] [PubMed] [Google Scholar]
- 12.Patel HJ, Herbert MA, Paone G, et al. The midterm impact of transcatheter aortic valve replacement on surgical aortic valve replacement in michigan. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.02.106. [DOI] [PubMed] [Google Scholar]
- 13.Ailawadi G, LaPar DJ, Speir AM, et al. Contemporary costs associated with transcatheter aortic valve replacement: A propensity-matched cost analysis. Ann Thorac Surg. 2016;101(1):154–160. doi: 10.1016/j.athoracsur.2015.05.120. discussion 160. [DOI] [PubMed] [Google Scholar]
- 14.Osnabrugge RL, Speir AM, Head SJ, et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg. 2013;96(2):500–506. doi: 10.1016/j.athoracsur.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Grover FL, Vemulapalli S, Carroll JD, et al. 2016 annual report of the society of thoracic surgeons/american college of cardiology transcatheter valve therapy registry. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in patients with transcatheter aortic valve replacement and left main stenting: The tavr-lm registry. J Am Coll Cardiol. 2016;67(8):951–960. doi: 10.1016/j.jacc.2015.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramee S, Anwaruddin S, Kumar G, et al. The rationale for performance of coronary angiography and stenting before transcatheter aortic valve replacement: From the interventional section leadership council of the american college of cardiology. JACC Cardiovasc Interv. 2016;9(23):2371–2375. doi: 10.1016/j.jcin.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the partner trial. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S11–16. doi: 10.1016/j.jtcvs.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy FH, McDermott KM, Anwaruddin S, et al. Abstract 22: Cost of transcatheter aortic valve replacement in medicare patients. Circulation: Cardiovascular Quality and Outcomes. 2015;8(Suppl 2):A22. [Google Scholar]
- 20.Reynolds MR, Lei Y, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol. 2016;67(1):29–38. doi: 10.1016/j.jacc.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin AP, Bach RG, Novak E, Lasala JM, Singh J. Abstract 21: Variation in hospital costs of percutaneous coronary intervention. Circulation: Cardiovascular Quality and Outcomes. 2014;7(Suppl 1):A21. [Google Scholar]
- 22.Amin AP, House JA, Safley DM, et al. Costs of transradial percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6(8):827–834. doi: 10.1016/j.jcin.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds MR, Magnuson EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: Results of the partner (placement of aortic transcatheter valves) trial (cohort a) J Am Coll Cardiol. 2012;60(25):2683–2692. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Grover FL, Shahian DM, Clark RE, Edwards FH. The sts national database. Ann Thorac Surg. 2014;97(1 Suppl):S48–54. doi: 10.1016/j.athoracsur.2013.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.