Abstract
Aims
To investigate the obesity paradox and association of extreme obesity with long-term outcomes among older ST-segment elevation myocardial infarction (STEMI) patients.
Methods and results
Nineteen thousand four hundred and ninety-nine patients ≥65 years with STEMI surviving to hospital discharge in NCDR ACTION Registry-GWTG linked to Centers for Medicare and Medicaid Services outcomes between 2007 and 2012 were stratified by body mass index (BMI) (kg/m2) into normal weight (18.5–24.9), overweight (25–29.9), class I (30–34.9), class II (35–39.9), and class III/extreme obese (≥40) categories. Multivariable-adjusted associations were evaluated between BMI categories and mortality by Cox proportional hazards models, and days alive and out of hospital (DAOH) by generalized estimating equations, within 3 years after discharge. Seventy percent of patients were overweight/obese and 3% extremely obese. Normal weight patients were older and more likely to smoke; while extremely obese patients were younger and more likely to be female and black, with lower socioeconomic status and more comorbidity (P ≤ 0.001). A U-shaped association was observed between BMI categories and mortality: patients with class I obesity were at lowest risk, while normal weight [hazard ratio (HR) 1.30, 95% confidence interval (CI) 1.15–1.47] and extremely obese patients (HR 1.33, 95% CI 1.02–1.74) had higher mortality. Normal weight [odds ratio (OR) 0.79, 95% CI 0.68–0.90] and extremely obese (OR 0.73, 95% CI 0.54–0.99) individuals also had lower odds of DAOH.
Conclusion
Mild obesity is associated with lower long-term risk in older STEMI patients, while normal weight and extreme obesity are associated with worse outcomes. These findings highlight hazards faced by an increasing number of older individuals with normal weight or extreme obesity and cardiovascular disease.
Keywords: Obesity, STEMI, Obesity paradox, Elderly, Extreme obesity, Primary PCI
Introduction
As the proportion of adults ≥65 years in the US population is projected to increase from 15 to 24% by 2060,1 understanding the interaction between obesity and ST-segment elevation myocardial infarction (STEMI) outcomes among older adults is increasingly relevant to public health. An obesity paradox exists among patients with myocardial infarction (MI) and other cardiovascular diseases whereby patients with normal body weight have a worse prognosis than overweight and mildly obese patients.2,3 A meta-analysis of 218 532 patients confirmed that obese patients with MI had a 30–40% lower mortality compared with normal weight patients after 1–3 years of follow-up.4 A large Swedish registry of coronary angiography patients also reported that mortality through 3 years was lowest among overweight/obese patients and highest among those who were underweight/normal weight.5
Many prior studies have not differentiated between MI type [STEMI vs. non-STEMI (NSTEMI)]6–8 or have focused on patients with NSTEMI.9–11 Although an obesity paradox is seen among older NSTEMI patients,12 it is unknown whether this paradox is seen over long-term follow-up among older STEMI patients. STEMI may differ from NSTEMI with regard to patient characteristics, pathophysiology, and complexity of coronary atherosclerosis. Moreover, findings have not generally been stratified by severity of obesity13 such that data on long term outcomes and processes of care among those with extreme obesity are limited.14,15 This knowledge gap is particularly relevant because the prevalence of extreme obesity is increasing even as overall obesity rates have stabilized.16
Therefore, we aimed to determine whether an obesity paradox is evident over long-term follow-up among older adults with mild obesity after STEMI and sought to further characterize the association of extreme obesity with long-term outcomes.
Methods
Data source
We studied older adults from a large representative patient sample in the National Cardiovascular Data Registry (NCDR) Acute Coronary Treatment and Intervention Outcomes Network Registry–Get with the Guidelines (ACTION Registry-GWTG). This study is a quality improvement registry database focused on patients admitted to the hospital with acute myocardial infarction. Details of the ACTION Registry-GWTG have been previously described.17 Briefly, trained hospital personnel from >1000 participating centres across the USA collect data from medical records using a standardized case report form (https://www.ncdr.com/webncdr/action/ home/datacollection). Abstracted data include demographics, clinical information, medical therapies, use and timing of cardiac procedures, and in-hospital outcomes. The registry ensures uniform data entry and transmission and is subject to quality checks. Because data are abstracted retrospectively and anonymously without patient identifiers, institutional review boards by policy waive the need for written informed consent.
Longitudinal outcomes were ascertained using the Centers for Medicare and Medicaid Services (CMS) longitudinal administrative database, which includes inpatient and outpatient claims and the corresponding denominator files through 2012. We linked the ACTION Registry-GWTG data to Medicare claims using a combination of indirect identifiers including age, sex, admission date, procedure date, and hospital identification for the examination of mortality, hospital admissions, and major adverse cardiac events (MACE).18
Study population
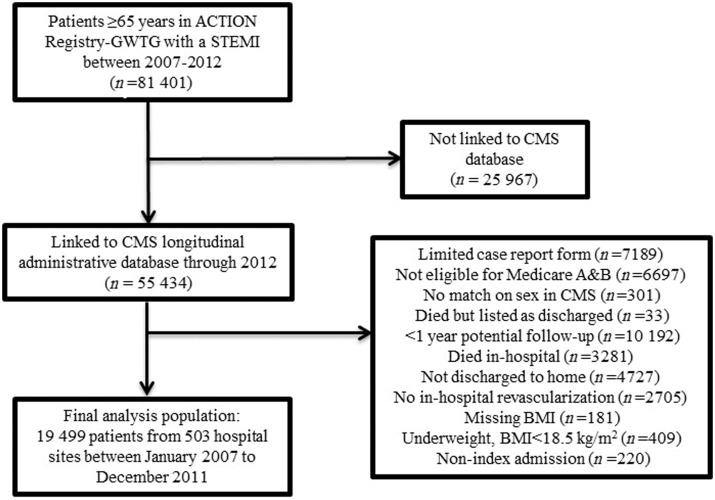
For the present study, we included all patients in ACTION Registry-GWTG with an acute STEMI at least 65 years old between 2007 and 2012 (n = 81 401 from 852 hospitals). 55 434 patients (68%) from 809 hospitals were successfully matched to CMS longitudinal administrative data through the end of 2012. After exclusions described in the Supplementary material, the final analysis population included 19 499 patients from 503 hospitals between 3 January 2007 and 31 December 2011 (Figure 1). Baseline characteristics were generally similar between those included vs. excluded from the analysis (see Supplementary material online, Table S1).
Figure 1.
Flow diagram of patient selection. BMI, body mass index; CMS, Center for Medicare and Medicaid Services; STEMI, ST-segment elevation myocardial infarction.
Outcomes
Outcomes assessed included all-cause mortality, days alive and out of hospital (DAOH), and a 4-point composite of MACE (mortality, readmission for myocardial infarction, stroke, or heart failure) through 3 years post-hospital discharge. The DAOH endpoint was calculated as the difference between total potential follow-up time and the sum of days spent in the hospital plus days dead. This endpoint captures the totality of a disease burden by incorporating both mortality and morbidity. It has a number of advantages over time-to-event measures including accounting for both number and duration of multiple hospitalizations, more clinical relevance by assigning greater weight to mortality, more patient-centric accounting for time spent outside the hospital with potential for better quality of life, and because it is not affected by issues of adjudication required for cause-specific outcomes.19 Other variable definitions are described in detail in the Supplementary material online.
Statistical analysis
Patients were stratified according to World Health Organization body mass index (BMI) categories13 into normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), class I obese (mild, 30–34.9 kg/m2), class II obese (moderate, 35–39.9 kg/m2), and class III obese (extreme, ≥40 kg/m2) categories. Trends in baseline characteristics, in-hospital treatment, and discharge medications were compared across BMI groups using the appropriate Cochran–Mantel–Haenszel statistics for categorical and continuous variables. Kaplan–Meier curves were generated to estimate the probability of all-cause mortality within 3 years post-discharge by BMI category. Comparisons across BMI groups were performed with the log rank statistic. Multivariable Cox proportional hazards models were used to evaluate the association between BMI category and mortality adjusted for clinical and socioeconomic status (SES) characteristics and in-hospital outcomes; results are reported as hazard ratio (HR) and 95% confidence interval (CI). Covariates used for adjustment included those in the ‘Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines (CRUSADE)’ long-term mortality model in older patients20: age, female sex, race, current or recent smoking status, prior myocardial infarction, prior percutaneous coronary intervention (PCI), prior coronary artery bypass graft surgery (CABG), prior heart failure, prior stroke, prior peripheral arterial disease, heart failure at presentation, heart rate at presentation, initial haemoglobin level, initial troponin level, initial creatinine level, and initial systolic blood pressure. STEMI specific additional covariates included: primary PCI at index admission, CABG at index admission, number of diseased vessels, left ventricular ejection fraction at index admission, in-hospital cardiogenic shock, and prescription for angiotensin converting enzyme inhibitor or angiotensin receptor blocker at discharge. Models were also adjusted for SES and the Charlson comorbidity index.21 SES scores were calculated according to Diez Roux et al.22 reflecting summed information about wealth and income, education, and occupation. Higher scores represent more favourable SES. Robust standard errors were used to account for clustering of patients within hospitals.
Differences across BMI categories in DAOH and MACE event rates were also assessed. For the MACE endpoint, the modelling strategy described above was used. For the DAOH endpoint, generalized estimating equations method was used to model DAOH as a proportion of days possible out of 1095 days (3 years). A logit link was used to reflect the fact that DAOH is bound on 2 sides by 0 and 1095 days. Robust empirical variance estimates were used to account for the fact that the data are not necessarily independently binomial and the potential for clustering of patients within hospitals. Sensitivity analyses were performed to help determine the potential role of reverse causality of existing non-cardiovascular diseases/deaths on the BMI-mortality relationship by (i) excluding patients who died within 6 months, and separately within 1 year, of hospital discharge, (ii) excluding patients with a history of cancer (defined by ICD-9 codes, see Supplementary material online) within 1 year of the index admission for myocardial infarction, and (iii) subgroup analyses stratifying patients by current smoking status and separately by Charlson comorbidity index (<3 vs. ≥3). All continuous variables were evaluated for nonlinearity with the outcome, and restricted cubic splines were used for those that did not meet the linear criteria. Variables had <2% missing values. When modelling outcomes, missing values for continuous covariates were imputed to sex-specific median of the non-missing values. For categorical variables, missing values were imputed to the most frequently occurring group. A 2-sided P-value of 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used to perform all analyses in the study.
Results
Baseline characteristics of the study population
Among the 19 499 patients included in the analysis, mean age was 74.8 years with 62.1% male and 90.4% white patients. In all, 30.4% were normal weight, 40.9% overweight, 19.6% were mildly obese, 6.1% were moderately obese, and 3.0% were extremely obese. Baseline characteristics of the study population stratified by BMI category are shown in Table 1. As BMI increased, there was a significant trend towards younger age, female sex, black race, and less smoking (P < 0.001). Higher mean SES and lower comorbidity were seen among those with normal weight/overweight whereas obese patients had lower mean SES and greater comorbidity, with a widening gap seen as BMI increased (see Supplementary material online, FigureS1). Increasing BMI was also associated with a stepwise trend towards higher left ventricular ejection fraction at presentation and less in-hospital cardiogenic shock (P < 0.001 for both) whereas a U-shaped relationship was seen with in-hospital major bleeding, with rates highest at the extremes of BMI (P < 0.001). No significant differences by BMI category were seen for rates of cardiac arrest, provision of antiplatelet, anticoagulation, statin, or reperfusion therapy, door to balloon time, or cardiac rehabilitation referral (P all >0.05) (Table 2).
Table 1.
Baseline demographics and medical history of the study population
| Patient characteristics | Normal weight (n = 5920) | Overweight (n = 7982) | Obese class I (n = 3837) | Obese class II (n = 1192) | Obese class III (n = 568) | P-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 76 (71–82) | 74 (69–79) | 72 (68–77) | 71 (68–76) | 71 (68–75) | <0.001 |
| Male, number (%) | 3306 (55.8) | 5411 (67.8) | 2459 (64.1) | 689 (57.8) | 237 (41.7) | <0.001 |
| Race/ethnicity, number (%) | <0.001 | |||||
| White | 5344 (90.3) | 7269 (91.1) | 3447 (89.8) | 1065 (89.4) | 501 (88.2) | |
| Black | 262 (4.4) | 340 (4.3) | 211 (5.5) | 73 (6.1) | 50 (8.8) | |
| Hispanic | 144 (2.4) | 231 (2.9) | 123 (3.2) | 27 (2.3) | 11 (1.9) | |
| Insurance status, number (%) | 0.02 | |||||
| Medicare | 2913 (49.2) | 3816 (47.8) | 1786 (46.6) | 556 (46.6) | 275 (48.4) | |
| Private | 2920 (49.3) | 4050 (50.7) | 2007 (52.3) | 625 (52.4) | 283 (49.8) | |
| Other | 84 (1.4) | 114 (1.4) | 42 (1.1) | 11 (0.9) | 10 (1.8) | |
| Body mass index, kg/m2 | 23.1 (21.6–24.1) | 27.3 (26.1–28.5) | 31.9 (30.9–33.2) | 36.7 (35.8–37.9) | 43.4 (41.4–47.4) | <0.001 |
| Socioeconomic status scorea | 0.80 (3.5) | 0.66 (3.4) | 0.54 (3.4) | 0.53 (3.2) | 0.07 (3.0) | <0.001 |
| Hospital region, number (%) | <0.001 | |||||
| West | 693 (11.7) | 886 (11.1) | 406 (10.6) | 139 (11.7) | 49 (8.6) | |
| Northeast | 434 (7.3) | 518 (6.5) | 260 (6.8) | 97 (8.1) | 46 (8.1) | |
| Midwest | 1743 (29.4) | 2615 (32.8) | 1306 (34.0) | 423 (35.5) | 204 (35.9) | |
| South | 3050 (51.5) | 3963 (49.7) | 1865 (48.6) | 533 (44.7) | 269 (47.4) | |
| Medical history | ||||||
| Hypertension, number (%) | 3971 (67.1) | 5755 (72.1) | 3029 (78.9) | 948 (79.5) | 464 (81.7) | <0.001 |
| Dyslipidaemia, number (%) | 3063 (51.7) | 4730 (59.3) | 2411 (62.8) | 760 (63.8) | 372 (65.5) | <0.001 |
| Diabetes mellitus, number (%) | 903 (15.3) | 1853 (23.2) | 1294 (33.7) | 503 (42.2) | 274 (48.2) | <0.001 |
| Current/recent smoker, number (%) | 1526 (25.8) | 1558 (19.5) | 649 (16.9) | 177 (14.9) | 88 (15.5) | <0.001 |
| Prior MI, number (%) | 1083 (18.3) | 1499 (18.8) | 748 (19.5) | 246 (20.6) | 114 (20.1) | 0.03 |
| Prior HF, number (%) | 280 (4.7) | 356 (4.5) | 209 (5.5) | 84 (7.1) | 52 (9.2) | <0.001 |
| Prior PCI, number (%) | 1162 (19.6) | 1695 (21.2) | 860 (22.4) | 287 (24.1) | 123 (21.7) | <0.001 |
| Prior CABG, number (%) | 517 (8.7) | 807 (10.1) | 394 (10.3) | 124 (10.4) | 43 (7.6) | 0.03 |
| Prior stroke, number (%) | 369 (6.2) | 481 (6.0) | 177 (4.6) | 75 (6.3) | 42 (7.4) | 0.07 |
| Peripheral arterial disease, number (%) | 537 (9.1) | 576 (7.2) | 257 (6.7) | 86 (7.2) | 38 (6.7) | <0.001 |
| Charlson comorbidity indexa | 1.92 (1.03) | 1.89 (1.04) | 1.98 (1.02) | 2.11 (1.11) | 2.15 (1.08) | <0.001 |
Data represented as median (IQR) or number (%). P-value was calculated by comparing only non-missing row values; all P-values are 5-group comparisons. BMI (kg/m2) categories are according to the World Health Organization classification: normal weight (18.5–24.9), overweight (25–29.9), class I obese (30–34.9), class II obese (35–39.9), and class III obese (≥40).
CABG, coronary artery bypass graft; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; BMI, body mass index; IQR, interquartile range.
Mean (standard deviation) presented for this variable.
Table 2.
Presentation and in-hospital characteristics and discharge interventions of the study population
| Patient characteristics | Normal Weight (n = 5920) | Overweight (n = 7982) | Obese class I (n = 3837) | Obese class II (n = 1192) | Obese class III (n = 568) | P-value |
|---|---|---|---|---|---|---|
| Presentation and in-hospital characteristics | ||||||
| Heart rate, bpm | 74 (62–88) | 74 (63–88) | 75 (63–88) | 77 (65–90) | 78.5 (66–92) | <0.001 |
| Systolic blood pressure, mmHg | 138 (116–158) | 141 (120–162) | 142 (122–163) | 143 (122–164) | 145 (121–164) | <0.001 |
| Signs of HF, number (%) | 453 (7.7) | 641 (8.0) | 309 (8.1) | 106 (8.9) | 49 (8.6) | 0.17 |
| Initial haemoglobin, g/dL | 13.5 (12.3–14.7) | 14.0 (12.8–15.1) | 14.0 (12.9–15.2) | 13.9 (12.7–15.1) | 13.5 (12.5–14.7) | <0.001 |
| Initial creatinine, mg/dL | 1.0 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | <0.001 |
| Initial troponin, xULN | 1.2 (0.3–11.9) | 1.0 (0.3–10.8) | 1.0 (0.2–9.8) | 1.0 (0.3–9.9) | 1.4 (0.3–15.2) | 0.005 |
| LVEF, number (%) | <0.001 | |||||
| ≥50% | 2661 (46.8) | 3906 (50.8) | 1908 (51.6) | 563 (49.6) | 303 (55.8) | |
| 25–50% | 2785 (49.0) | 3515 (45.8) | 1665 (45.0) | 546 (48.1) | 225 (41.4) | |
| <25% | 224 (3.9) | 239 (3.1) | 100 (2.7) | 23 (2.0) | 12 (2.2) | |
| Multi-vessel coronary disease, number (%) | 4018 (68.4) | 5479 (69.1) | 2623 (68.8) | 785 (66.4) | 356 (63.1) | 0.10 |
| Overall reperfusion therapy | 5361 (96.2) | 7157 (95.8) | 3440 (95.3) | 1081 (96.4) | 527 (97.4) | 0.41 |
| Primary PCI | 4922 (89.1) | 6527 (88.1) | 3103 (86.7) | 985 (88.9) | 484 (90.1) | 0.03 |
| Door to balloon time (minutes) | 64.0 (49.0–81.0) | 63.0 (48.0–79.0) | 64.0 (48.0–80.0) | 64.0 (50.5–82.0) | 64.0 (48.0–81.5) | 0.72 |
| In-hospital CABG, number (%) | 329 (5.6) | 605 (7.6) | 288 (7.5) | 70 (5.9) | 24 (4.2) | 0.02 |
| In-hospital cardiogenic shock, number (%) | 289 (4.9) | 310 (3.9) | 140 (3.7) | 39 (3.3) | 20 (3.5) | <0.001 |
| In-hospital major bleeding, number (%) | 723 (12.2) | 801 (10.0) | 365 (9.5) | 127 (10.7) | 71 (12.5) | <0.001 |
| Aspirin within 24 h, number (%) | 5788 (98.9) | 7787 (98.9) | 3747 (98.9) | 1160 (98.9) | 553 (99.1) | 0.91 |
| P2Y12 receptor inhibitor within 24 h, number (%) | 5417 (91.5) | 7240 (90.7) | 3457 (90.1) | 1097 (92.0) | 536 (94.4) | 0.65 |
| Statin within 24 h, number (%) | 4154 (72.7) | 5611 (72.9) | 2709 (72.9) | 856 (75.0) | 418 (75.3) | 0.22 |
| Discharge interventions | ||||||
| Aspirin, number (%) | 5747 (98.9) | 7748 (99.0) | 3719 (98.9) | 1157 (99.1) | 545 (98.4) | 0.97 |
| P2Y12 receptor inhibitor, number (%) | 5552 (94.2) | 7365 (92.7) | 3531 (92.4) | 1116 (93.9) | 534 (94.9) | 0.04 |
| Statin, number (%) | 5440 (94.6) | 7349 (94.9) | 3552 (95.0) | 1108 (96.1) | 518 (94.5) | 0.15 |
| Beta blocker, number (%) | 5413 (97.4) | 7408 (97.8) | 3587 (97.5) | 1112 (97.1) | 532 (97.8) | 0.97 |
| ACE inhibitor/ARB, number (%) | 4200 (76.8) | 5823 (78.3) | 2928 (81.1) | 909 (81.3) | 446 (82.9) | <0.001 |
| Aldosterone antagonist, number (%) | 235 (4.1) | 331 (4.2) | 180 (4.8) | 61 (5.2) | 28 (5.1) | 0.02 |
| Cardiac rehabilitation referral, number (%) | 4665 (84.7) | 6440 (85.0) | 3126 (85.3) | 973 (84.6) | 454 (85.3) | 0.58 |
Data represented as median (IQR) or number (%). P-value was calculated by comparing only non-missing row values; all P-values are 5-group comparisons. BMI (kg/m2) categories are according to the World Health Organization classification: normal weight (18.5–24.9), overweight (25–29.9), class I obese (30–34.9), class II obese (35–39.9), and class III obese (≥40).
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BPM, beats per minute; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; HF, heart failure; PCI, percutaneous coronary intervention; ULN, upper limit of normal; BMI, body mass index.
Relation of body mass index to long-term mortality and hospitalization after ST-segment elevation myocardial infarction
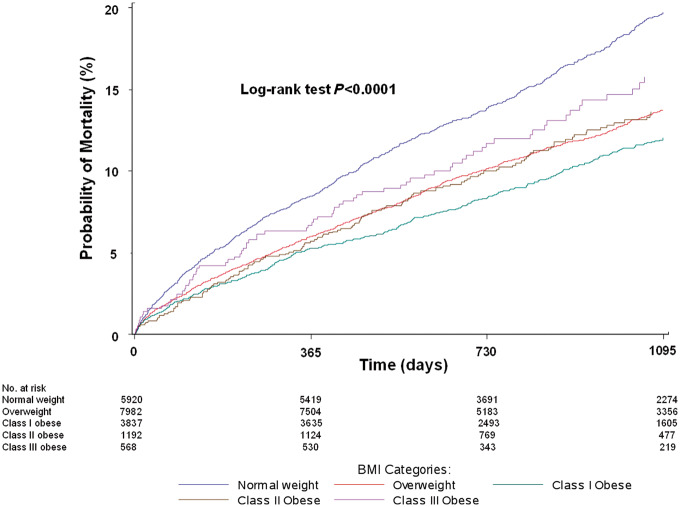
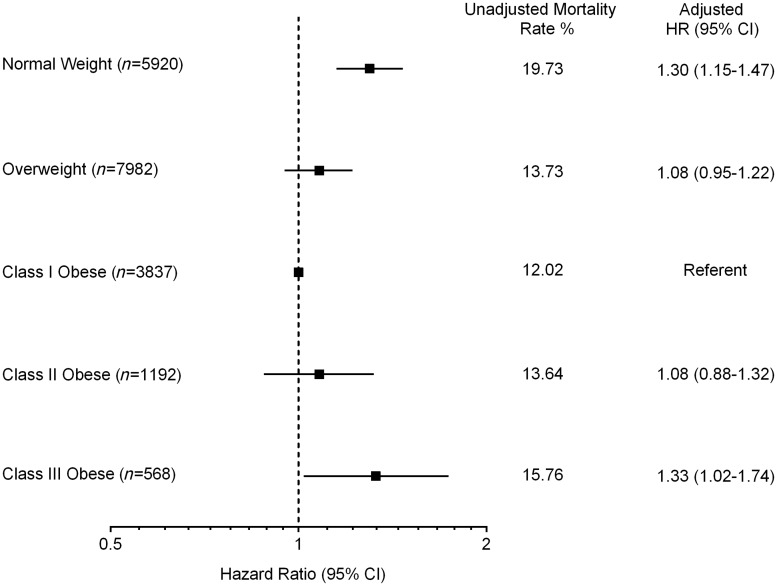
Over a median follow-up period of 2.6 years, 15.3% of patients died. In unadjusted analyses, a U-shaped association was seen between BMI and mortality, with higher mortality among normal weight individuals (19.7%) and those with extreme obesity (15.8%), and the lowest mortality among those with mild obesity (12.0%) (Figure 2). After multivariable adjustment, the U-shaped association persisted: patients with mild (class I) obesity were at lowest risk (referent group), while normal weight (HR 1.30, 95% CI 1.15–1.47) and extremely obese patients (HR 1.33, 95% CI 1.02-–1.74) had higher all-cause mortality (Figure 3). The adjusted association between normal weight and higher mortality persisted even after excluding patients who died within 6 months, and separately within 1 year, of hospital discharge; and excluding patients with a history of cancer within 1 year of the index admission (see Supplementary material online, TablesS2–S4). Results were generally similar in subgroup analyses stratified by current smoking status and separately by Charlson comorbidity index (<3 vs. ≥3), with a persistently higher adjusted hazard for mortality among normal weight compared with mildly obese patients even among non-smokers and those with low comorbidity (see Supplementary material online, TablesS5and S6). Results using unbiased continuous BMI linear splines were consistent with those using clinical BMI categories.
Figure 2.
Unadjusted probability of mortality (%) by 3 years post-discharge by BMI category. BMI (kg/m2) categories are according to the World Health Organization classification: normal weight (18.5–24.9, blue), overweight (25–29.9, red), class I obese (30–34.9, green), class II obese (35–39.9, brown), and class III obese (≥40, purple).
Figure 3.
Unadjusted and multivariable-adjusted risk of death at 3 years post-hospital discharge by BMI category; observed hazard and hazard ratios (HR) with 95% confidence intervals (CI) are displayed. Model adjusted for age, female sex, race, current or recent smoking status, prior myocardial infarction, percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG), heart failure, stroke, peripheral arterial disease, heart failure at presentation, heart rate at presentation, initial haemoglobin level, initial troponin level, creatinine level, and systolic blood pressure, primary PCI or CABG at index admission, number of diseased vessels, left ventricular ejection fraction, in-hospital cardiogenic shock, prescription for angiotensin converting enzyme inhibitor or angiotensin receptor blocker at discharge, socioeconomic status and Charlson comorbidity index. BMI categories same as in Figure 2.
A U-shaped distribution across BMI categories was also seen with DAOH (see Supplementary material online, FigureS2). Individuals with mild obesity had the greatest number of DAOH in the 3 years post-hospital discharge (mean 832 ± 293) whereas normal weight and extremely obese patients had the least (mean 802 ± 315 and 802 ± 308, respectively, P < 0.001). After multivariable adjustment, the odds of spending more time alive and out of hospital at 3 years post-discharge remained significantly lower for normal weight (OR 0.79, 95% CI 0.68–0.90) and class III obese (OR 0.73, 95% CI 0.54–0.99) patients compared with class I obese patients. There was no significant difference in DAOH for overweight or class II obese patients compared with class I obese patients.
Relation of body mass index to major adverse cardiovascular events after ST-segment elevation myocardial infarction
Within 3 years after hospital discharge, 27.3% of patients experienced a MACE event. Unadjusted MACE event rates also demonstrated a U-shaped distribution, with the lowest event rate among class I obese (24.8%) and the highest event rates among normal weight (30.8%) and extremely obese (30.5%) patients. However, after multivariable adjustment, the excess MACE risk among normal weight patients was attenuated and was no longer different from class I obese patients; similar results were seen with overweight individuals. However, class II (HR 1.18, 95% CI 1.02–1.36) and class III patients (HR 1.23, 95% CI 1.02–1.49) remained at excess risk for MACE compared with patients with class I obesity (Table 3).
Table 3.
Risk of a major adverse cardiovascular event at 3 years post-hospital discharge across body mass index categories
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| BMI category | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Normal weight (n = 5920) | 1.29 (1.19–1.41) | <0.001 | 1.05 (0.96–1.15) | 0.30 |
| Overweight (n = 7982) | 1.04 (0.95–1.13) | 0.42 | 0.99 (0.91–1.09) | 0.87 |
| Class I obese (n = 3837) | Referent | Referent | ||
| Class II obese (n = 1192) | 1.28 (1.12–1.47) | <0.001 | 1.18 (1.02–1.36) | 0.03 |
| Class III obese (n = 568) | 1.28 (1.07–1.53) | 0.006 | 1.23 (1.02–1.49) | 0.03 |
Referent: Class I Obese. MACE: all-cause mortality or readmission for myocardial infarction, stroke, or heart failure. BMI (kg/m2) categories are according to the World Health Organization classification: normal weight (18.5–24.9), overweight (25–29.9), class I obese (30–34.9), class II obese (35–39.9), and class III obese (≥40).
CI, confidence interval; HR, hazard ratio; BMI, body mass index; MACE, major adverse cardiac event.
Discussion
In this study of the relationship between BMI and long-term mortality and morbidity outcomes in 19 499 older patients with STEMI, we found that (i) the majority (70%) were overweight or obese; (ii) 3% had extreme obesity; (iii) extreme obesity was associated with female sex, black race, and lower SES; (iv) an obesity paradox was present among older STEMI patients such that those with mild-moderate obesity were at lower risk for death and lived longer outside of hospital compared with normal weight or extremely obese individuals; (v) the improved outcomes observed in mild obesity did not extend to patients with extreme obesity, who remained at significantly increased risk for all adverse outcomes; and (vi) the obesity paradox did not extend to composite MACE events, which demonstrated a linear association with BMI. Although the possibility of reverse causality cannot be completely ruled out, our sensitivity analyses show that the increased hazard for mortality among normal BMI patients (compared with mild obesity) after STEMI persists despite excluding early deaths and patients with existing cancer, and is consistent across smoking status and low vs. high comorbidity, all situations in which excess mortality could potentially be attributed to non-cardiovascular causes.
An obesity paradox has been reported in older NSTEMI patients in a long-term follow-up study from the CRUSADE Registry.12 However, this NSTEMI population was older with more women and diabetes and lower rates of smoking and in-hospital cardiogenic shock, and less cardiac catheterization. In contrast, 88% of the patients in our study underwent primary PCI, and were well treated with secondary prevention with minor differences between BMI groups. Thus, the observed differences in outcome are not likely explained by differences in treatment of the index STEMI. In a prior study limited to in-hospital outcomes among STEMI patients from NCDR ACTION Registry-GWTG, the protective association of obesity on in-hospital mortality also did not extend to extremely obese patients.15 However, this study was limited to in-hospital outcomes and the extremely obese patients were generally younger, limiting generalizability to an older STEMI population. In the current study limited to those ≥65 years, our results suggest that the favourable outcomes associated with mild-moderate obesity do not extend to patients with extreme obesity at any age after STEMI.
The excess mortality seen with class III obesity suggests a ‘threshold effect’, such that exposure to BMI ≥40 kg/m2 attenuates or reverses any protective effects present from surplus energy reserves in milder obesity. Higher in-hospital bleeding rates were also observed in this group that may be associated with increased risk for long-term recurrent bleeding, MACE, and mortality after PCI.23 Adverse effects on haemodynamics and cardiac structure and function may contribute to mortality risk in extreme obesity such as increased total blood volume required to perfuse a growing adipose organ with greater required cardiac output and workload.2 Alterations in the autonomic nervous system may also result from extreme obesity and potentiate its metabolic effects.24 Finally, although referral rates for cardiac rehabilitation were not different across BMI groups in our study, individuals with extreme obesity are more likely to have orthopedic/musculoskeletal problems and impaired physical fitness. These individuals may not be able to effectively participate in cardiac rehabilitation and therefore may not derive the same long-term benefit as individuals with lower BMI.
The increased long-term risk seen among normal weight patients after STEMI despite a more favourable SES and less medical comorbidity warrants discussion. Many researchers have postulated that the worse prognosis in normal weight patients may be explained by the effect of residual confounding,15 such that normal body weight in a contemporary cardiovascular disease population reflects the presence of serious comorbidity not adequately captured. Therefore, evidence for an obesity paradox should not be misinterpreted to recommend higher target BMIs after STEMI since reverse causality may be present.3 Importantly, whereas older studies found excess risk limited to underweight individuals, this is now seen with BMI in the ‘normal’ range in our contemporary study. We believe this reflects the overall shift in the BMI distribution among patients with STEMI, and illustrates the sobering reality that it is now so ‘abnormal’ to present with STEMI and BMI < 25 that the possibility of unrecognized medical illness or frailty must be considered.
The clinical implications of these findings are increasingly relevant given the static prevalence of obesity despite substantial clinical and policy efforts. The National Health and Nutrition Examination Survey (NHANES) reported an upward trend towards more severe forms of obesity among children25 and a recent study from Canada reported that the prevalence of extreme obesity increased by 400% over the past 2 decades.26 An analysis from NHANES reported an overall prevalence of extreme obesity of 5.5% in US men and 9.9% in women, with increases in the prevalence of overall obesity and of extreme obesity among women between 2005 and 2014.16 A similar trend was observed specifically among women older than 60.27 It is important to note that the impact of extreme obesity on mortality grows significantly stronger with increasing age.28 Our findings also have economic and patient-centred implications since increased rates of readmission and longer time in the hospital lead to higher costs and decreased quality of life. These findings highlight a need for aggressive prevention and treatment for this high risk group. Whether treatment paradigms after STEMI, such as approaches to medical therapy or multi-vessel revascularization, influence outcomes and differ by BMI class remains to be determined.
Our study has several limitations. First, differences in post-discharge processes of care/treatment may have influenced the protective association seen with mild obesity after STEMI. Also, alternative metrics of adiposity were not captured in the dataset, limiting the ability to explore variation in body composition or body fat distribution. Since studies have shown that more contemporary measures of central obesity such as waist circumference and waist to hip ratio are more robust predictors of cardiovascular outcomes,29 more detailed metrics of central adiposity such as body composition and fat distribution30 will be important to consider in future studies. Frailty or physical fitness, important factors that may influence the association of BMI with outcomes, were not available. Our findings may not be generalizable to other racial/ethnic populations, such as South Asians, in whom different BMI risk cut-offs for mortality and cardiovascular disease may exist,31 or to younger individuals <65 years. Lastly, because obese STEMI patients are often younger than their normal weight counterparts, it is possible that selection bias exists in which data on only the healthiest obese patients that survive long enough to hospital discharge was captured. However, since overweight and obese patients had lower SES and more medical comorbidity, our findings are unlikely to be explained by healthy selection bias. Our findings should also be taken within the context of their observational registry design with the inherent limitations that were are unable to fully account for unmeasured confounding and that the results should be considered hypothesis generating.
In summary, the obesity paradox persists over long-term follow-up in older STEMI patients but does not extend to extreme obesity (Figure 4). Further study is needed to discern the sociobiological mechanisms underpinning these observations. The rapidly growing population with extreme obesity, characterized by lower SES and high proportions of women and blacks, represents a population at notably increased risk for adverse long term outcomes after STEMI.
Figure 4.
Long-term mortality in older adults after ST-segment elevation myocardial infarction by body mass index.
Supplementary material
Supplementary material is available at European Heart Journal—Quality of Care and Clinical Outcomes online.
Funding
This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) [K23 DK106520]; and by the Dedman Family Scholarship in Clinical Care from UT Southwestern [both to I.J.N.].
Conflict of interest : none declared.
Supplementary Material
References
- 1. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf (26 September 2016).
- 2. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 3. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force M. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niedziela J, Hudzik B, Niedziela N, Gasior M, Gierlotka M, Wasilewski J, Myrda K, Lekston A, Polonski L, Rozentryt P. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol 2014;29:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 2013;34:345–353. [DOI] [PubMed] [Google Scholar]
- 6. Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the "obesity paradox" in the get with the Guidelines database. Am J Cardiol 2007;100:1331–1335. [DOI] [PubMed] [Google Scholar]
- 8. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002;39:578–584. [DOI] [PubMed] [Google Scholar]
- 9. Diercks DB, Roe MT, Mulgund J, Pollack CV Jr, Kirk JD, Gibler WB, Ohman EM, Smith SC Jr, Boden WE, Peterson ED. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J 2006;152:140–148. [DOI] [PubMed] [Google Scholar]
- 10. Buettner HJ, Mueller C, Gick M, Ferenc M, Allgeier J, Comberg T, Werner KD, Schindler C, Neumann FJ. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J 2007;28:1694–1701. [DOI] [PubMed] [Google Scholar]
- 11. Mahaffey KW, Tonev ST, Spinler SA, Levine GN, Gallo R, Ducas J, Goodman SG, Antman EM, Becker RC, Langer A, White HD, Aylward PE, Col JJ, Ferguson JJ, Califf RM, Investigators ST. Obesity in patients with non-ST-segment elevation acute coronary syndromes: results from the SYNERGY trial. Int J Cardiol 2010;139:123–133. [DOI] [PubMed] [Google Scholar]
- 12. O'Brien EC, Fosbol EL, Peng SA, Alexander KP, Roe MT, Peterson ED. Association of body mass index and long-term outcomes in older patients with non-ST-segment-elevation myocardial infarction: results from the CRUSADE Registry. Circ Cardiovasc Qual Outcomes 2014;7:102–109. [DOI] [PubMed] [Google Scholar]
- 13. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 14. Herrmann J, Gersh BJ, Goldfinger JZ, Witzenbichler B, Guagliumi G, Dudek D, Kornowski R, Brener SJ, Parise H, Fahy M, McAndrew TC, Stone GW, Mehran R. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol 2014;114:9–16. [DOI] [PubMed] [Google Scholar]
- 15. Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2011;58:2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, Cannon CP. A call to ACTION (acute coronary treatment and intervention outcomes network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2009;2:491–499. [DOI] [PubMed] [Google Scholar]
- 18. Goyal A, de Lemos JA, Peng SA, Thomas L, Amsterdam EA, Hockenberry JM, Peterson ED, Wang TY. Association of patient enrollment in medicare Part D with outcomes after acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2015;8:567–575. [DOI] [PubMed] [Google Scholar]
- 19. Ariti CA, Cleland JG, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, McMurray JJ, Michelson EL, Ostergren J, Yusuf S. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J 2011;162:900–906. [DOI] [PubMed] [Google Scholar]
- 20. Roe MT, Chen AY, Thomas L, Wang TY, Alexander KP, Hammill BG, Gibler WB, Ohman EM, Peterson ED. Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am Heart J 2011;162:875–883.e1. [DOI] [PubMed] [Google Scholar]
- 21. D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993;32:382–7. [PubMed] [Google Scholar]
- 22. Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 23. Rao SV, Dai D, Subherwal S, Weintraub WS, Brindis RS, Messenger JC, Lopes RD, Peterson ED. Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. JACC Cardiovasc Interv 2012;5:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015;2015:341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr 2014;168:561–566. [DOI] [PubMed] [Google Scholar]
- 26. Twells LK, Gregory DM, Reddigan J, Midodzi WK. Current and predicted prevalence of obesity in Canada: a trend analysis. CMAJ Open 2014;2:E18–E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health 2013;103:1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality–fat or fiction? Nat Rev Cardiol 2011;8:233–237. [DOI] [PubMed] [Google Scholar]
- 30. Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, Khera A, Vega GL, McGuire DK, Grundy SM, de Lemos JA. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol 2015;65:2150–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Araneta MR, Kanaya AM, Hsu WC, Chang HK, Grandinetti A, Boyko EJ, Hayashi T, Kahn SE, Leonetti DL, McNeely MJ, Onishi Y, Sato KK, Fujimoto WY. Optimum BMI cut points to screen asian americans for type 2 diabetes. Diabetes Care 2015;38:814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.