Shared decision making (SDM) is a process whereby clinicians collaboratively help patients to reach evidence-informed and value-congruent medical decisions. This process is especially relevant in screening for conditions in which there is a close trade-off between harms and benefits. Many screening recommendations from the Canadian Task Force on Preventive Health Care (CTFPHC) are graded as weak recommendations in the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system, indicating that there is potential for both benefits and harms of screening. In these circumstances, individual patients might make different screening decisions depending on their individual values and preferences. Thus, SDM is essential for implementing weak recommendations that are congruent with patient values and preferences.
Shared decision making offers a structured way to incorporate evidence as well as patient values and preferences into medical decision making. This process can support conversations leading to better-informed decisions congruent with what matters most to patients. Just how does SDM happen in practice? Let’s start with a clinical case.
John is 66 years old; you’ve known him and his family for years. John takes a daily statin and a baby aspirin for primary prevention of coronary artery disease. Since the age of 18, he has smoked a pack a day. At a recent office visit, he tells you about another recent attempt to quit. This time, a nicotine patch was of no help and so he now asks you to prescribe varenicline. In conversation about this prescription, he reminds you that his father died of lung cancer. You then recall that the College of Family Physicians of Canada endorsed a recommendation on screening for lung cancer from the CTFPHC.
For adults aged 55 to 74 years with at least a 30 pack-year smoking history who currently smoke or quit fewer than 15 years ago, we recommend annual screening with low-dose computed tomography (CT) of the chest, up to 3 consecutive times. Screening should only be carried out in health care settings with expertise in early diagnosis and treatment of lung cancer (weak recommendation; low-quality evidence).1
Before this visit, John had no pre-formed opinion about screening for lung cancer. Like many people, he did not know this type of screening was possible. However, low-dose CT chest screening is available in your community. You ask if he has heard about this option. What are his views? You make it explicit to John that he faces a decision about whether to be screened or not and that you are willing to support him as he works through the options. And so begins the process of SDM around whether he should be screened.
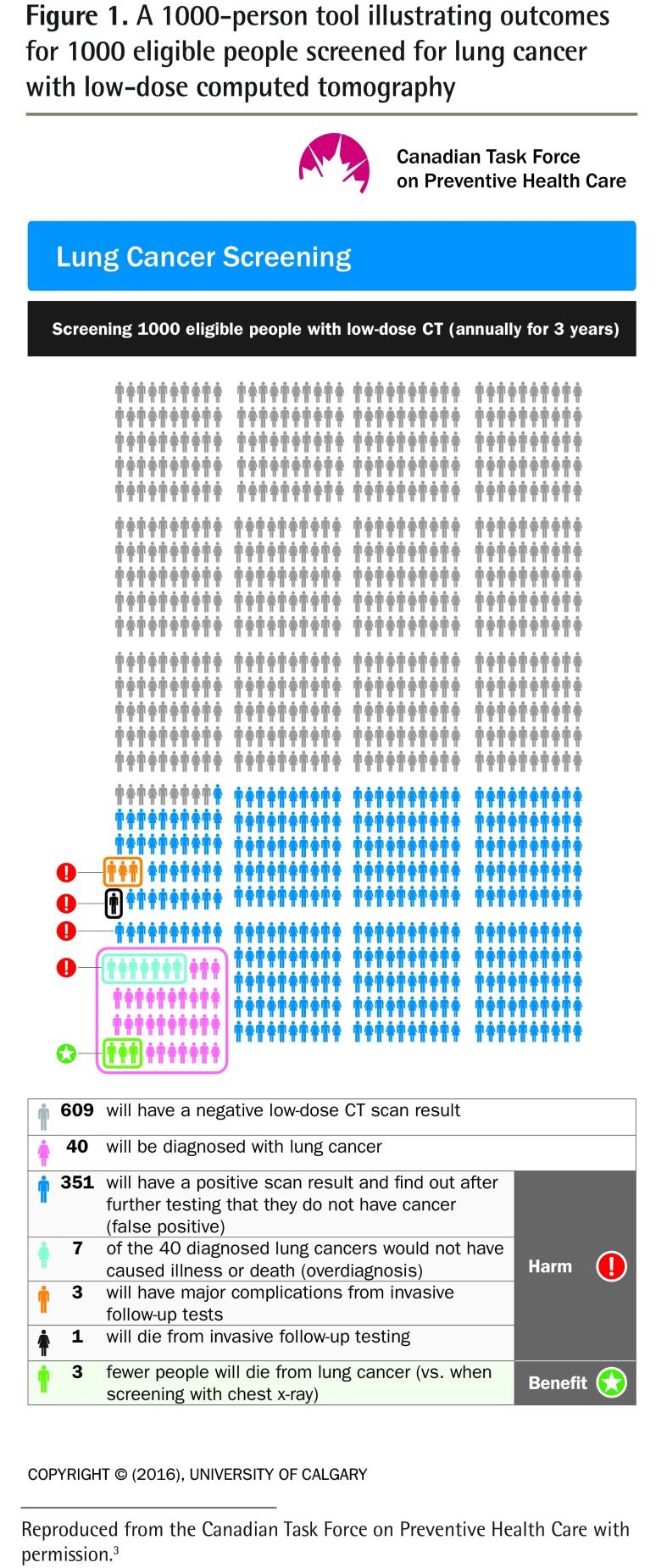
In smokers like John, about 16 people per 1000 would die from lung cancer over a median follow–up of 6.5 years.2 To better explain the options for whether to be screened, you ask John to consider the harms and benefits of screening for lung cancer. You do this by showing him the 1000-person tool in Figure 1.3
Figure 1.
A 1000-person tool illustrating outcomes for 1000 eligible people screened for lung cancer with low-dose computed tomography
Reproduced from the Canadian Task Force on Preventive Health Care with permission.3
This tool shows that among 1000 people screened annually for 3 years with low-dose CT, 3 fewer will die from lung cancer after treatment (vs when screened with a chest x-ray scan). However, 351 persons will be told that the findings from their scan were not normal but will learn after follow-up testing that they do not have cancer (false positives), and 1 person will die from invasive follow-up testing. An additional 7 will be diagnosed with a type of lung cancer that would not have caused illness or death. This is overdiagnosis, defined as the detection of a condition that would not have caused harm if it remained undetected.
As Marshall reminds us, even when the benefits of screening interventions are apparent, they are enjoyed only by very few.4,5 In screening for lung cancer with a CT scan, the task force 1000-person tool illustrates the close balance between the potential for harm and the potential for benefit. There is no one “correct” choice—rather each person is encouraged to decide for themselves whether to be screened based on the evidence and on what matters most to them.
What SDM is and what it is not
Let’s start by stating what it is not: With respect to a decision at hand, SDM is not about convincing the patient to follow the doctor’s recommendation. Nor is it about giving the patient whatever test or treatment he or she requests, or about leaving your patient to decide on his or her own.
There are 2 core elements to SDM: risk communication and values clarification. The former happens when we attempt to communicate the benefits and harms of interventions based on evidence. Values clarification involves clarifying what matters most to the patient and his or her family. Shared decision making becomes embedded within a process in which a health care provider and a patient relate to and influence each other as they collaborate in making a choice about health care.6 The choice must be congruent with what matters to the patient—his or her values and preferences are to be incorporated into the decision.
Preferences are inclinations toward or away from an option. Values are the underlying feelings that help determine preferences. They represent concepts relevant to the decision that matter to patients or their family members and include attributes relevant to a decision (eg, efficacy, side effects, cost, and related concepts such as patient priorities, life philosophies, and life circumstances).7,8
Steps of SDM
Shared decision making involves the following steps of medical decision making, and a decision might require more than one visit.
Identify a clear decision point: Does the patient know about the options (to be screened or not) and wish to be screened for lung cancer?
At this step, ensure both the patient and the clinician understand and make explicit what the decision is about and what the options are.
Provide information about the clinical problem and options at the decision point.
This involves the provision of balanced, evidence-based information regarding the options under consideration. The information could include what the evidence tells us about both the good and the bad outcomes and over what time period; the applicability of this information to individuals like the person who is making the decision; the robustness of the evidence, such as the extent of uncertainty around the estimate of effect; and the local availability of the options. Tools that compare the outcomes among screened and unscreened persons facilitate SDM.9,10
Elicit the patient perspective: Assess the patient’s view on what matters most.
Clinical teams play important roles in encouraging and supporting patients to become more active in health-related decisions. This is a learnable skill. Clinicians might wish to ask about any previous experiences, any related concerns, and more important, patient values and preferences regarding the different outcomes associated with the options under consideration.
Guide the patient toward a final decision.
This involves the challenge of providing guidance without being overly directive. In support of informed, value-based decisions on preventive health care, many clinicians will tell their patients about guideline recommendations from the CTFPHC. But a general recommendation (especially a weak recommendation, as in John’s case) about preventive health care is not targeted at specific individuals and their circumstances. Rather, it is based on the estimated benefits and harms across the entire target population. Clinicians know how the care of patients depends on their personal circumstances and yields choices that might not fit with any general recommendation.
Assess how comfortable the patient is with his or her decision.
At the end of the process, as a decision is made, the clinician can assess patient comfort with the decision by asking 4 brief questions, using the SURE screening test (Table 1).11 This can help both clinician and patient understand how much the patient feels informed, clear about his or her values, and supported. A negative response to 1 of the 4 items will flag any remaining issues for further attention.11
Table 1.
The SURE test: A response of yes scores 1 and a response of no scores 0; a score of < 4 is a positive result for the patient to be at risk of clinically significant decisional conflict.
| SURE ACRONYM | TEST |
|---|---|
| Sure of myself | Do you feel SURE about the best choice for you? |
| Understand information | Do you know the benefits and risks of each option? |
| Risk-benefit ratio | Are you clear about which benefits and risks matter most to you? |
| Encouragement | Do you have enough support and advice to make a choice? |
Copyright O’Connor and Légaré, 2008.
Reprinted from Légaré et al.11
In John’s case
After a pause in the conversation, John stands up, thanks you for the prescription for varenicline, and heads for the door. He says nothing more about his decision on screening for lung cancer. You are a bit surprised, as you never really had the chance to clarify his values and preferences on this matter.
As you start to type your clinical note, you glance again at the 1000-person tool on your screen. You reflect on how strange it was, in this brief clinical encounter, to have expected a decision on whether to be screened or not for lung cancer. For the future, you decide to offer patients the 1000-person tool on lung cancer screening from the CTFPHC to reflect upon and revisit later.
KEY POINTS
Shared decision making (SDM) offers a structured process to incorporate evidence as well as patient values and preferences into screening decisions.
Shared decision making is most relevant when there is a close trade-off between the harms and the benefits of a screening decision that could be altered by individual patient values and preferences.
The core elements of SDM are risk communication and values clarification. Values clarification considers both patient values and patient preferences. Preferences are inclinations toward or away from an option. Values are the underlying feelings that help determine preferences.
Patient decision aids are knowledge translation tools that facilitate SDM, but individuals might require more than one office visit to arrive at a decision about screening.
Footnotes
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de septembre 2017 à la page e377.
Competing interests
All authors have completed the International Committee of Medical Journal Editors’ Unified Competing Interest form (available on request from the corresponding author) and declare that they have no competing interests.
Suggested additional reading
1. Hoffmann TC, Légaré F, Simmons MB, McNamara K, McCaffery K, Trevena LJ, et al. Shared decision making: what do clinicians need to know and why should they bother? Med J Aust 2014;201(1):35-9.
2. Légaré F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns 2014;96(3):281-6. Epub 2014 Jul 3.
References
- 1.Lewin G, Morissette K, Dickinson J, Bell N, Bacchus M, Singh H, et al. Recommendations on screening for lung cancer. CMAJ. 2016;188(6):425–32. doi: 10.1503/cmaj.151421. Epub 2016 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. Epub 2011 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canadian Task Force on Preventive Health Care . Patient tool—benefits vs harms. Lung cancer screening. Calgary, AB: University of Calgary; 2016. Available from: http://canadiantaskforce.ca/wp-content/uploads/2016/05/ctfphclung-cancerharms-and-benefitsfinal.pdf. Accessed 2017 Jul 18. [Google Scholar]
- 4.Marshall KG. Prevention. How much harm? How much benefit? 4. The ethics of informed consent for preventive screening programs. CMAJ. 1996;155(4):377–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall KG. Screening for prostate cancer. How can patients give informed consent? Can Fam Physician. 1993;39:2385–90. [PMC free article] [PubMed] [Google Scholar]
- 6.Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32(2):276–84. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz A, Weiner SJ, Binns-Calvey A, Weaver FM. Providers contextualise care more often when they discover patient context by asking: meta-analysis of three primary data sets. BMJ Qual Saf. 2016;25(3):159–63. doi: 10.1136/bmjqs-2015-004283. Epub 2015 Jul 22. [DOI] [PubMed] [Google Scholar]
- 8.Lee YK, Low WY, Ng CJ. Exploring patient values in medical decision making: a qualitative study. PLoS One. 2013;8(11):e80051. doi: 10.1371/journal.pone.0080051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Zipkin DA, Umscheid CA, Keating NL, Allen E, Aung K, Beyth R, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161(4):270–80. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 11.Légaré F, Kearing S, Clay K, Gagnon S, D’Amours D, Rousseau M, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56:e308–14. Available from: www.cfp.ca/content/cfp/56/8/e308.full.pdf. Accessed 2017 Jul 14. [PMC free article] [PubMed] [Google Scholar]