Abstract
Background
Whether or not there is a relationship between the newly-discovered irisin hormone and bone healing is not yet known. The aim of this study was to investigate what effect irisin hormone has on the bone healing process.
Material/Methods
The study included 21 adult patients with a diagnosed fracture of the lower extremity (femur or tibia). Informed consent was obtained from all the patients. A total of four venous blood samples were taken from the patients: before fracture stabilization, then postoperatively on days 1, 10, and 60. In patients with femoral neck fracture who had hip prosthesis applied, bone tissue samples were taken from the removed femur head and irisin was determined immunohistochemically in muscle biopsies taken from the same patients.
Results
In analysis, it was revealed that the mean value of irisin 60 days after operation is significantly higher than the values of irisin before operation, 1 day after operation, and 15 day after operation (p<0.001, p<0.001, p<0.001, respectively). Intense staining was observed in compact bone tissue, muscle tissue, and in hypertrophic vascular endothelium within the Havers canal.
Conclusions
The level of irisin hormone increased in the bone union process and affects fracture healing due to irisin receptors in human bone tissue.
MeSH Keywords: Bone and Bones; Bone Diseases, Endocrine; Fracture Healing
Background
Bone healing occurs as a result of the complex events requiring sequential interaction between progenitor cells, osteoblasts, and mediators found in the region [1–3]. Approximately 7.9 million fractures occur per year in the USA, and in 5–20% of these, severe morbidity is associated with delayed healing and causes a reduction in productivity [4–6].
Irisin, which was discovered by Böstrom et al. in 2012, is formed of molecular 112 amino acids and is synthesized primarily in muscle tissue, and several other tissues such as the liver, pancreas saliva, and sweat glands [7]. Although many molecules play a role in fracture healing, irisin hormone has recently been reported to show an osteoblastic effect [8–11]. To the best of our knowledge, there has been no study to date that has investigated the relationship between fracture union and the recently discovered irisin hormone. The measurement of the levels of this hormone in patients with a bone fracture could provide an idea of the pathophysiology of fracture healing.
The aim of this prospective study was to determine the change in the level of irisin hormone in the blood and to show irisin hormone in human bone and muscle tissue immunohistochemically.
Material and Methods
Approval for the study was granted by the Clinical Research Ethics Committee of Kirikkale University Medical Faculty (2015/10–14). Informed consent was obtained from all the study participants. The study included a total of 21 adult patients who met the study inclusion criteria. All the patients had a lower extremity long bone fracture (femur or tibia) that was treated with closed intramedullary nailing. Patients were excluded from the study if they had an open or pathological fracture, required blood transfusion, had osteogenesis imperfecta, rickets, suspected malignancy, diabetes, chronic kidney failure, osteomyelitis, a history of gastric or intestinal surgery, hepatic or hematological disorder, thyroid dysfunction, or a hormonal disorder. All patients were operated on within 24 hours of presentation at the Emergency Department. A detailed anamnesis was taken from all the cases and a thorough physical examination was done.
Hormonal and biochemical measurements
Venous blood samples of 5 mL were taken in the morning between 0800 and 1000 from the patients a total of four times; before fracture stabilization and on postoperative days 1, 15, and 60. As peptides are easily separated by proteases within the cell, a protease inhibitor containing aprotinin was added to the tubes with the blood samples to be able to correctly measure the amount of irisin. After centrifugation, 1 N HCl was added to the obtained samples up to a volume of 1/10 and this ensured that the samples could remain stable for up to a year at −20°C. To determine the irisin levels in the collected samples, Active Human Irisin ELISA kit was used (YH Biosearch Laboratory Cat.No: YHB1765Hu).
Pathological examinations and irisin-specific immunoperoxidase test
In patients with a femoral neck fracture where hip arthroplasty was applied, the level of irisin hormone expression was examined in tissues using bone tissue samples taken from the femur head and muscle biopsy samples taken from the same patients. Samples extirpated from the femoral head during the operation were fixed in 4% buffered paraformaldehyde for 48 hours and were then decalcified with commercial EDTA disodium salt (Bio-optica, Italy W01030799) changed every other day. After 15 days the samples were trimmed to a thickness of 1 cm, and without washing the decalcified bone tissue under tap water, tissues of 3–5 mm were transferred to cassettes. For routine follow-up procedure, the tissues were passed through a series of alcohol (70%, 80%, 90%, 96% and 99.5%) and xylol, then embedded in paraffin and sections of 4–5 μm thickness were again cut from the paraffin blocks. The histology slices were deparaffinized and dehydrated. Then, to inhibit endogenous peroxidase activity, they were incubated for five minutes with 3% hydrogen peroxidase solution. For the antigen retrieval procedure, the sections were boiled for 30 minutes in a pH 6.0 citrate buffer and to prevent non-specific binding, they were incubated for seven minutes in 10% bovine serum albumin. Then the sections were incubated for 60 minutes in mouse anti-irisin (FNDC5) monoclonal primer antibody (Biorbyt, orb229904, UK) diluted to a ratio of 1: 200.
According to the recommendations in the horse-radish peroxidase chromogen kit (Invitrogen, USA), the sections were then processed first for 30 minutes with secondary biotin antibody, then for 30 minutes in an avidin-peroxidase solution. When antigenic structures became apparent with AEC chromogen substrate, all the samples were counter-stained with Mayer’s hematoxylin. The skeletal muscle biopsy samples taken during the operation were accepted as positive controls and sections applied with phosphate buffered saline rather than primer antibody were used as negative controls. Antigenic structures stained red with AEC were evaluated as positive and microphotographs were taken with an Olympus BX51 trinocular microscope.
Radiological evaluations
The Lane and Sandhu [12] radiological scoring system was used to evaluate the formation of new bone in the fracture line on anterior-posterior and lateral radiographs taken in the first, third, and sixth month postoperatively.
Statistical Methods
Statistical analysis was applied using SPSS v.16.0 software (SPSS Inc., Chicago, USA). Obtained data was assessed by a confidence interval (CI) of 95% and a two-tailed p<0.05 were determined to be statistically significant for all of the analyses. The numerical data was analyzed with Shapiro-Wilk test in terms of assessment of data whether they were parametric. The differences in the values of irisin before operation, 1 day after operation, 15 days after operation and 60 days after operation were assessed with general linear model. If there would be a significant difference in first assessment, Bonferroni test was used to evaluate the pairwise comparisons. All of the numerical data were expressed as the mean SD values. The differences categorical variables were assessed using the χ2 test.
Results
No statistically significant difference was determined in respect of age, gender, treatment applied, and the affected extremity (p>0.05). The mean values of irisin was before operation, 1 day after operation, 15 days after operation and 60 days after operation were 6.38±1.05 μg/mL, 6.35±1.01 μg/mL, 6.87±0.97 μg/mL and 12.87±1.66 μg/mL respectively (Figures 1, 2). In multi-variate testing, it was found that there were significant differences in repeated measures of irisin (F=182.6, partial eta squared=0.945, p<0.001). In post-hoc analysis, it was revealed that the mean value of irisin 60 days after operation was significantly higher than the values of irisin before operation, 1 day after operation, and 15 days after operation (p<0.001, p<0.001, p<0.001). The mean value of irisin 15 days after operation was also significantly higher than the values of irisin before operation, and 1 day after operation (p<0.001, p<0.001). The mean values of irisin before operation and 1 day after operation were found to be similar (p=0.98).
Figure 1.
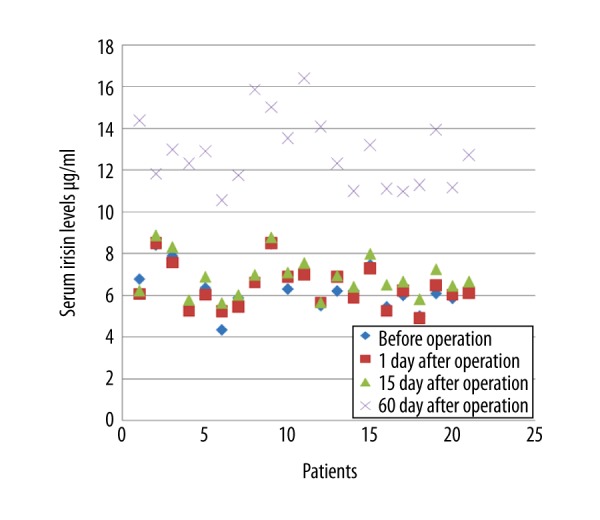
Serum irisin levels in patients with fracture before fracture stabilization and on postoperative days 1, 15, and 60.
Figure 2.
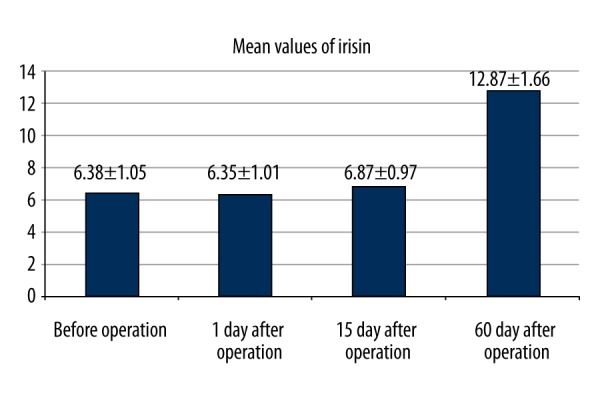
Serum irisin levels (mean ± standard error of the mean) before operation and on postoperative days 1, 15, and 60.
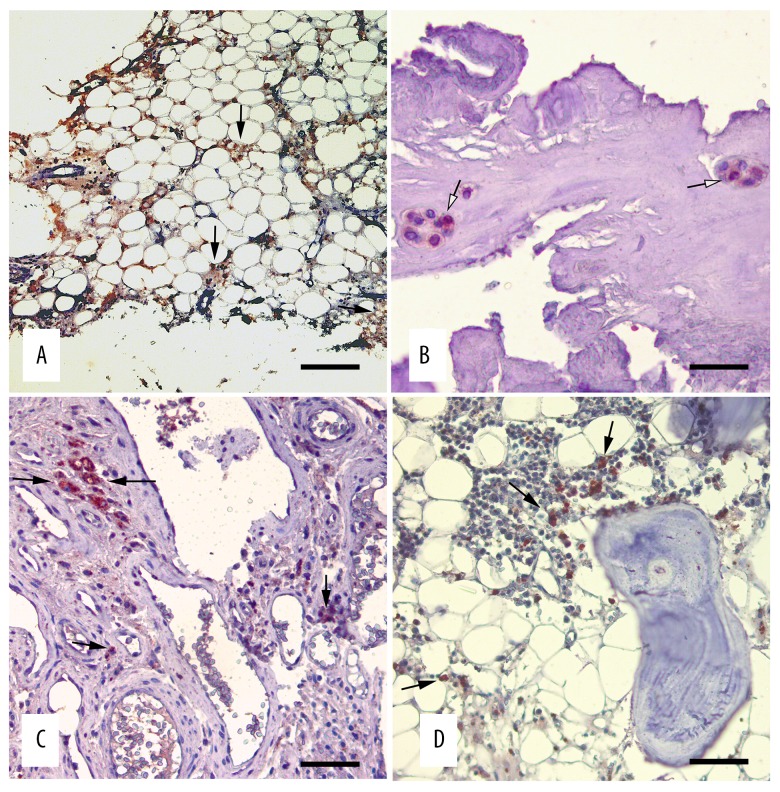
According to the irisin antibody staining test with avidin-biotin peroxidase, cytoplasmic intense immunopositive staining was observed in the vascular cells in the vesicular nucleus structure and extensive cytoplasm showing osteoblast morphology, especially in spongy bone tissue (Figure 3A, 3B). At the same time, a mild level of irisin antigen immunopositive reaction was seen in bone marrow cells (Figure 3C) between the spongy tissue.
Figure 3.
Irisin immunoreactivities in the spongy and compact bone tissue. Avidin-biotin immunoperoxidase technique, anti-irisin mouse antibody, Mayer’s hematoxylin counterstain. (A) Irisin activity in the capillary endothelia and osteoblasts (arrows), bar=250 micrometer (B) Irisin positive immunostaining in the osteoblast cytoplasm (arrows), bar=60 micrometer (C) Compact tissue irisin activity (arrows), bar=90 micrometer (D) Irisin immunopositive reactions in the bone marrow cells (arrows), bar=120 micrometer.
Although the osteoid matrix in compact bone tissue showed a mild degree of irisin activity, most of the hypertrophic vascular endothelium in Havers canals showed intense staining (Figure 3D). In the muscle tissue used as the positive control group, irisin immunopositive staining generally showed an intense and diffuse nature within the sarcoplasm. While irisin immunoreactivity was observed in interstitial vascular endothelium and in some mid-diameter artery media layers, there was no staining in any of the negative control sections (Figure 4).
Figure 4.
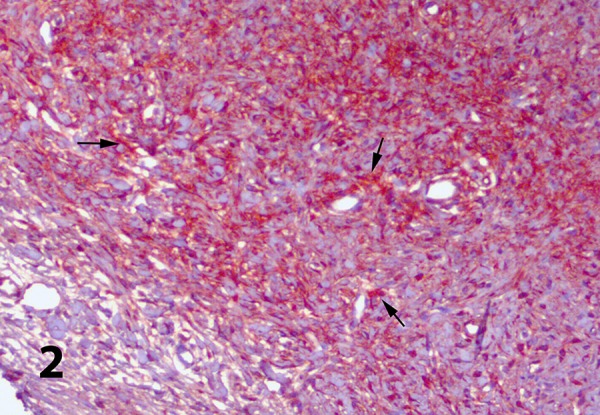
Irisin immunoreactivities in the skeletal muscle tissue. Avidin-biotin immunoperoxidase technique, anti-irisin mouse antibody, Mayer’s hematoxylin counterstain, bar=120 micrometer.
According to the Lane and Sandhu radiological evaluation results, osseous union was seen to have started in all patients in the radiological examinations made three months postoperatively. In the postoperative six-month examination, full osseous union was seen in all patients.
Discussion
The most important results of this study were the increase in the amount of irisin hormone in the blood in the bone union process and the finding that irisin receptors were seen in human bone cells. The mechanisms involved in the process of fracture healing and the sequence of events continue to be a subject attracting great attention in orthopedic science. Factors affecting fracture healing and research into the acceleration of healing are popular topics of research. Many different studies have been made on the subject of accelerating fracture healing in particular [13–16].
The effect of irisin on bone metabolism is one of the important subjects still under discussion [17]. Despite the association of reduced muscle strength with vitamin D deficiency and the risk of falling, no correlation has been shown between irisin and 25 (OH) D levels [9,18,19]. However, it has been shown that recombinant irisin (r-irisin) applied at a low dose to young mice increased cortical bone mineral density and changed bone geometry in a positive way. Furthermore, with an increase in irisin activating transcription factor 4 expression there was seen to be a positive effect on osteoblasts by an increase in the activity and differentiation of bone building cells [20–22]. Anastasilakis et al. reported a relationship between previous osteoporotic fractures and low irisin levels in the circulation of postmenopausal females with low bone mass [9]. In an in vitro study by Colaianni et al., it was shown that irisin increased osteoblast differentiation via the Wnt-β-catenin signaling pathway and osteoclast differentiation was prevented by the suppression of the RANK/RANKL pathway [23]. In another study, Zhang et al. reported that irisin facilitated osteoblast differentiation in osteoblastic cells and inhibited osteoclast differentiation by suppressing the c1 nuclear factor kappa B ligand (RANKL)/receptor activator of active T cells (NFAT) in Raw264.7 cells [24]. These findings suggest that irisin could be a bone anabolic factor marker.
In another study, it was shown that irisin partially supported osteoblast differentiation by the bone morphogenic protein (BMP) pathway, and inhibited osteoclast differentiation by suppressing the RANKL-Akt1/MITF/PU1-NFATc1 pathway [25]. In this way, irisin can be a mediator of the positive effects of exercise on the skeleton and could constitute a therapeutic target for bone diseases, including osteoporosis [26,27].
Conclusions
The results of this study showed that irisin levels in the blood increased in the fracture union process, and as irisin receptors were in human bone tissue, fracture union was affected. This study could form the basis of future research. As the presence of irisin has been shown in fracture union, perhaps in future studies, non-union could be eliminated with compensatory irisin hormone applied to bones with non-union of fractures.
Acknowledgments
We thank Professor Dr. Oğuz Kul from Kirikkale University, Faculty of Veterinary, Department of Pathology, who provided assistance in immunohistochemically evaluation in this study.
Footnotes
Conflict of interests
None.
Source of support: This study was funded by Kirikkale University Scientific Research Project (2015/001)
References
- 1.Cruess RL, Dumont J. Fracture healing. Can J Surg. 1975;18:403–13. [PubMed] [Google Scholar]
- 2.Simmons DJ. Fracture healing perspectives. Clin Orthop Relat Res. 1985;200:100–13. [PubMed] [Google Scholar]
- 3.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–55. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einhorn TA, Lane JM. Significant advances have been made in the way surgeons treat fractures. Clin Orthop Relat Res. 1998;355:2–3. [PubMed] [Google Scholar]
- 5.Calori GM, Albisetti W, Agus A, et al. Risk factors contributing to fracture non-unions. Injury. 2007;38:11–18. doi: 10.1016/s0020-1383(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 6.Zeckey C, Mommsen P, Andruszkow H, et al. The aseptic femoral and tibial shaft non-union in healthy patients – an analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop J. 2011;5:193–97. doi: 10.2174/1874325001105010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-likedevelopment of white fat and thermogenesis. Nature. 2012;481(7382):463–68. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9–10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 9.Anastasilakis AD, Polyzos SA, Makras P, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25:1633–42. doi: 10.1007/s00198-014-2673-x. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Liu H-J, Guo W-C, Yang J. Low serum concentrations of irisin are associated with increased risk of hip fracture in Chinese older women. Joint Bone Spine. 2017 doi: 10.1016/j.jbspin.2017.03.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Guo B, Zhang ZK, Liang C, et al. Molecular communication from skeletal muscle to bone: A review for muscle-derived myokines regulating bone metabolism. Calcif Tissue Int. 2017;100(2):184–92. doi: 10.1007/s00223-016-0209-4. [DOI] [PubMed] [Google Scholar]
- 12.Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18:213–25. [PubMed] [Google Scholar]
- 13.Serbest S, Tiftikci U, Tosun HB, et al. Is there a relationship between fracture healing and mean platelet volume? Ther Clin Risk Manag. 2016;12:1095–99. doi: 10.2147/TCRM.S108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calori GM, Albisetti W, Agus A, et al. Risk factors contributing to fracture non-unions. Injury. 2007;38:11–18. doi: 10.1016/s0020-1383(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 15.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Cai M. Leptin influences healing in the sprague dawley rat fracture model. Med Sci Monit. 2017;23:258–65. doi: 10.12659/MSM.899259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchis-Gomar F, Alis R, Pareja-Galeano H, et al. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine. 2014;46(3):674–77. doi: 10.1007/s12020-014-0170-9. [DOI] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Fredman L, et al. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010;95:5266–73. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: The effect of vitamin D on falls: A systematic review and metaanalysis. J Clin Endocrinol Metab. 2011;96(10):2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 20.Colaianni G, Grano M. Role of Irisin on the bone–muscle functional unit. Bonekey Rep. 2015;4:765. doi: 10.1038/bonekey.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colaianni G, Mongelli T, Colucci S, et al. Crosstalk between muscle and bone via the muscle-myokine irisin. Curr Osteoporos Rep. 2016;14:132–37. doi: 10.1007/s11914-016-0313-4. [DOI] [PubMed] [Google Scholar]
- 22.Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157–62. doi: 10.1073/pnas.1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colaianni G, Cuscito C, Mongelli T, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol. 2014;2014:902186. doi: 10.1155/2014/902186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Cheng J, Tu Q, Chen JJ. Effects of irisin on bone metabolism and its signal mechanism. J Bone Miner Res. 2013;28(Suppl 1):SA0185. [Google Scholar]
- 25.Zhang J, Wu Y, Yu L, et al. Exercise strengthens bone through myokine irisin. J Bone Miner Res. 2012;27(Suppl 1):1222. [Google Scholar]
- 26.DiVasta AD, Gordon CM. Exercise and bone: where do we stand? Metabolism. 2013;62:1714–17. doi: 10.1016/j.metabol.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Qiao YQ, Nie Y, Ma YX, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6:18732. doi: 10.1038/srep18732. [DOI] [PMC free article] [PubMed] [Google Scholar]