Abstract
Background and purpose
There is a paucity of data on persistence of secondary prevention medications among stroke survivors in resource-limited settings where stroke is on a rapid upward trajectory and its management severely challenged. To avert new cardiovascular events after stroke, preventive medications should be promptly instituted and used continuously. We report 1-year rates and determinants of persistent utilization of secondary prevention therapies after stroke in Ghana.
Methods
A retrospective observational study involving 418 stroke survivors enrolled into a Neurology clinic in a tertiary institution in central Ghana between January 2011 and December 2013. Data on demography, stroke type, risk factor profile and five secondary risk prevention medication classes namely antihypertensive, antiplatelet, statins, antidiabetic and anticoagulants were collected from patient charts. Persistence within first year after stroke was defined as continuation of all secondary preventive medications prescribed at enrollment to the Neurology clinic and it excluded 126 (≈ 30%) patients who could not complete 12 month follow up. Data was closed for analysis in June 2015 to allow for at least 12 months of follow-up.
Results
Rates of utilization of secondary preventive medications and its intensity were influenced by stroke type and prevailing vascular risk factors. In decreasing order, antihypertensive, lipid-modifying, anti-platelet, anti-diabetic medications and anti-coagulants were prescribed at frequencies (%) of 394 (94.3%), 303 (72.5%), 274 (65.6%), 61 (14.6%) and 2 (0.5%) respectively at enrollment into the Neurology clinic (n = 418). Overall, 92.1% of subjects (n = 292) under follow-up for 1 year were persistent on secondary prevention medications initiated at enrollment into the neurology clinic with medication class specific rates of 97.5% for antihypertensive, 94.8% for anti-platelets, 94.1% for statins, 85.7% for anti-diabetic and 50% for anticoagulants. Abuse of alcohol was significantly associated with non-persistence, adjusted OR (95% CI) of 3.08 (1.13–8.38).
Conclusion
Persistence of secondary preventive medications among stroke survivors in this resource-limited setting is excellent and comparable to those in resource-replete countries. There is however the need to investigate the causes of high attrition rates from care.
Highlights
-
•
Prompt initiation of CVD preventive drugs is recommended after stroke.
-
•
Hypertension, dyslipidemia, diabetes commonest modifiable CVD risk factors identified among Ghanaian stroke survivors.
-
•
Antihypertensive, lipid-modifying, anti-platelet agents prescribed at 94%, 73% and 66% respectively.
-
•
30% Loss to follow-up among stroke survivors at month 12.
-
•
92% of stroke survivors persisted on CVD preventive drugs at month 12.
1. Introduction
Antihypertensive agents, antithrombotic agents, lipid modifiers, anticoagulant, and anti-diabetic therapies form the foundation of modern secondary preventive strategies for strokes and other cardiovascular diseases. Prompt initiation and sustained utilization of these interventions are prescribed by various international guidelines [1], [2] and are associated with reduced stroke recurrences, and improved overall cardiovascular morbidity and mortality. Reports emanating from high-income countries (HIC) suggest that continual utilization of secondary prevention medications is challenged with short-to-medium term persistence ranging between 60 and 90% [3], [4], [5], [6].
Data on rates of utilization and persistence of evidence-based cardiovascular risk reduction therapies among stroke survivors from Low-to-Middle Income countries (LMIC) in particular sub-Saharan Africa are scarce. It is currently unknown whether the poor short- and long-term post-stroke outcomes in LMICs [7] are contributed to by the lack of implementation of guideline recommended interventions. This is because, although there is a commonality to the profile of risk factors for stroke globally, profound regional differences in the magnitude and direction of vascular risk factors exist [8], [9], [10]. Furthermore, the control of vascular risk factors world-wide is hampered by geographical variations in access and utilization of cardiovascular preventive medications as well as adherence to these therapies [11], [12]. Consequently, stroke mortality and mortality rates particularly for stroke are disproportionately higher in LMICs compared with HICs [13].
There is an urgent need to bridge the gap in knowledge on secondary risk prevention among stroke survivors in LMICs to facilitate the design of context-specific and culturally relevant interventions due to the projected increase in stroke burden in these settings. We therefore sought to evaluate the rates of utilization of antihypertensive agents, anti-thrombotic agents, lipid modifiers, anticoagulant and anti-diabetic therapy among stroke survivors and the 1-year persistence of these interventions in a tertiary referral hospital in Kumasi, Ghana. Our preliminary hypothesis was that rates of utilization of and 1-year persistence on secondary preventive medications may be lower among stroke survivors in a LMIC compared with data from HIC.
2. Methods
This was a retrospective study approved by the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, and the Komfo Anokye Teaching Hospital, Kumasi, Ghana. The study was enacted at the Neurology Clinic of the Komfo Anokye Teaching Hospital in Kumasi, Ghana. Kumasi is the second largest city in Ghana with an estimated population of 4 million inhabitants. The Neurology clinic was established in 2011 by FSS and runs once a week receiving referrals for adults > 16 years with neurologic disorders from 6 out of the 10 administrative regions of Ghana and serves an estimated population of 10 million as previously described [14]. In Ghana, patients registered on the National Health Insurance Scheme have the cost of secondary prevention medications and indeed drugs considered essential covered by the scheme. 90% of stroke survivors in this cohort were registered with NHIS.
Stroke survivors are referred to the Neurology clinic upon discharge from the ward as in-patients or from surrounding hospitals and clinics for follow-up care mainly for secondary prevention and rehabilitation. At enrollment into the clinic, patient charts from in-patient are used at the Neurology clinic for follow-up. A data collection form was designed to extract the relevant variables from the medical charts of subjects. Data collected for the present analysis include age, gender, marital status, occupation, religion, type of stroke, blood pressure measurements on admission and discharge as in-patients and vascular risk factors as well as medications including anti-hypertensive, anti-thrombotic agents (antiplatelets and anticoagulants), statins and anti-diabetic medications prescribed on discharge, at enrollment and during the first 12 months of follow-up at the Neurology Clinic. Typically stroke patients are scheduled for follow-up visits on months 1, 3, 6 and 12 with non-scheduled visits where necessary. At clinic visits, stroke survivors are routinely evaluated by the attending Neurologists (FSS or JA) and their findings are documented in patients' medical charts. The present analysis involves all 418 stroke survivors who enrolled into the Neurology clinic between January 2011 through to December 2013 and data was closed for analysis in June 2015 to allow for at least 12 months of follow-up. The following definitions were used:
-
1.
Stroke was defined as a sudden onset of focal or global cerebral, spinal or retinal dysfunction of a vascular cause with evidence of infarction of the central nervous system tissue. Stroke types were determined for those with cranial CT scans performed within 10 days post-stroke to allow for delineation of ischemic from hemorrhagic strokes and rule out stroke mimics. For subjects without the means to perform neuroimaging studies, stroke diagnosis was made on clinical grounds.
-
2.
We assessed utilization rates of drug classes by dividing the number of subjects on the drug by the total number at each time point (at in-patient discharge, at enrollment into the Neurology clinic, at months 3 and 12 of follow-up) and by stroke type.
-
3.
Persistence on medications over 12 months was defined as continuation of all medications prescribed at enrollment into the Neurology clinic. To be eligible for determination of persistence, documentation of being on a specific medication or class of medication at enrollment into Neurology clinic was required, as was requirement for subjects to have completed 12 months of follow-up. Persistence was determined for the following 5 medication classes: antihypertensive, lipid modifier, antithrombotic, anticoagulant, and anti-diabetic medications.
2.1. Statistical analysis
Means and medians were compared using the Student's t-test or Mann-Whitney's U test for paired comparisons and Analysis of variance or Kruskal Wallis tests for > 2 group comparisons. Proportions were compared using the Chi-squared test. A multivariable logistic regression model was used to evaluate the demographic and clinical determinants on non-persistence on any of the 5 medication classes at month 12. Regimen non-persistence was analyzed as an all-or-none variable (i.e. subjects who remained on all medication classes prescribed at enrollment visit at Neurology clinic at the 12-month follow-up were considered persistent, whereas subjects who stopped at least one class of medication prescribed at enrollment were non-persistent). In all analysis, two-tailed p-values < 0.05 were considered statistically significant with no adjustments for multiple comparisons. Data missing at random were excluded from analysis. Statistical analysis was performed using SPSS version 19.
3. Results
3.1. Demographic & clinic characteristics of stroke survivors at presentation to Neurology clinic according to stroke types
Of the 418 stroke survivors who enrolled into the Neurology clinic, 139 had ischemic stroke (33.2%), 77 had hemorrhagic stroke (18.4%) and 203 (48.4%) were undetermined due either to lack of a cranial CT scan examination due to inability afford the investigation or late presentation after incident stroke thus decreasing sensitivity of radiological typing of stroke. Compared with ischemic stroke subjects, hemorrhagic stroke patients were significantly younger, likely to be employed and married as shown in Table 1. Furthermore, 94% of hemorrhagic stroke survivors versus 85% of ischemic stroke survivors (p = 0.02) had hypertension while 26% of ischemic stroke patients compared with 12% (p = 0.003) of hemorrhagic stroke survivors had diabetes mellitus. Median (IQR) duration of hospitalization of hemorrhagic stroke survivors of 8 (6–12) days was significantly longer than that of ischemic and undetermined stroke survivors of 6 (5–9) and 6 (4–8) days respectively, p < 0.0001.
Table 1.
Comparison of demographic, risk factors and clinical features among subjects according to stroke types at discharge.
| Characteristic | Ischemic stroke N = 139 (33.2%) |
Hemorrhagic stroke N = 77 (18.4%) |
Undetermined stroke type n = 202 (48.4%) |
p-value | p-value# |
|---|---|---|---|---|---|
| Age, mean ± SD | 62.5 ± 14.3 | 53.7 ± 12.8 | 61.1 ± 13.2 | < 0.0001 | < 0.0001 |
| Male gender, n (%) | 65 (46.8) | 49 (63.6) | 95 (47.0) | 0.03 | 0.02 |
| Currently employed, n (%) | 63 (45.3) | 55 (71.4) | 93 (46.0) | 0.0002 | 0.0002 |
| Married, n (%) | 62 (44.6) | 46 (59.7) | 90 (44.6) | 0.06 | 0.03 |
| Frequency of vascular risk factors, n (%) | |||||
| Hypertension | 119 (85.6) | 73 (94.8) | 188 (93.1) | 0.04 | 0.02 |
| Dyslipidemia | 43 (51.2) | 22 (44.0) | 49 (44.5) | 0.60 | 0.42 |
| Diabetes mellitus | 36 (25.9) | 10 (13.0) | 37 (18.3) | 0.06 | 0.03 |
| Alcohol abuse | 22 (15.8) | 18 (23.4) | 22 (10.9) | 0.03 | 0.17 |
| Cigarette smoking | 6 (4.3) | 3 (4.0) | 7 (3.5) | 0.92 | 0.88 |
| SBP on admission, mean ± SD | 151.6 ± 36.5 | 171.3 ± 33.1 | 169.2 ± 35.2 | < 0.0001 | 0.0003 |
| DBP on admission, mean ± SD | 95.6 ± 21.2 | 106.4 ± 21.3 | 101.4 ± 22.0 | < 0.0001 | < 0.0001 |
| SBP on discharge, mean ± SD | 130.1 ± 21.8 | 134.3 ± 15.8 | 132.0 ± 17.1 | 0.35 | 0.17 |
| DBP on discharge, mean ± SD | 78.9 ± 13.4 | 84.0 ± 14.7 | 80.7 ± 12.1 | 0.05 | 0.02 |
| Duration of admission, median (IQR) | 6 (5–9) | 8 (6–12) | 6 (4–8) | < 0.0001 | 0.02 |
| MRS at discharge, median (IQR) | 3 (2–4) | 3.5 (1–5) | 3 (2–4) | 0.71 | 0.72 |
55, 27, 93 subjects with ischemic, hemorrhagic and undetermined stroke type respectively did not have fasting lipid results.
p-value for comparison between ischemic and hemorrhagic stroke.
3.2. Secondary prevention medications prescribed at discharge, enrollment and follow-up at Neurology clinic
In decreasing order, antihypertensive, lipid-lowering, anti-platelet, diabetes medications and anti-coagulants were prescribed at frequencies (%) of 394 (94.3%), 303 (72.5%), 274 (65.6%), 61 (14.6%) and 2 (0.5%) respectively at enrollment into the Neurology clinic for care. However as shown in Table 2, the rates of prescription of these secondary preventive therapies varied significantly according to stroke type and the presence of vascular risk factors such as hypertension, diabetes mellitus and dyslipidemia. Among 139 subjects with CT scan confirmed ischemic strokes who were eligible for antihypertensive agents, lipid modifiers and antithrombotic agents according guidelines, 94 (67.6%) were initiated on all three agents, 36 (25.9%) were on 2 out of 3, 7 (5.0%) were on 1 out of 3 and only 2 (1.4%) was not on any of these three agents.
Table 2.
Comparison of frequencies of utilization of antihypertensive, antithrombotic, statin and anti-diabetic medications at enrollment into Neurology clinic according stroke types.
| Medication | Ischemic stroke n = 139 (33.2%) |
Hemorrhagic stroke n = 77 (18.4%) |
Undetermined stroke type n = 202 (48.4%) |
Total N = 418 |
p-value | p-value# |
|---|---|---|---|---|---|---|
| Antihypertensive therapy | 123 (88.5) | 76 (98.7) | 195 (96.5) | 394 (94.3) | 0.001 | 0.008 |
| ACE-Inhibitors | 65 (46.8) | 53 (68.8) | 107 (53.0) | 225 (53.8) | 0.007 | 0.002 |
| ARB | 61 (43.9) | 31 (40.3) | 80 (39.6) | 172 (41.1) | 0.72 | 0.52 |
| Beta blockers | 3 (2.2) | 6 (7.8) | 13 (6.4) | 22 (5.3) | 0.12 | 0.05 |
| Calcium channel blockers | 90 (64.7) | 65 (84.4) | 161 (79.7) | 316 (75.6) | 0.0009 | 0.002 |
| Diuretics | 23 (16.5) | 33 (42.9) | 58 (28.7) | 114 (27.3) | 0.0001 | < 0.0001 |
| Hydrallazine | 1 (0.7) | 5 (6.5) | 13 (6.4) | 19 (4.5) | 0.03 | 0.01 |
| Methyldopa | 16 (11.5) | 27 (35.1) | 43 (21.3) | 86 (20.6) | 0.0002 | < 0.0001 |
| # of antihypertensive, median (IQR) | 2 (1–2) | 3 (2–4) | 2 (2–3) | < 0.0001 | < 0.0001 | |
| Antiplatelet therapy | 118 (84.9) | 5 (6.5) | 151 (74.8) | 274 (65.6) | < 0.0001 | < 0.0001 |
| Aspirin | 116 (98.3) | 5 (100.0) | 147 (97.4) | 268 (64.1) | ||
| Clopidogrel | 2 (1.7) | 0 (0.0) | 4 (2.6) | 6 (1.4) | ||
| Anticoagulants | 2 (2.2) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 0.13 | 0.29 |
| Statin therapy | 120 (86.3) | 41 (53.2) | 142 (70.3) | 303 (72.5) | < 0.0001 | < 0.0001 |
| Low intensity | 4 (3.3) | 3 (7.3) | 20 (14.1) | 27 (6.5) | ||
| Moderate intensity | 93 (77.5) | 27 (65.9) | 100 (49.5) | 220 (72.6) | ||
| High intensity | 23 (19.2) | 11(26.8) | 22 (15.4) | 56 (13.4) | ||
| Anti-diabetic therapy | 25 (18.0) | 7 (9.1) | 29 (14.4) | 61 (14.6) | 0.04 | 0.08 |
| Insulin | 2 | 0 | 2 | 4 (1.0) | ||
| Oral hypoglycemics | 25 | 7 | 29 | 61 (14.6) | ||
| Total # medications, median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.04 | 0.10 |
High intensity statin = Rosuvastatin 20–40 mg or Atorvastatin 40–80 mg.
p-value for comparison between ischemic and hemorrhagic stroke.
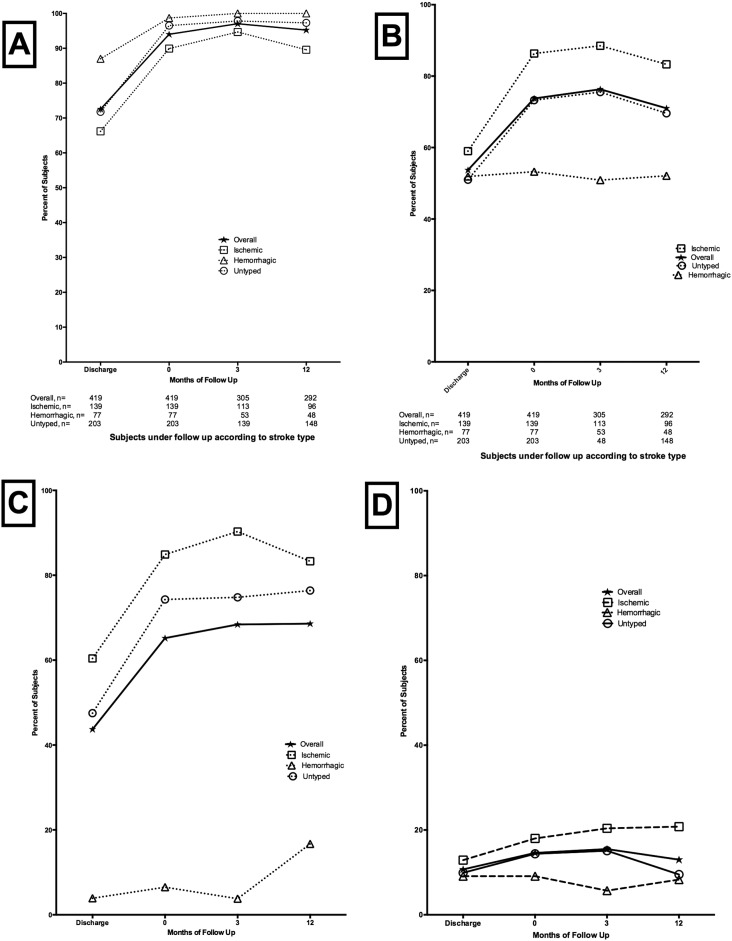
Rates of utilization of all secondary preventive interventions increased at enrollment into the Neurology clinic compared to the discharge from the ward. During follow-up, rates of utilization remained > 90% overall for antihypertensive medications, > 70% for statins, > 65% for anti-platelets, > 15% for anti-diabetic medications for the proportion of patients who remained under follow-up. (Fig. 1A–D).
Fig. 1.
Utilization of secondary preventive medications on discharge, enrollment and 12-month follow-up at Neurology clinic according to stroke type. A. Antihypertensive medications, B. Statins, C. Anti-platelets, D. Anti-diabetic medications.
3.3. Persistence of secondary preventive therapy at year 1 after stroke
To calculate 1-year persistence rates, we excluded 126 (30%) subjects who did not complete 12-months follow-up. As shown in Table 3, there were non-significant differences in the demographic or clinical characteristics except for alcohol abuse which was commoner among defaulters.
Table 3.
Comparison of demographic and clinical features of defaulters versus non-defaulters.
| Characteristic | Non-defaulters N = 292 |
Defaulters N = 126 |
p-value |
|---|---|---|---|
| Age, mean ± SD | 60.0 ± 13.5 | 60.8 ± 14.7 | 0.56 |
| Male, n (%) | 146 (49.8) | 63 (50.0) | 0.97 |
| Employed, n (%) | 142 (48.5) | 70 (55.5) | 0.18 |
| Stroke type, n (%) | 0.27 | ||
| Ischemic | 97 (33.2) | 29 (23.0) | |
| Hemorrhagic | 48 (16.4) | 42 (33.3) | |
| Undetermined | 147 (50.4) | 55 (43.7) | |
| Modified Rankin Score on discharge, median (IQR) | 3 (1–4) | 3 (2–5) | 0.15 |
| Risk factors, n (%) | |||
| Hypertension | 266 (91.9) | 114 (90.5) | 0.92 |
| Dyslipidemia | 81 (27.7) | 33 (26.2) | 0.72 |
| Diabetes mellitus | 55 (18.8) | 28 (14.6) | 0.42 |
| Alcohol excess | 35 (12.0) | 27 (21.4) | 0.01 |
| Cigarette smoking | 11 (3.8) | 5 (4.0) | 0.92 |
Overall, 92.1% of subjects under follow-up were persistent on secondary prevention medications initiated at enrollment into the Neurology clinic. The rates for medication classes were 97.5% for antihypertensive, 94.8% for anti-platelets, 94.1% for statins, 85.7% for anti-diabetic and 50% for anticoagulants (Table 4). Predictors associated with persistence overall in a bivariate analysis identified alcohol abuse and employment status to be significantly associated while dyslipidemia and cumulative number of medications were marginally associated. After adjustment for confounding variables, only alcohol abuse retained a significant association with non-persistence with adjusted OR (95% CI) of 3.08 (1.13–8.38), p = 0.03 (Table 5).
Table 4.
Persistence of secondary preventive medications at month 12 by drug type and class.
| Drug class/drug | Prescription at enrollment at Neurology clinic (%)a (total n = 292) |
Persistence, n (%) |
|---|---|---|
| Overall | 292 (100.0) | 269 (92.1) |
| Antihypertensive | 276 (94.5) | 269 (97.5) |
| ACE-I | 151 (51.7) | 129 (85.4) |
| ARB | 117 (40.1) | 110 (94.0) |
| Beta-blockers | 16 (5.5) | 15 (93.8) |
| CCB | 226 (77.4) | 213 (94.2) |
| Diuretics | 87 (29.8) | 72 (82.8) |
| Hydralazine | 15 (5.1) | 10 (66.7) |
| Methyldopa | 67 (22.9) | 59 (88.1) |
| Anti-platelets | 192 (65.8) | 182 (94.8) |
| Aspirin | 190 (65.1) | 179 (94.2) |
| Clopidogrel | 3 (1.0) | 3 (100.0) |
| Statins | 203 (69.5) | 191 (94.1) |
| Low-intensity | 23 (7.9) | 18 (78.3) |
| Moderate-intensity | 153 (52.4) | 142 (92.8) |
| High-intensity | 27 (9.2) | 22 (81.5) |
| Anti-diabetics | 42 (14.4) | 36 (85.7) |
| Insulin | 3 (1.0) | 3 (100.0) |
| Oral agents | 42 (14.4) | 36 (85.7) |
| Anticoagulant (Warfarin) | 2 (0.68) | 1 (50.0) |
This excludes 126 patients who did not complete 12-month follow-up.
Table 5.
Predictors of non-persistence on secondary preventive medications at month 12.
| Predictor | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) |
p-value |
|---|---|---|---|---|
| Age (each 10-year increase) | 0.86 (0.63–1.18) | 0.35 | ||
| Gender | ||||
| Male | 1.00 | 0.83 | ||
| Female | 1.10 (0.47–2.58) | |||
| Employment status | ||||
| Unemployed | 1.00 | 0.05 | 1.00 | 0.13 |
| Employed | 2.52 (1.00–6.32) | 2.07(0.80–5.36) | ||
| Stroke type | ||||
| Ischemic stroke | 1.00 | |||
| Hemorrhagic | 0.79 (0.23–2.67) | 0.71 | ||
| Undetermined | 0.57 (0.22–1.45) | 0.24 | ||
| Hypertension | 0.65 (0.18–2.36) | 0.52 | ||
| Diabetes mellitus | 2.01 (0.79–5.16) | 0.15 | ||
| Alcohol abuse | 3.77 (1.43–9.94) | 0.007 | 3.08 (1.13–8.38) | 0.03 |
| Cigarette smoking | 1.18 (0.14–9.63) | 0.88 | ||
| Dyslipidemia | 0.36 (0.11–1.15) | 0.08 | ||
| Number of medications on discharge (each increase) | 1.34(0.97–1.85) | 0.08 | ||
| Modified Rankin Scale on discharge | ||||
| 3 or less | 1.00 | 0.57 | ||
| > 4 | 1.31(0.52–3.29) |
4. Discussion
Among this West African cohort of stroke survivors, we found that the rates of utilization of secondary preventive medications within the first year after stroke were influenced by the frequencies of vascular risk factors and stroke type. Accordingly antihypertensive medications- for the cardinal vascular risk for stroke - were the most prescribed secondary preventive therapy among the 5 drug classes studied. The moderate utilization rates of statins and anti-platelets observed were driven by the high proportions of subjects with hemorrhagic and undetermined stroke types where indications for the use of these agents are not clearly defined or supported by guidelines. The low rates of utilization of anti-coagulants may reflect the lower rates of prevalent or detected cardio-embolic strokes in this relatively younger stroke population compared with those in advanced world.
The persistent use of secondary preventive medications among patients under follow-up remained well over 90% for antihypertensive, statins and anti-platelets in our cohort and is comparable with data from high-income settings. The Preventing Recurrence Of Thromboembolic Events through Coordinated Treatment (PROTECT) [5] study was a single-center quality improvement interventional study involving 128 patients followed for 12 months. In PROTECT, 89% maintained ACE-inhibitors or ARB compared with 85.4% and 94% respectively in the present cohort, while statins and antithrombotic were maintained at 99% and 98% respectively in that study compared with 94% and 95% in the present study. Our data on overall rates of persistence is also in accord with reports from a study among Canadian stroke survivors where self-reported persistence for all categories of stroke prevention medications were > 90% [15]. However, in the multi-center Adherence evaluation After Ischemic stroke Longitudinal (AVAIL) Registry [4] in the United States only 66% were regimen persistent after 12 months follow-up similar to the Swedish Riks-Stroke Register with persistence rates of 74% for antihypertensive medications, 56% for statins over a 2-year period [3]. In Low-to-Middle Income countries, data on regimen persistence after stroke is scanty. Among Chinese cohorts, antihypertensive persistence at year 1 among stroke survivors was reported at only 37% of subjects [16] and up to a third of stroke survivors were non-persistent by month 3 post-stroke [17]. Overall, our data suggest that higher persistence rates are possible in resource-limited settings within the context of a dedicated neurology service which may translate into reduced risk for recurrent vascular events, although attrition rates were admittedly high.
A recent review of data from the multicenter Vitamin Intervention for Stroke Prevention (VISP) trial involving 3680 recent noncardioembolic US stroke patients aged 35 years or older who were followed for 2 years to assess the effects of optimal combination of evidence-based drug therapies including antihypertensive agents, lipid modifiers, and antithrombotic agents on the risk of recurrent vascular events after stroke has been reported. Patients in that study were categorized by appropriateness level 0 to III depending on the number of drugs prescribed divided by the number of drugs potentially indicated for each patient (0 = none of the indicated medications prescribed and III = all indicated medications prescribed). The investigators found that compared with level 0: the adjusted hazard ratio of recurrent stroke for level I was 0.51 (95% CI, 0.21–1.25), level II 0.50 (0.23–1.09), and level III 0.39 (0.18–0.84) and similarly for the composite risk of stroke/coronary heart disease/vascular death strongly highlighting that optimal combination of secondary prevention medication classes after a recent non-cardioembolic stroke is associated with a significantly lower risk of stroke, major vascular events, and death [18]. Although we did not assess recurrent vascular events in the present study, up to 68% of ischemic stroke subjects in the present cohort were prescribed all three medication classes, 26% on two of three and only 5% on one out of the three evidence based secondary preventive medications. It is however important to note the following differences between our cohort and those from the high-income countries. First, ≈ 50% had stroke type undetermined and 18% had hemorrhagic strokes compared with the scenario in developed world setting where > 80% have ischemic stroke phenotype. Hence previously published studies on persistence have focused primarily on ischemic strokes and transient ischemic attacks [4], [5], [15], [16], [17] although 9.5% of subjects in the Swedish cohort had hemorrhagic strokes [3]. Second, guideline recommendations on use of statins and anti-platelets in hemorrhagic or undetermined strokes, a common scenario in routine practice in LMIC, are not clearly defined. It is noteworthy that ≈ 75% and 70% of subjects with undetermined stroke types were initiated on anti-platelets and statin therapy. Third, persistence on therapy was assessed during each clinic visit using prescriptions of medications and medication possession at hospital visit due to absence of an electronic prescription register in our setting. Fourth, a high attrition rate ≈ 30% was observed in our cohort and the reasons for this are currently being investigated by our group but it is likely that a proportion of defaulting patients might have either died or resorted to alternative medicines. In the AVAIL registry, only 15% attrition rate was reported [4]. However, the high default rate in the present cohort may not have led to a systematic bias since profound differences were not noted in the demographic and clinical characteristics of defaulters compared with non-defaulters.
Interventions to improve patient adherence and persistence on secondary preventive medicines has been at the forefront of recent discussions by the World Health Organization [19] for which various interventions including tele-medicine approaches are being evaluated some of which have shown preliminarily promising results with others on-going and due to report [20], [21], [22], [23]. Implementation and widespread penetration of these interventions into routine care particularly in LMICs is anticipated as well as strategies to simplify treatment for stroke survivors such as the use of the Cardiovascular Polypill which contain antihypertensive agents, lipid modifiers and anti-thrombotic agents which are also undergoing experimental trials to evaluate their efficacy in both primary and secondary prevention [24], [25], [26], [27], [28], [29].
It is noted as a limitation that this is a single-center study in a tertiary institution in a low-income country and may not reflect practice in many institutions of care in developing countries where post-stroke care is handled by General physicians and Physician Assistants due to the perennial shortage of neurologists. Adherence rates were not reported in the present study due to the lack of documentation of medication and therapeutic lifestyle adherence in patient records. As part of routine care, nurses and clinicians educate stroke survivors on adherence to secondary prevention therapy. The observed associations between non-persistence and excessive use of alcohol may not be causal owing to the retrospective study design.
In conclusion we report that 1-year persistence of > 90% to secondary preventive therapy after stroke is achievable in a resource-limited setting. The reasons for high default rates among stroke survivors need a closer interrogation in subsequent studies.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Grant U01 NS079179 and R21 NS094033 from the National Institute of Neurological Disorder and Stroke.
References
- 1.Kernan W.N., Ovbiagele B., Black H.R. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 2.The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee Guidelines for management of ischemic stroke and transient ischemic attack 2008. Cerebrovasc. Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 3.Glader E.L., Sjolander M., Eriksson M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41(2):397–401. doi: 10.1161/STROKEAHA.109.566950. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell C.D., Olson D.M., Zhao X. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77(12):1182–1190. doi: 10.1212/WNL.0b013e31822f0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovbiagele B., Kidwell C., Selco S. Treatment adherence rates one year after initiation of a systematic hospital-based stroke prevention program. Cerebrovasc. Dis. 2005;20:280–282. doi: 10.1159/000087711. [DOI] [PubMed] [Google Scholar]
- 6.Hamann G.F., Weimar C., Glahn J. Adherence to secondary stroke prevention strategies- results from the German Stroke Data Bank. Cerebrovasc. Dis. 2003;15:282–288. doi: 10.1159/000069490. [DOI] [PubMed] [Google Scholar]
- 7.Owolabi M.O., Akarolo-Anthony S., Akinyemi R. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015;26:S27–S38. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donnell M.J., Xavier D., Liu L. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 9.Lavados P.M., Hennis A.J., Fernandes J.G. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6(4):362–372. doi: 10.1016/S1474-4422(07)70003-0. [DOI] [PubMed] [Google Scholar]
- 10.Feigin V.L., Roth G.A., Naghavi M. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016 June;9 doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 11.Khatib R., McKee M., Shannon H. PURE Study investigators. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387(10013):61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S., Islam S., Chow C.K. PURE study investigators. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378(9798):1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthi R.V., Moran A.E., Feigin V.L. Stroke prevalence, mortality and disability-adjusted life years in adults aged 20–64 years in 1990–2013: data from the global burden of disease 2013 study. Neuroepidemiology. 2015;45(3):190–202. doi: 10.1159/000441098. [DOI] [PubMed] [Google Scholar]
- 14.Sarfo F.S., Akassi J., Badu E. Profile of neurological disorders in an adult neurology clinic in Kumasi, Ghana. eNeurologicalSci. 2016;3:69–74. doi: 10.1016/j.ensci.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lummis H., Sketris I., Gubitz G. Medication persistence rates and factors associated with persistence in patients following stroke: a cohort study. BMC Neurol. 2008;10:25. doi: 10.1186/1471-2377-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J., Ju Y., Wang C. Patterns and predictors of antihypertensive medication used 1 year after ischemic stroke or TIA in urban China. Patient Prefer. Adherence. 2013;7:71–79. doi: 10.2147/PPA.S39800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji R., Liu G., Shen H. Persistence of secondary prevention medications after acute ischemic stroke or transient ischemic attack in Chinese population: data from China National Stroke Registry. Neurol. Res. 2013;35(1):29–36. doi: 10.1179/1743132812Y.0000000107. [DOI] [PubMed] [Google Scholar]
- 18.Park J.H., Ovbiagele B. Optimal combination secondary prevention drug treatment and stroke outcomes. Neurology. 2015;84:50–56. doi: 10.1212/WNL.0000000000001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . 2003. Adherence to Long-term Therapies: Evidence for Action. [PubMed] [Google Scholar]
- 20.Jakobsson S., Irewall A.L., Bjorklund F. Cardiovascular secondary prevention in high-risk patients: a randomized controlled trial sub-study. BMC Cardiovasc. Disord. 2015;15:125. doi: 10.1186/s12872-015-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irewall A.L., Ogren J., Bergstrom L. Nurse-led, telephone-based, secondary preventive follow-up after stroke or transient ischemic attack improves blood pressure and LDL cholesterol: results from the first 12 months of the randomized, controlled NAILED stroke risk factor trial. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal A.K., Shaikh Q., Pasha O. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored short messaging service (SMS)-SMS4Stroke study. BMC Neurol. 2015;15:212. doi: 10.1186/s12883-015-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarfo F.S., Treiber F., Jenkins C. Phone-based intervention under nurse guidance after stroke (PINGS): study protocol for a randomized controlled trial. Trials. 2016;17(1):436. doi: 10.1186/s13063-016-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusuf S. The International Polycap Study 3 (TIPS) 2015. ClinicalTrials.govwww.clinicaltrials.gov (Identifier: NCT0164637)
- 25.Heart Outcomes Prevention Evaluation-3 (HOPE-3) 2015. https://clinicaltrials.gov/ct2/show/NCT00468923
- 26.Heart Outcomes Prevention and Evaluation 4 (HOPE-4). https://clinicaltrials.gov/ct2/show/NCT01826019, (NCT01826019), 2016.
- 27.Mant J. 2015. PROPS-preventative Role of a Fixed Dose Combination Pill in Stroke: A Multi-centre Open Label Randomized Controlled Trial of a Fixed Dose Combination Pill Versus Standard Care for Secondary Prevention of Stroke in a Primary Care Setting (ISRCTN58452386) ISRCTN Registry. [Google Scholar]
- 28.Secondary Prevention of Cardiovascular Disease in the Elderly Trial (SECURE). https://clinicaltrials.gov/ct2/show/NCT02596126, NCT02596126, 2015.
- 29.Ostovaneh M.R., Poustchi H., Hemmeing K. Polypill for the prevention of cardiovascular disease (PolyIran): study design and rationale for a pragmatic cluster randomized controlled trial. Eur. J. Prev. Cardiol. 2015;22:1609–1617. doi: 10.1177/2047487314550803. [DOI] [PMC free article] [PubMed] [Google Scholar]