Abstract
Recent evidence from embryonic stem cells suggests that the aryl hydrocarbon receptor (AHR) plays a central role in the regulation of pluripotency, a short-lived property of cells in the early blastula inner cell mass (ICM). Four key observations support this conclusion. The first is the temporal association between upregulation of AHR expression and the onset of cell differentiation, which argues for the AHR as a determinant of cell fate decisions. The second is the repression of the pluripotency factors OCT4 and NANOG by the AHR, which depresses their function and contributes to the cell's exit from pluripotency. The third is the temporal association between changes in global DNA methylation and stage-dependent AHR expression, which parallel each other during embryonic development, suggesting that AHR helps configure a repressive chromatin structure that controls differentiation. The fourth is the incidence of early developmental aberrations that take place in Ahr-null mice and cause the disruption of their embryonic program, which is likely to be a consequence of the loss of pluripotency of the Ahr−/− ICM cells.
In this short review, we will focus on the modulation of pluripotency as a novel function of the AHR, and on the potentially detrimental developmental outcomes that may result from exposure to environmental toxicants. This line of enquiry brings us to the tantalizing conclusion that by activating mechanisms that modulate pluripotency, AHR regulates embryonic development. The likelihood that exposure to environmental AHR ligands might disrupt developmental processes is a reasonable corollary to this conclusion.
Keywords: Aryl hydrocarbon receptor, embryonic development, pluripotency, cell cycle regulation, cell fate decisions, epigenetic reprogramming
1. Introduction
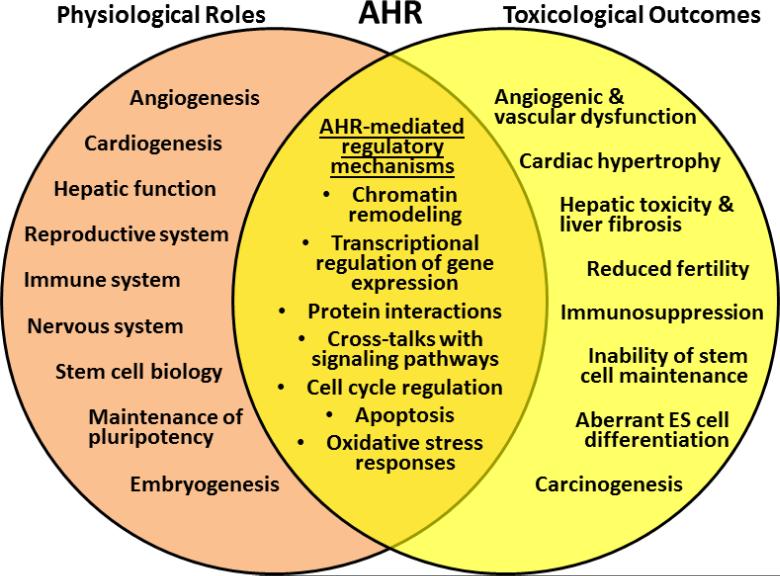
For nearly three decades, the primary function of the AHR, the only member of the bHLH/PAS family of transcription factors activated by known ligands [1], was thought to mediate the toxic responses to environmental contaminants such as PAHs, HAHs, and co-planar PCBs. However, discoveries of endogenous ligands and clues from animal studies during the last 10 - 15 years have uncovered new functions for this receptor in the regulation of normal physiology, cellular differentiation, and embryonic development [2-11]. Due to its functional duality between toxicity and normal physiology (Fig. 1) the AHR is uniquely positioned not only to convert signals from environmental toxicants into alterations of embryonic development, but to trigger a sustained state of cardiovascular insufficiency that increases the risk of adult disease [2, 8, 12-16]. Consonant with the postulates of the Barker Theory of the Developmental Origins of Health and Disease [17], developmental cardiac insufficiency may translate in the adult into congenital heart disease.
Fig. 1.
AHR dual functions in toxicity and normal physiology.
Disruption of endogenous AHR signaling, either by gene deletion or by exposure to dioxin or other DLCs, leads to alterations of embryonic development and results in pathogenic phenotypes, pointing at the possibility that one of AHR's endogenous functions might be to regulate aspects of the differentiation program defined by cell type specific epigenomic processes. Several lines of evidence support the view that the AHR safeguards normal embryogenesis by coordinating the choices between proliferation and differentiation, two concerted processes that eventually bring about pluripotency loss [18-20]. Appropriate control of AHR expression appears to be essential to maintain pluripotency, an objective that the blastomeres attain by maintaining the AHR in a state of positive repression mediated by the pluripotency factors themselves in combination with Polycomb Group repressors [18, 21]. AHR repression may provide the ICM cells with the opportunity to reprogram gene expression, implementing epigenetic mechanisms that bring about global changes in DNA methylation [22-27]. In addition, the AHR might also play a significant role in the determination of cell fate decisions, as suggested by the correlation between AHR expression and lineage specification after implantation [9, 28]. The fact that the pattern of AHR expression parallels these changes suggests that the association of the receptor with a repressive chromatin configuration might ultimately play a determinant role in differentiation. In this context, the presence of multiple early embryonic defects in Ahr-null mice suggests that the lack of AHR may disrupt embryonic programming [28-35].
We have focused this commentary on the regulation of pluripotency by the AHR and on the potentially deleterious outcomes that result from exposure to environmental toxicants. We will discuss the evidence showing the involvement of AHR in the maintenance of the stem cell pool and the determination of cell fate decisions, including an analysis of phenotypes arising in the Ahr knockout mice. In closing, we will discuss the mechanisms by which AHR might regulate pluripotency.
2. AHR and the homeostasis of pluripotency
2.1 AHR expression and DNA methylation during mouse embryonic development
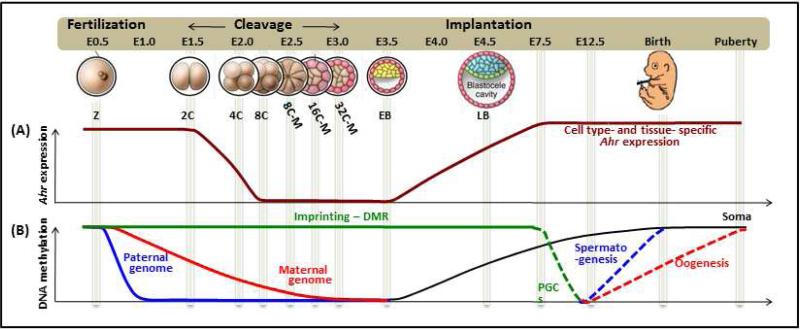
The first few cleavage divisions of embryonic development set in motion the transition from the totipotency of the cells in the morula to the pluripotency of the ICM cells in the blastocyst and reset the epigenomic plasticity of the embryo. Among the biochemical modifications that reconfigure chromatin structure and mark this transition, DNA methylation and epigenomic plasticity appear to proceed in opposite directions: the earlier the embryonic time, the greater the epigenomic plasticity and the lesser the extent of DNA methylation [26]. Later, as the embryo implants, the DNA methylome is established in a cell type specific manner that correlates with its fate specification. Interestingly, AHR expression parallels the global demethylation progress of the maternal DNA (Fig. 2). Fertilized eggs have high levels of Ahr mRNA and/or protein and their expression is maintained in the embryos up to the 4-cell stage morula, at which time individual blastomeres can be segregated according to the level of Ahr expression [22, 23]. Ahr mRNA expression completely disappears during the cleavage divisions and becomes de novo detectable in early blastocysts [24, 25, 27]. After implantation, the earliest that AHR expression has been detected is in E7.5 embryos [9], becoming thereafter detectable in almost all developing organs [24, 28]. These observations suggest that the AHR may play a stage-dependent developmental function, possibly behaving as a differentiation-promoting factor that opposses pluripotency. Consistent with this hypothesis, our recent study suggests that Ahr expression must be repressed lest the ES cells would slow down proliferation and lose pluripotency [18, 21]. A similar AHR expression pattern and regulatory function is found in the differentiation of hematopoietic stem cells and cancer stem cells [36-39] suggestive of a widespread AHR function in the ontogeny of diverse stem cell lineages.
Fig. 2. Schematic illustration of Ahr expression and genome-wide DNA methylation throughout the mouse life cycle.
(A): High levels of Ahr can be detected in early-fertilized eggs until the 4-cell stage morula. The expression completely disappears during cleavage cycles and becomes de novo detectable in the early blastocysts. After implantation, AHR expression can be detected starting in E7.5 embryos and thereafter can be found in almost all developing-organs. (B): Global changes of DNA methylation during epigenetic reprogramming in embryos (from reference [26]). After fertilization, the paternal genome undergoes active demethylation, whereas the demethylation of the maternal genome is slower and depends on DNA replication (by passive demethylation). These post-fertilization demethylation events do not include the differentially methylated regions of genomic imprinting loci. As blastocysts implant and the lineages start to be specified, new DNA methylation profiles are acquired during the specification of embryonic cells. Abbreviations: Z - zygote, 2C - 2-cell stage embryo, 4C - 4-cell stage embryo, 8C - 8-cell stage embryo, 8C-M - 8-cell stage morula, 16C-M - 16-cell stage morula, 32C-M - 32-cell stage morula, EB - early blastocyst, LB - late blastocyst, DMR - differentially methylated region, PGC - primordial germ cell.
Recently, TCDD exposure was showed to demethylate the promoter of the hepatic Cyp1a1 gene and induce persistent gene expression changes through the crosstalk of AHR signaling with the thymine DNA glycosylase TDG and the ten-eleven translocation methyldioxygenases TET2 and TET3 [40]. The parallel between AHR expression level and the amount of globally methylated DNA suggests that the AHR may be functionally associated with the organization of a repressive chromatin configuration. If this were the case, disruption of AHR signaling would lead to aberrant DNA methylation and ultimately to altered ontogeny. In good agreement with this prediction, maternal exposure to TCDD was shown to lead to DNA hypermethylation of murine CD8+ T cells and cause long-lasting effects in response to influenza virus infection later in life [13]. Similarly, rats exposed to TCDD in utero showed a good correlation between hypermethylation of the Brca1 gene promoter in mammary tissues and the incidence of breast cancer in adulthood [41]. Furthermore, TCDD exposure during in vitro ES cell differentiation decreased the expression levels of the DNA methyltransferase genes Dnmt3a and Dnmt3b [9], suggesting that AHR may also regulate the expression of enzymes responsible for de novo DNA methylation. In conclusion, the AHR appears to have a stage-dependent developmental function that coordinates the organization of the embryonic epigenome through modulation of the DNA methylome.
2.2 AHR function in the maintenance of pluripotency
Due to the limitations inherent to the use of ICM cells in vivo, the molecular mechanisms involved in maintaining pluripotency have been more often studied with ICM-derived ES cells in vitro, in which cells maintenance of pluripotency appears to be the result of the concerted outcome of epigenetic regulation, cell cycle monitored self-renewal, and balanced transcription [42-44]. In these cells, AHR could regulate pluripotency by controlling both self-renewal and gene expression at the transcriptional level, balancing the expression of the pluripotency factors OCT4, NANOG and SOX2 and of differentiation related markers [18]. Under physiological conditions, Ahr is repressed in the ES cells, but its untimely derepression causes pluripotency loss and mitotic delay. These observations suggest that AHR may control the pluripotent population by disrupting their self-renewal through lengthening mitosis. The same is true in hematopoietic stem cells, where knockout of Ahr or treatment with an AHR antagonist promotes their expansion and proliferation, indicative of the role of AHR in the maintenance of the stem cell pool [38, 39, 45-47]. Unlike in ES cells, however, AHR maintains the hematopoietic stem cell population by restricting their exit from quiescence. Altogether, these observations point at the possibility that AHR, activated by endogenous signals/ligands, may regulate the maintenance of pluripotency by modulating the size of the stem cell pool, a property closely associated with self-renewal ability.
2.3 AHR and cell differentiation
Observations from in vivo studies indicate that the premature expression of pioneer transcription factors during embryonic development determines the cell fate and composition of the succeeding cell populations [48, 49]. In fact, it has been proposed that trophoblast and ICM fates could be predicted from the inter-blastomere expression variability of several genes in embryos at the 4-cell-stage [23], the Ahr being one of these genes. Thus like other genes, such as Sox21, that have a cell-fate determining function, Ahr might also be engaged in the determination of preimplantation embryonic fates. However, when the AHR is untimely derepressed in ES cells, this function causes them to restrict their spontaneous differentiation into cardiomyocytes and to initiate a short-lived commitment to neuroglia [18]. Hence, by priming lineage specification, the AHR may function as a pioneer transcription factor. The possibility of segregating blastomeres on the basis of Ahr expression suggests that AHR might be associated with the determination of preimplantation embryonic fates. Consistent with this hypothetical AHR function, Ahr−/− ES cells, which have higher OCT4 expression levels than wild type, also have a delayed cardiomyocyte differentiation [10]. Furthermore, in vivo studies in Ahr knockout mice have shown that, although loss of the gene is not lethal, many embryos are unable to survive through in utero development [29] and of those that develop to term, the few that survive the perinatal period grow up with multi-organ dysfunction [30-35]. These observations suggest that the developmental program of Ahr−/− embryos is abnormal, likely because of alterations of pluripotency in the Ahr−/− ICM cells. We surmise that, as a result of unbalanced pluripotency, Ahr−/− embryos engage in biased cell fate decisions that either cause them to discontinue embryonic development and die in utero or to survive with multi-organ defects.
3. Potential mechanisms through which AHR modulates pluripotency
3.1 Regulation of cell cycle
The anti-proliferative function of the AHR is conserved between somatic and pluripotent stem cells [18, 50, 51] and, by regulating ES cell proliferation, could modulate pluripotency. In fact, we have recently shown that AHR controls the expression and activity of several mitotic regulators in ES cells, including MID1, 14-3-3, PP2A-B55α, CDK1, Y15pCDK1, and CDK1 [18]. Ahr derepression causes the loss of pluripotency and delayed mitotic progression through the disruption of MID1-PP2A-CDC25B-CDK1 signaling. These results illustrate the significance of mitotic control in the maintenance of pluripotency, highlighting how untimely Ahr derepression may perturb it. Appropriate control of mitosis has also been linked to developmental reprogramming, as indicated by the high responsiveness of the cytoplasm of mitotic zygotes to somatic nuclear transfer [52]. Furthermore, at the 4-cell stage morula, a time when embryonic decisions between proliferation and apoptosis are made, blastomeres can be segregated on the basis of Ahr expression, suggesting that the AHR may function as a regulator of early embryonic cell cycle [23, 53]. Taking into account the high levels of AHR expression in early embryos (up to the 4-cell stage), it seems reasonable to propose that by regulating mitosis the AHR functions to maintain pluripotency.
3.2 Interplay between AHR and pluripotency factors in stem cells
In ES cells, the AHR and the pluripotency factors OCT4 and SOX2 mutually regulate each other's expression. The mouse Ahr gene is repressed by the binding of OCT4/SOX2/NANOG complexes on a distal silencer domain of its promoter and is quickly derepressed as the cells differentiate [21]. Untimely AHR derepression downregulates the expression of OCT4 and SOX2 and reduces pluripotency [18]. Hence, although Ahr expression in ES cells is under the control of the network of pluripotency factors, an increase of AHR expression is likely to counteract the maintenance of pluripotency and to induce exit from the pluripotent state. Consistent with this idea, recent studies reveal that AHR turns off the expression of OCT4 and NANOG through Alu-transposon transcription during differentiation of human embryonic teratocarcinoma cells [37], suggesting that AHR may activate a mechanism that controls the expression of pluripotency genes in both pluripotent and differentiation states.
3.3 Epigenetic regulation through DNA methylation
As mentioned earlier, changes on the embryonic DNA methylome could be a potential mechanism for AHR to modulate pluripotency. Although the molecular mechanisms involved in this AHR function remain to be identified, disruption of endogenous AHR signaling by TCDD exposure or Ahr ablation during either embryonic development or ES differentiation attest to the potential involvement of AHR in the regulation of DNA methylome changes associated with lineage specification [9, 13, 41, 54, 55]. These effects of AHR signaling disruption may alter the determination of cell fate decisions during embryonic development by modulating the cell-type specific DNA methylome.
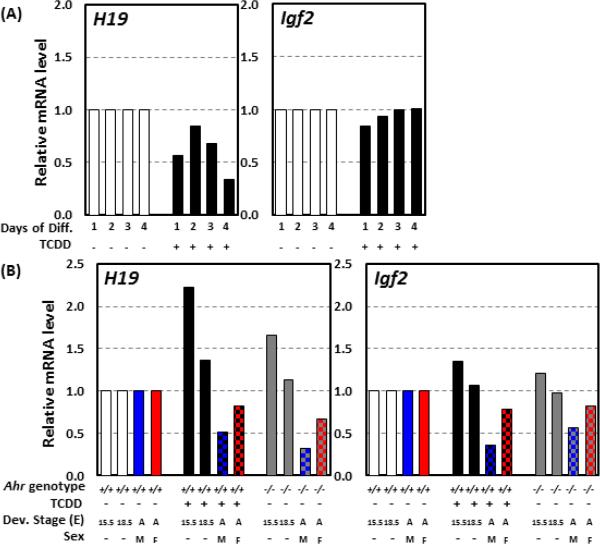
A case in point is the regulation of the imprinted H19-Igf2 locus, which is targeted by AHR during early embryogenesis. Embryos arising from TCDD-exposed fertilized eggs show decreased expression of both H19 and Igf2, accompanied by hypermethylation of the locus DMR [56]. Similar results were observed in our studies on TCDD treatment of differentiating ES cells, which resulted in downregulation of H19 expression and a trend to decrease Igf2 mRNA levels [9] (Fig. 3A). In vivo, TCDD exposure in utero or knockout of the Ahr gene led to altered expression of H19 and Igf2 in embryonic cardiac tissues. Both genes were upregulated in E15.5 and E18.5 sinoatrial node areas of the right atrium, an effect that persisted in both male and female adult mice, in which both genes were downregulated in the right atrium [2, 11] (Fig. 3B). This effect in cardiac tissues, common to Ahr knockout and TCDD exposure, suggests that TCDD disrupts endogenous AHR functions in the developing heart. Conceivably, one of the developmental functions of the AHR would be to regulate cardiogenesis by modulating the cardiac DNA methylome and the expression of imprinting genes. TCDD exposure may cause heart dysfunction by disrupting AHR's endogenous signaling. In this regard however, the likelihood that the potential disruptive effect of TCDD and other DLCs may depend on their biopersistence, would suggest that more easily metabolized ligands may be much less toxic to the endogenous AHR functions.
Fig. 3. H19 and Igf2 expression during in vitro differentiation of ES cells and in mouse embryonic and adult heart tissues.
(A): Expression levels of H19 and Igf2 at 1, 2, 3, and 4 days of ES differentiation with and without TCDD exposure. (B): Expression of H19 and Igf2 in cardiac sinoatral node of Ahr−/− mice and Ahr+/+ mice exposed in utero to TCDD or corn oil isolated from E15.5 and E18.5 embryonic hearts and in male and female right atria from adult heart. Abbreviations: Diff. - differentiation, Dev. - developmental, E - embryonic day, A - adult, M - male, and F - female.
4. Conclusion
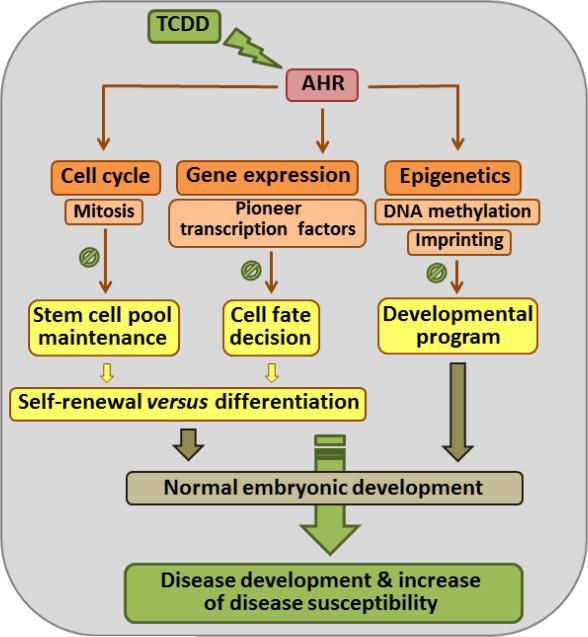
Whether and how AHR may regulate pluripotency is an open question. Here, we discuss the evidence and suggest potential mechanisms by which AHR might fulfill this function (Fig. 4). We propose that through activation by endogenous ligands AHR participates in the coordination of multiple biological processes that define pluripotency and embryonic development. One possibility is that AHR might control stem cell self-renewal by regulating the cell cycle. Alternatively, AHR might control the expression of both pluripotency factors and differentiation-related markers and might participate in priming cell fate decisions by regulating the balanced transcription of pluripotency genes. Yet another possibility is that AHR might help coordinate the embryonic epigenome by regulating the DNA methylome and imprinting during embryonic development. The appropriate coordination of these AHR functions would promote the development of embryos with normal phenotypes, while exposure to environmental contaminants could derail endogenous AHR signaling and perturb embryonic ontogeny to cause congenital developmental disease and increased risk of adult disease. Paradoxically, the adult impact of developmental exposure might turn out to be much more significant than the impact of direct exposure to the adult itself.
Fig. 4. Summary of potential mechanisms by which AHR may regulate embryonic development by modulating pluripotency.
AHR could regulate embryogenesis by modulating the essential mechanisms that define pluripotency. The involvement of AHR in cell cycle regulation, notably mitotic progression, in early embryonic cells could have a major impact in the maintenance of the stem cell pool. The regulatory role of AHR in the expression levels of pluripotency factors could interfere with the determination of cell fate decisions as stem cells differentiate. AHR could also participate in the acquisition of epigenome plasticity by modulating the cell type specific DNA methylome and genomic imprinting. Collectively, normal AHR functions result in the development of embryos with normal phenotypes; to the contrary, ablation of Ahr or derailed endogenous signaling caused by exposure to environmental toxicants would lead to disease and/or increased disease susceptibility.
Highlights.
The Ah receptor slows down stem cell mitotic progression, playing a central role in the regulation of pluripotency
Early developmental aberrations in Ahr-null mice cause the disruption of their embryonic program.
By activating mechanisms that modulate pluripotency, the Ah receptor regulates embryonic development.
Acknowledgements
We thank Dr. Ying Xia for a critical reading of the manuscript. The work in our lab was supported by NIH grants R01ES06273, R01ES010807, R01ES024744, and P30ES006096.
Abbreviations
- AHR
Aryl Hydrocarbon Receptor
- DLC
Dioxin-like Compounds
- DMR
Differentially Methylated Region
- ES
Embryonic Stem
- HAH
Halogenated Aryl Hydrocarbon
- HSC
Hematopoietic Stem Cells
- ICM
Inner Cell Mass
- PAH
Polycyclic Halogenated Hydrocarbon
- PCB
Polychlorinated Biphenyl
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TDG
Thymine DNA glycosylase
- TET
Ten-Eleven Translocation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Furness SG, Lees MJ, Whitelaw ML. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS letters. 2007;581:3616–3625. doi: 10.1016/j.febslet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 2••.Carreira VS, Fan Y, Kurita H, Wang Q, Ko CI, Naticchioni M, Jiang M, Koch S, Zhang X, Biesiada J, Medvedovic M, Xia Y, Rubinstein J, Puga A. Disruption of Ah Receptor Signaling during Mouse Development Leads to Abnormal Cardiac Structure and Function in the Adult. PloS ONE. 2015;10:e0142440. doi: 10.1371/journal.pone.0142440. [This article shows that cardiac defects resulting from in utero TCDD exposure could progress into heart abnormalities in the adult.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 4.Esser C. The Aryl Hydrocarbon Receptor in Immunity: Tools and Potential. Methods Mol. Biol. 2016;1371:239–257. doi: 10.1007/978-1-4939-3139-2_16. [DOI] [PubMed] [Google Scholar]
- 5.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug. Metab. Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeger C, Tischkau SA. Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction. Environ. Health Insights. 2016;10:133–141. doi: 10.4137/EHI.S38343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laiosa MD, Tate ER, Ahrenhoerster LS, Chen Y, Wang D. Effects of Developmental Activation of the Aryl Hydrocarbon Receptor by 2,3,7,8-Tetrachlorodibenzo--dioxin on Long-Term Self-Renewal of Murine Hematopoietic Stem Cells. Env. Health Perspect. 2016;124(7):957–965. doi: 10.1289/ehp.1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Chen J, Ko CI, Fan Y, Carreira V, Chen Y, Xia Y, Medvedovic M, Puga A. Disruption of aryl hydrocarbon receptor homeostatic levels during embryonic stem cell differentiation alters expression of homeobox transcription factors that control cardiomyogenesis. Env. Health Perspect. 2013;121:1334–1343. doi: 10.1289/ehp.1307297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Kurita H, Carreira V, Ko CI, Fan Y, Zhang X, Biesiada J, Medvedovic M, Puga A. Ah Receptor Activation by Dioxin Disrupts Activin, BMP, and WNT Signals During the Early Differentiation of Mouse Embryonic Stem Cells and Inhibits Cardiomyocyte Functions. Toxicol. Sci. 2016;149:346–357. doi: 10.1093/toxsci/kfv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreira VS, Fan Y, Wang Q, Zhang X, Kurita H, Ko CI, Naticchioni M, Jiang M, Koch S, Medvedovic M, Xia Y, Rubinstein J, Puga A. Ah Receptor Signaling Controls the Expression of Cardiac Development and Homeostasis Genes. Toxicol. Sci. 2015;147:425–435. doi: 10.1093/toxsci/kfv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahrenhoerster LS, Leuthner TC, Tate ER, Lakatos PA, Laiosa MD. Developmental exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin attenuates later-life Notch1-mediated T cell development and leukemogenesis. Toxicol. Appl. Pharmacol. 2015;283:99–108. doi: 10.1016/j.taap.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boule LA, Burke CG, Fenton BM, Thevenet-Morrison K, Jusko TA, Lawrence BP. Developmental Activation of the AHR Increases Effector CD4+ T Cells and Exacerbates Symptoms in Autoimmune Disease-Prone Gnaq+/− Mice. Toxicol. Sci. 2015;148:555–566. doi: 10.1093/toxsci/kfv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boule LA, Winans B, Lambert K, Vorderstrasse BA, Topham DJ, Pavelka MS, Jr., Lawrence BP. Activation of the aryl hydrocarbon receptor during development enhances the pulmonary CD4+ T-cell response to viral infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L305–313. doi: 10.1152/ajplung.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongan M, Meng Q, Wang J, Kao WW, Puga A, Xia Y. Gene-Environment Interactions Target Mitogen-activated Protein 3 Kinase 1 (MAP3K1) Signaling in Eyelid Morphogenesis. J. Biol. Chem. 2015;290:1977019779. doi: 10.1074/jbc.M115.665729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Winans B, Nagari A, Chae M, Post CM, Ko CI, Puga A, Kraus WL, Lawrence BP. Linking the aryl hydrocarbon receptor with altered DNA methylation patterns and developmentally induced aberrant antiviral CD8+ T cell responses. J. Immunol. 2015;194:4446–4457. doi: 10.4049/jimmunol.1402044. [This article shows that developmental TCDD exposure changed the DNA methylome of CD8+ T cells and reduced their response during influenza virus infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker DJ. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 18••.Ko CI, Fan Y, de Gannes M, Wang Q, Xia Y, Puga A. Repression of the Aryl Hydrocarbon Receptor is Required to Maintain Mitotic Progression and Prevent Loss of Pluripotency of Embryonic Stem Cells. Stem Cells. 2016 doi: 10.1002/stem.2456. Epub ahead of print. [This article shows the regulatory role of AHR in the expression of pluripotency factors and the level of pluripotency. Additional results from this article show that AHR regulates the maintenance of the embryonic stem cell pool by modulating mitotic progression and mitotic MID1-PP2A-CDC25B-CDK1 signaling.] [DOI] [PubMed] [Google Scholar]
- 19.Mulero-Navarro S, Fernandez-Salguero PM. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016;4:45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J, Sheng B, Qiu Y, Yang K, Xiao W, Yang H. Role of AhR in positive regulation of cell proliferation and survival. Cell Prolif. 2016;49:554–560. doi: 10.1111/cpr.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko CI, Wang Q, Fan Y, Xia Y, Puga A. Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem cell Res. 2014;12:296–308. doi: 10.1016/j.scr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey A, Nebert DW. Markedly increased constitutive CYP1A1 mRNA levels in the fertilized ovum of the mouse. Biochem. Biophys. Res. Commun. 1998;251:657–661. doi: 10.1006/bbrc.1998.9519. [DOI] [PubMed] [Google Scholar]
- 23••.Goolam M, Scialdone A, Graham SJ, Macaulay IC, Jedrusik A, Hupalowska A, Voet T, Marioni JC, Zemicka-Goetz M. Heterogeneity in Oct4 and Sox2 Targets Biases Cell Fate in 4-Cell Mouse Embryos. Cell. 2016;165:61–74. doi: 10.1016/j.cell.2016.01.047. [This article shows that blastomeres of the 4-cell stage morula can be segregated into high Ahr- and low Ahr-expressing cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech. Dev. 1998;73:117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 25.Peters JM, Wiley LM. Evidence that murine preimplantation embryos express aryl hydrocarbon receptor. Toxicol. Appl. Pharmacol. 1995;134:214–221. doi: 10.1006/taap.1995.1186. [DOI] [PubMed] [Google Scholar]
- 26.Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28:33–42. doi: 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Ohsako S, Baba T, Miyamoto K, Tohyama C. Effects of 2,3,7,8-tetrachlorodibenzo-pdioxin (TCDD) on preimplantation mouse embryos. Toxicology. 2002;174:119–129. doi: 10.1016/s0300-483x(02)00047-1. [DOI] [PubMed] [Google Scholar]
- 28.Abbott BD, Probst MR. Developmental expression of two members of a new class of transcription factors: II. Expression of aryl hydrocarbon receptor nuclear translocator in the C57BL/6N mouse embryo. Dev. Dyn. 1995;204:144–155. doi: 10.1002/aja.1002040205. [DOI] [PubMed] [Google Scholar]
- 29.Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JJ. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Salguero PM, Pineau T, Hibert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–776. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 32.Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahvis GP, Pyzaiski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol. Pharmacol. 2005;67:714–720. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- 34.Lund AK, Agbor LN, Zhang N, Baker A, Zhao H, Fink GD, Kanagy NL, Walker MK. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension. 2008;51:803–809. doi: 10.1161/HYPERTENSIONAHA.107.100586. [DOI] [PubMed] [Google Scholar]
- 35.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem cell Rev. 2012;8:1223–1235. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Morales-Hernandez A, Gonzalez-Rico FJ, Roman AC, Rico-Leo E, Alvarez-Barrientos A, Sanchez L, Macia A, Heras SR, Garcia-Perez JL, Merino JM, Fernandez-Salguero PM. Alu retrotransposons promote differentiation of human carcinoma cells through the aryl hydrocarbon receptor. Nuclear Acids Res. 2016;44:4665–4683. doi: 10.1093/nar/gkw095. [This article suggests that AHR participates in the downregulation of OCT4 and NANOG expression through Alu-transposons transcription.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cell. Dev. 2011;20:769–784. doi: 10.1089/scd.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unnisa Z, Singh KP, Henry EC, Donegan CL, Bennett JA, Gasiewicz TA. Aryl Hydrocarbon Receptor Deficiency in an Exon 3 Deletion Mouse Model Promotes Hematopoietic Stem Cell Proliferation and Impacts Endosteal Niche Cells. Stem Cell. Int. 2016:4536187.44. doi: 10.1155/2016/4536187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amenya HZ, Tohyama C, Ohsako S. Dioxin induces Ahr-dependent robust DNA demethylation of the Cyp1a1 promoter via Tdg in the mouse liver. Sci. Rep. 2016;6:34989. doi: 10.1038/srep34989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aluru N, Kuo E, Helfrich LW, Karchner SI, Linney EA, Pais JE, Franks DG. Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters DNA methyltransferase (dnmt) expression in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2015;284:142–151. doi: 10.1016/j.taap.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boroviak T, Nichols J. The birth of embryonic pluripotency. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2014;369:1657. doi: 10.1098/rstb.2013.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Biol. 2013;20:282–289. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- 44.Christophersen NS, Helin K. Epigenetic control of embryonic stem cell fate. J. Exp. Med. 2010;207:2287–2295. doi: 10.1084/jem.20101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol. Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- 46.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rentas S, Holzapfel NT, Belew MS, Pratt GA, Voisin V, Wilhelm BT, Bader GD, Yeo GW, Hope KJ. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016;532:508–511. doi: 10.1038/nature17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Artus J, Chazaud C. A close look at the mammalian blastocyst: epiblast and primitive endoderm formation. Cellular and molecular life sciences : CMLS. 2014 Sep;71:3327. doi: 10.1007/s00018-014-1630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 50.Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor A MA. Puga, Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol. Cell. Biol. 2007;27:6127–6139. doi: 10.1128/MCB.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70:212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Egli D, Rosains J, Birkhoff G, Eggan K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007;447:679–685. doi: 10.1038/nature05879. [This article describes the reprogramming activity of the mitotic zygote cytoplasm.] [DOI] [PubMed] [Google Scholar]
- 53.Fabian D, Makarevich AV, Chrenek P, Bukovska A, Koppel J. Chronological appearance of spontaneous and induced apoptosis during preimplantation development of rabbit and mouse embryos. Theriogenology. 2007;68:1271–1281. doi: 10.1016/j.theriogenology.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 54.Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol. Carcinog. 2015;54:261–269. doi: 10.1002/mc.22095. [DOI] [PubMed] [Google Scholar]
- 55.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 56••.Wu Q, Ohsako S, Ishimura R, Suzuki JS, Tohyama C. Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2. Biol. Reprod. 2004;70:1790–1797. doi: 10.1095/biolreprod.103.025387. [This article shows that the H19-Igf2 imprinted locus of embryos developped from TCDD exposed fertilized eggs have a hypermethylated differentially methylated region and decreased expression of both H19 and Igf2.] [DOI] [PubMed] [Google Scholar]